HPLC method for detecting cyclopropylamine in lenvatinib mesylate
A technology of lenvatinib mesylate and cyclopropylamine, which is applied in the field of HPLC analysis of cyclopropylamine, can solve the problems of inability to control the content of cyclopropylamine, low detection sensitivity, single product, etc., and achieves simple and easy analysis cost , single product, accurate and reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
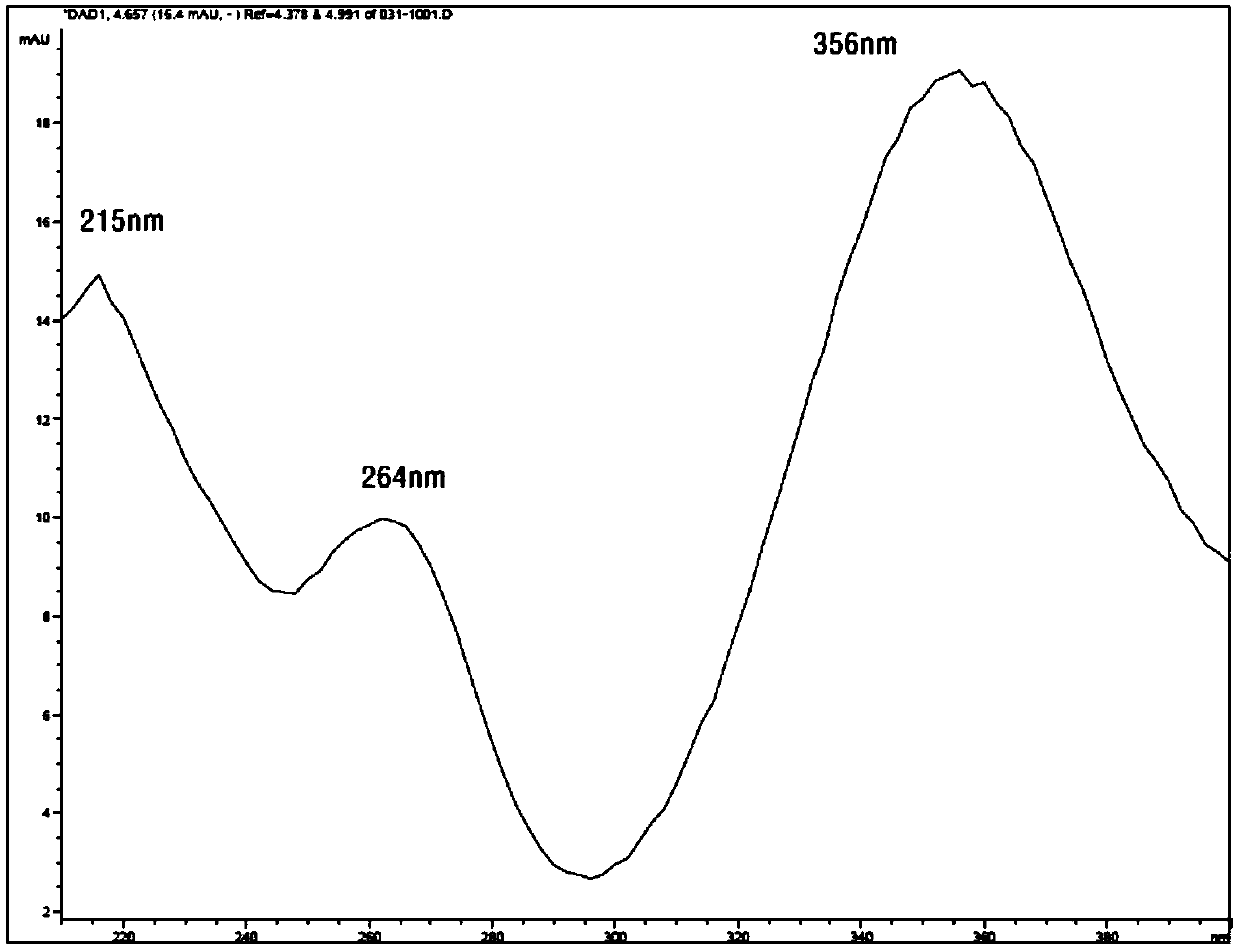
[0031] Take an appropriate amount of cyclopropylamine, add an appropriate amount of solvent [acetonitrile-water (80:20)], add a derivatization reagent 2,4-dinitrochlorobenzene solution to prepare a control solution, and use a diode array detector (DAD) to perform full-wavelength spectroscopy The results show that the maximum absorption of SCR-4341 derivatized chromatographic peaks is at 215nm, 264nm, and 356nm. At 356nm wavelength, the detection sensitivity is higher and the interference is less, so the 356nm wavelength is selected as the measurement wavelength of this product. See the detection spectrum figure 1 .
