Novel crystal form of lenvatinib mesylate
A mesylate salt and crystal form technology, applied in the preparation of organic compounds, organic chemistry, organic chemistry methods, etc., can solve problems affecting the bioavailability of drugs, affecting the dissolution and release of pharmaceutical compositions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
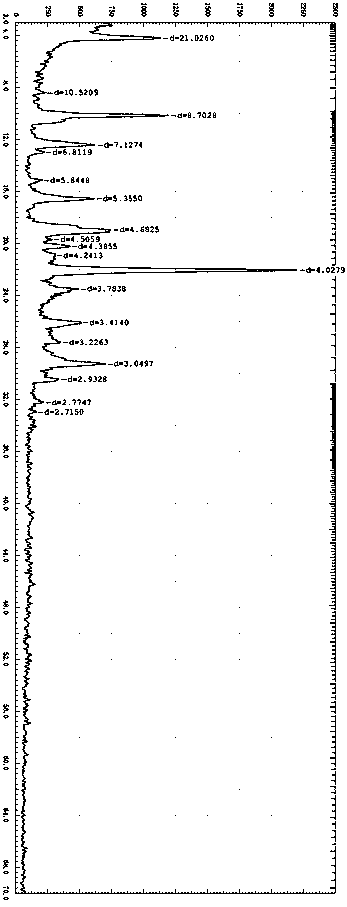
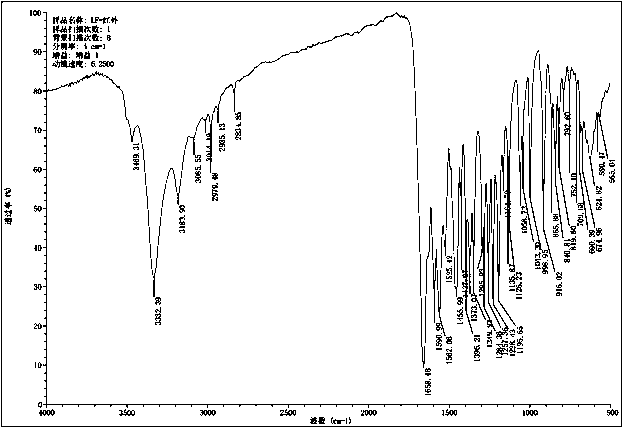
[0050] Get 10g of 4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxyl-6-quinoline carboxamide methanesulfonate and add it to a mixture with a volume ratio of 2:1 Organic solvent (N,N-dimethylformamide and dioxane) and aqueous solution, stirred and dissolved at room temperature, then lowered to 0-5 ° C, added 5 times the volume of water, stirred until crystals slowly precipitated; centrifuged Gained solid is collected, and the X-ray diffraction pattern of gained product is as follows figure 1 shown.
Embodiment 2
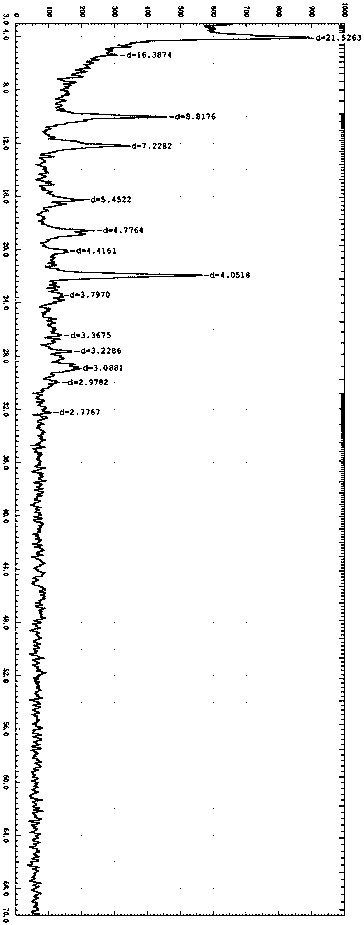
[0052] Get 30g of 4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxyl-6-quinoline carboxamide methanesulfonate and join in the mixing mixture whose volume ratio is 4:1 Organic solvent (N,N-dimethylformamide and dioxane) and aqueous solution, stirred and dissolved at room temperature, then lowered to 0-5°C, added 10 times the volume of water, stirred until crystals slowly precipitated; centrifuged The obtained solid was collected, and the X-ray diffraction spectrum of the product obtained was basically consistent with that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com