New crystal form of lenvatinib mesylate, and preparation method thereof
A technology for lenvatinib mesylate and mesylate, which is applied to the new crystal form of lenvatinib mesylate and the field of preparation thereof, can solve the problem that the preparation process of crystal form M is difficult to control, difficult to purify, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 The preparation method of lenvatinib mesylate salt crystal form 6
[0059] Put 1.0733 g of lenvatinib free base solid in a jacketed reaction flask, add 25 mL of acetonitrile and water mixed solvent (the volume ratio of acetonitrile and water is 99 / 1), and stir at 10°C. 155 μL of methanesulfonic acid (purity>99%) was added into 3 mL of acetonitrile, and the solution of methanesulfonic acid in acetonitrile was dropped into the reaction flask within 120 minutes. Stirring was continued for 22 hours at 10°C. After filtration, the filter cake was dried at room temperature to obtain a white solid.
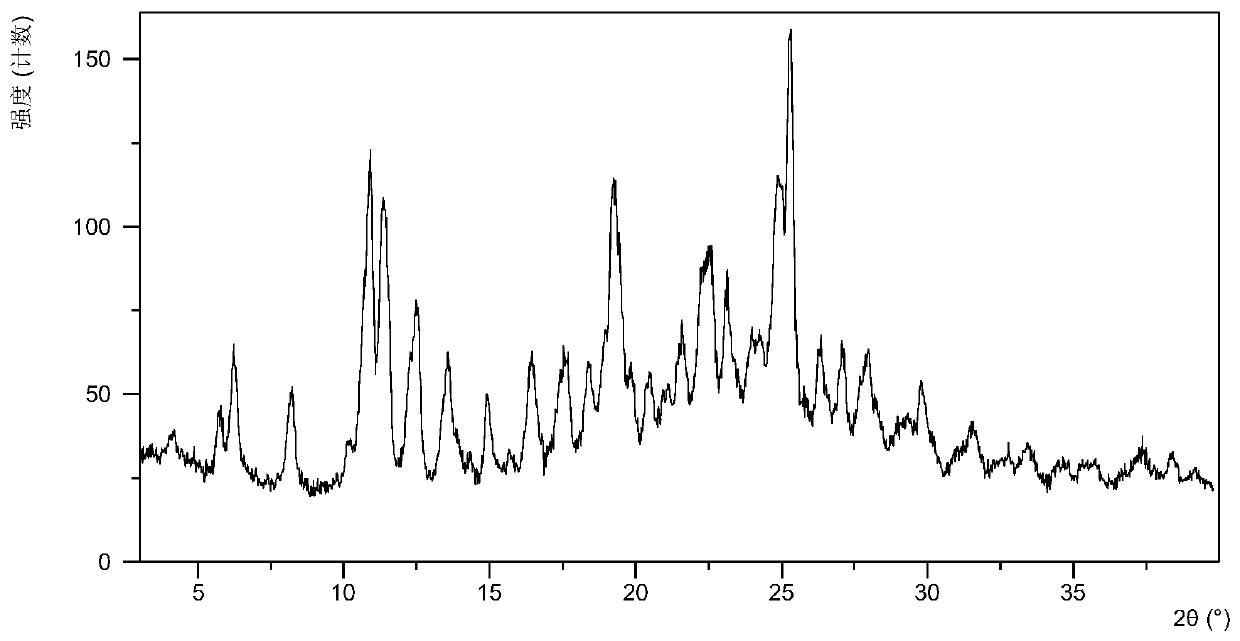
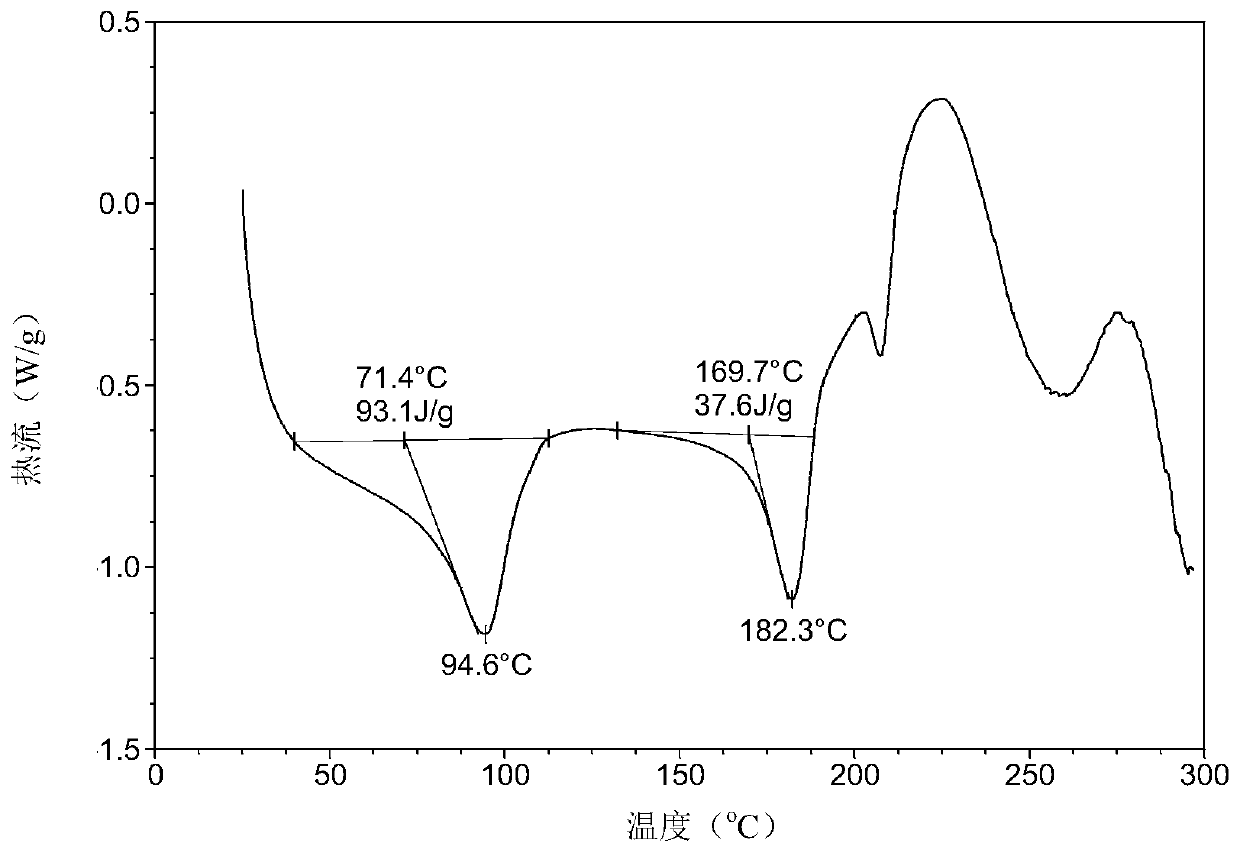
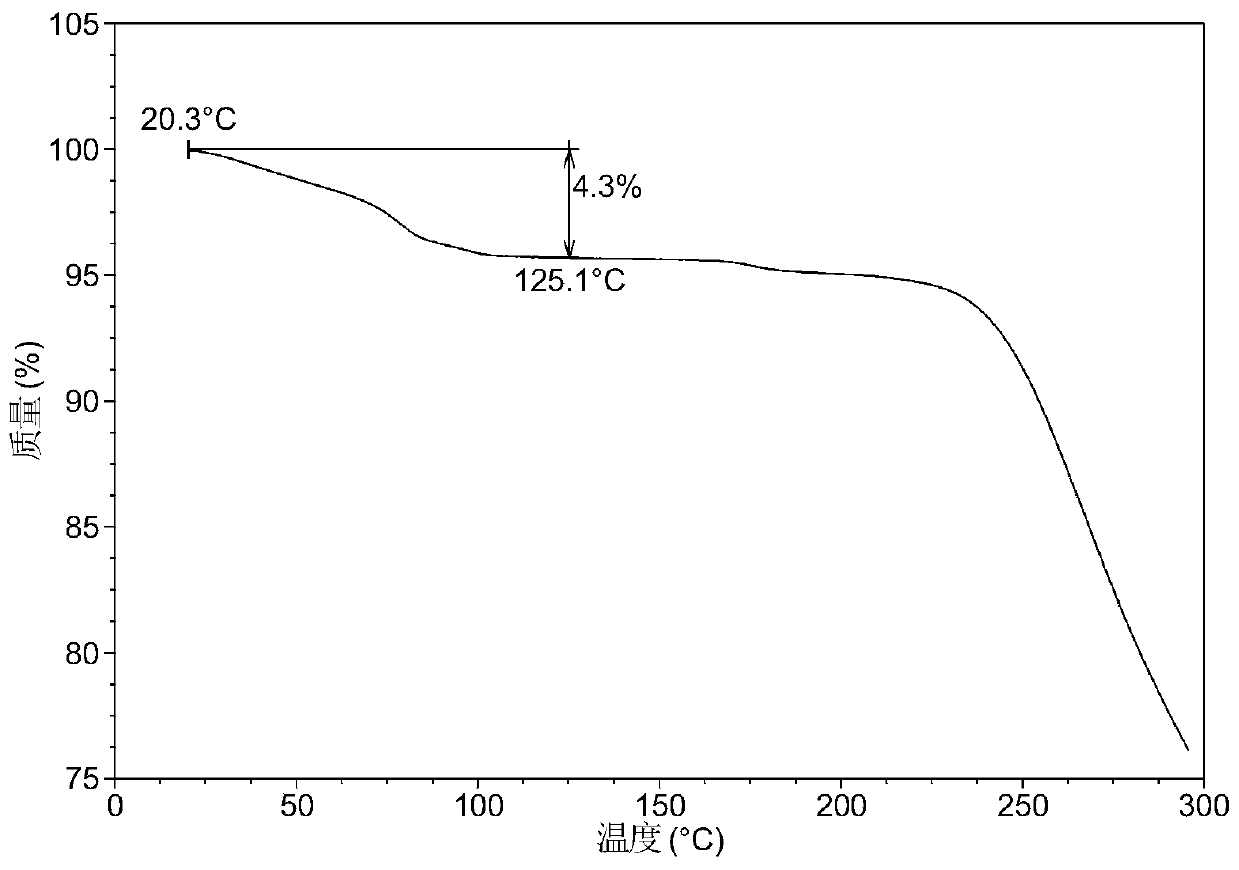
[0060] After testing, the white solid obtained in this experiment is the crystal form 6 of lenvatinib mesylate. Its X-ray powder diffraction data are as follows figure 1 , shown in Table 1. DSC of Form 6 as figure 2 As shown, when heated to 71°C, dehydration begins. TGA of Form 6 as image 3 As shown, when heated to 125 ° C, there is about 4.3% weight loss.
[00...
Embodiment 2
[0065] Stability test of embodiment 2 mesylate crystal form 6
[0066] Samples of crystal form 6 of the mesylate salt of the present invention were taken respectively and placed under the conditions of room temperature / 22.5% relative humidity and room temperature / 43.2% relative humidity for 13 weeks, and samples were taken to detect changes in the crystal form. The results are shown in Table 2. XRPD comparison pictures of mesylate salt form 6 before and after placement Figure 5 shown. Figure 5 The upper picture is before storage, the middle one is after 13 weeks at room temperature / 22.5% relative humidity, and the lower picture is after 13 weeks at room temperature / 43.2% relative humidity.
[0067] Table 2
[0068] starting crystal form condition placement time Crystalline change Mesylate salt form 6 Room temperature / 22.5% relative humidity 13 weeks constant Mesylate salt form 6 Room temperature / 43.2% relative humidity 13 weeks constan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com