A kind of lenvatinib mesylate solid dispersion and its preparation method and application
A solid dispersion, lenvatinib technology, applied in the field of medicine, can solve the problem of low solubility of lenvatinib mesylate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
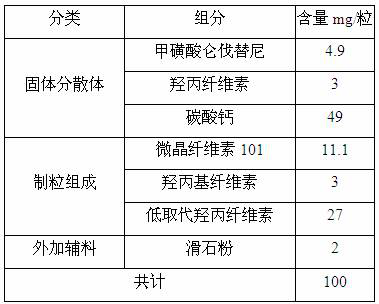
[0022] Embodiment 1: Preparation of lenvatinib mesylate solid dispersion (I)
[0023] Add 24.5g of lenvatinib mesylate into 150ml of acetone / water mixed solvent (mass ratio, 10:1), heat up to about 55°C, stir to dissolve, add 15g of hydroxypropyl cellulose evenly, stir to dissolve ,spare. Add 245g of calcium carbonate into the fluidized bed, set the inlet air temperature at 60°C, and when the material temperature reaches 25°C, spray the lenvatinib mesylate solution into the fluidized bed. After spraying, add 10mL of acetone / water mixed solvent (mass ratio, 10:1) to spray into the fluidized bed, and dry until the water content is below 1.0%. After grinding, 268.23 g of solid dispersion was obtained, yield 94.28%.
Embodiment 2
[0024] Embodiment 2: Preparation of lenvatinib mesylate solid dispersion (II)
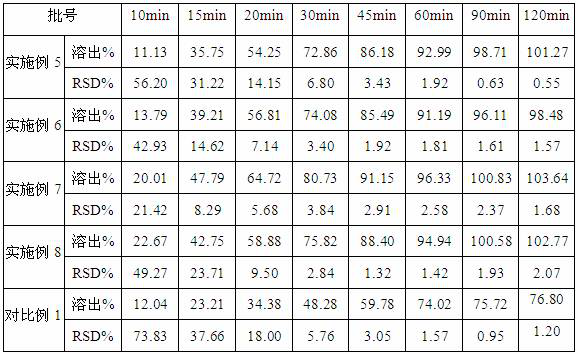
[0025] Add 49g of lenvatinib mesylate into 200ml of acetone / water mixed solvent (mass ratio, 10:1), heat up to about 55°C, stir to dissolve, add 30g of hypromellose evenly, stir to dissolve, spare. Add 490g of calcium carbonate into the high-shear wet granulator, open the cutting knife and the granulating knife, and atomize and spray the lenvatinib mesylate solution. After that, put the material into a blast drying oven at 60°C to dry to a moisture content below 1%. After grinding, the collected material was 529.21g, and the yield was 93.01%.
Embodiment 3
[0026] Embodiment 3: Preparation of lenvatinib mesylate solid dispersion (III)
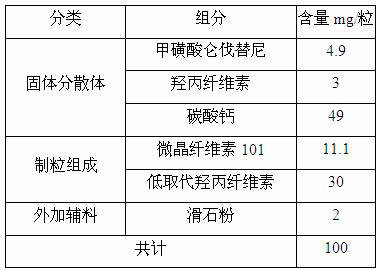
[0027] Add 25g of lenvatinib mesylate into 150ml of acetone / water mixed solvent (mass ratio, 10:1), heat up to about 55°C, stir to dissolve, add 15g of hydroxypropyl cellulose evenly, stir to dissolve, spare. Add 200g of mannitol into the fluidized bed, set the inlet air temperature to 60°C, and when the material temperature reaches 25°C, spray the lenvatinib mesylate solution into the fluidized bed. After spraying, add 10mL of acetone / water mixed solvent (mass ratio, 10:1) to spray into the fluidized bed, and dry until the water content is below 1.0%. After grinding, 213.34 g of solid dispersion was obtained, yield 88.89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com