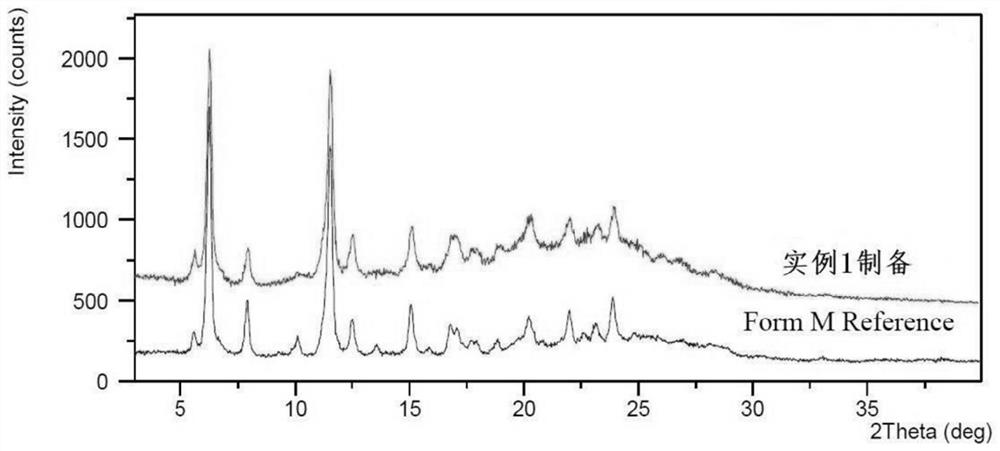
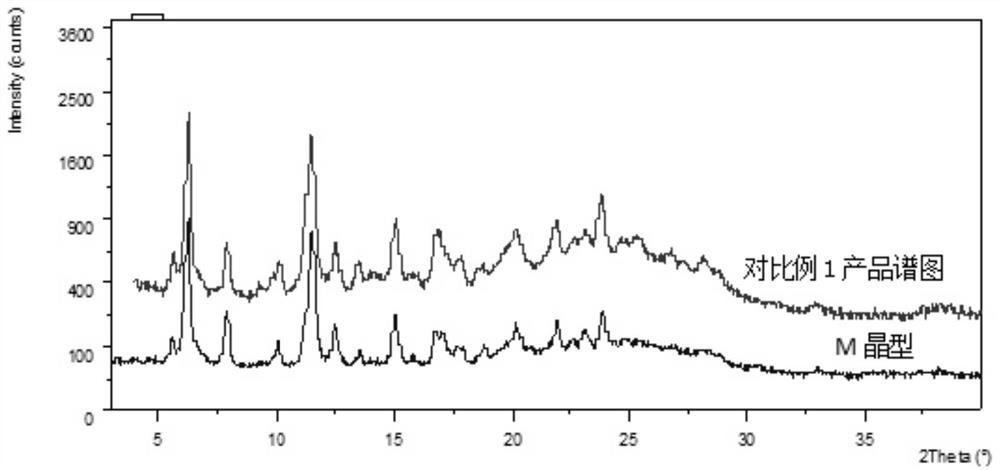
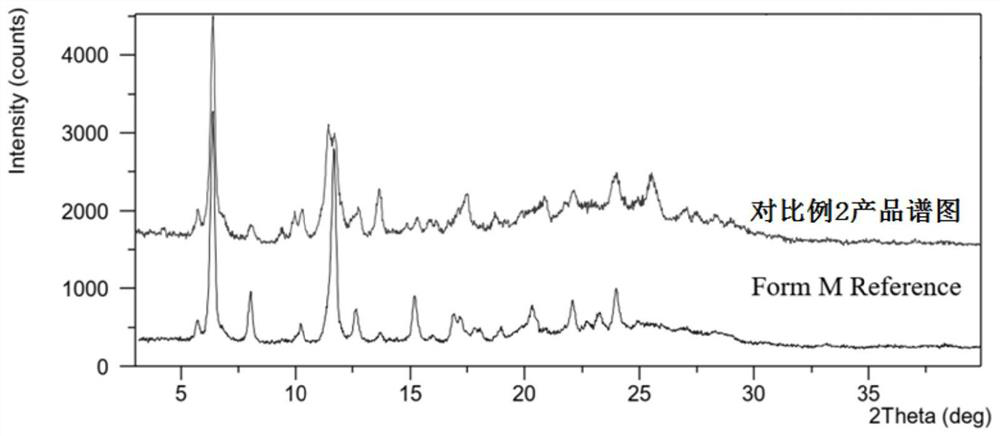
Preparation method of lenvatinib mesylate M crystal form
A technology of lenvatinib and methanesulfonic acid, which is applied in the field of stable preparation of lenvatinib mesylate M crystal form, and can solve the problem of poor repeatability of the crystal form M process, unqualified genotoxic impurity content, crystal transformation To achieve the effects of good physical and chemical stability, control of genotoxic impurity content, and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Put 6.0kg of lenvatinib and 120.5kg of acetonitrile into a 500L enamel kettle equipped with a nitrogen protection device to cool down to -10-0°C, add 1.35kg of methanesulfonic acid dropwise, and keep stirring for 8 hours. At an ambient temperature of 0-5° C. and a humidity of less than 50% RH, the reaction solution was filtered to obtain a wet product of lenvatinib mesylate. Put the obtained wet product into a desiccator, at 25-30°C, pass high-purity nitrogen gas, control the vacuum degree at -0.085--0.080MPa, and dry the wet product until the residual acetonitrile is less than the limit of 410ppm stipulated in the Pharmacopoeia. After drying, control the temperature of the desiccator at 5-15°C, and pass in 15-25% RH humidified nitrogen until the product moisture is 3.0-6.0%, and 6.97kg of light yellow lenvatinib mesylate salt M crystal form is obtained. l H-NMR (400MHz, DMSO-d6) δppm8.91(d, J=6.0Hz, 1H), 8.73(s, 1H), 8.36(d, J=9.1Hz, 1H), 8.04(s, 1H), 7.93 (s,1H),7.85...
Embodiment 2
[0029] Put 6.0kg of lenvatinib and 150.8kg of acetonitrile into a 500L enamel kettle equipped with a nitrogen protection device to cool down to -5-5°C, add 1.42kg of methanesulfonic acid dropwise, and keep stirring for 8 hours. At an ambient temperature of 0-5° C. and a humidity of less than 50% RH, the reaction solution was filtered to obtain a wet product of lenvatinib mesylate. Put the obtained wet product into a desiccator, at 25-30°C, pass high-purity nitrogen gas, control the vacuum degree at -0.085--0.080MPa, and dry the wet product until the residual acetonitrile is less than the limit of 410ppm stipulated in the Pharmacopoeia. After drying, control the temperature of the desiccator at 5-15°C, and pass in 15-25% RH humidified nitrogen until the product moisture is 3.0-6.0%, and 6.9 kg of light yellow lenvatinib mesylate salt M crystal form is obtained.
Embodiment 3
[0043] Take an appropriate amount of the crystals prepared in Example 1 and Comparative Example 5 and place them under the humidity conditions of 40% RH, 50% RH, 60% RH and 70% RH respectively to investigate the hygroscopicity of the crystals. The results are shown in Table 2.
[0044] Table 2 The results of the investigation on the hygroscopicity of crystallization
[0045]
[0046] As can be seen from Table 2, compared with the crystals prepared without water replenishment operation:
[0047] (1) Under the same humidity conditions, the weight gain of the crystals after water replenishment is smaller, that is, the crystals after water replenishment are less affected by the ambient humidity than the crystals without water replenishment, therefore, it is more conducive to the production of raw material drug screening process operate;
[0048] (2) Under the condition of 40% RH humidity, the crushing and sieving process is carried out. It can be seen from the weight gain by h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com