Embodiment 2
[0033] 1) Chromatographic conditions
[0034] Instrument: high performance liquid chromatography
[0035] Chromatographic column: Agilent Zorbax-Bonus-RP-C18 (150mm×4.6mm, 3.5μm)
[0036] Mobile phase: 0.05mol / L ammonium acetate solution (take 3.85g of ammonium acetate, add 1000ml of water to dissolve, add 1ml of glacial acetic acid to mix, adjust the pH to 5.0)-acetonitrile-methanol (18:11:11)
[0037] Solvent: acetonitrile - water (80:20)
[0038] Injection volume: 10μl
[0039] Detection wavelength: 356nm
[0040] 2) Sample preparation
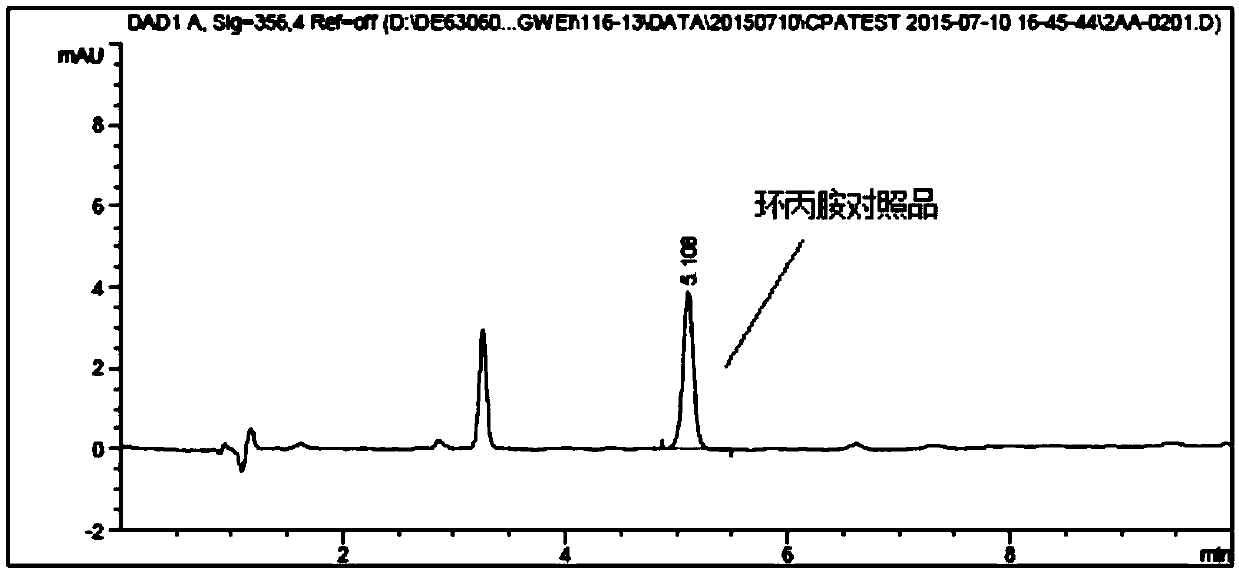
[0041] (1) Accurately weigh an appropriate amount of SCR-4341 reference substance, prepare a solution containing about 7.5mg of SCR-4341 per 1ml, accurately measure 2ml into a 20ml headspace bottle, add lenvatinib mesylate 10mg, and accurately measure Take 0.5ml of sodium carbonate aqueous solution (take 2.5g of sodium carbonate, add 25ml of water to dissolve it) 0.5ml, add derivatization reagent 2,4-dinitrochlorobenzene solution (take...
Embodiment 3
[0048] 1) Chromatographic conditions
[0049] Instrument: high performance liquid chromatography
[0050] Chromatographic column: Agilent Zorbax-Bonus-RP-C18 (150mm×4.6mm, 3.5μm)
[0051] Mobile phase: 0.05mol / L ammonium acetate solution (take 3.85g of ammonium acetate, add 1000ml of water to dissolve, add 1ml of glacial acetic acid to mix, adjust the pH to 5.0)-acetonitrile-methanol (18:11:11)
[0052] Solvent: acetonitrile - water (80:20)
[0053] Injection volume: 10μl
[0054] Detection wavelength: 356nm
[0055] 2) Sample preparation
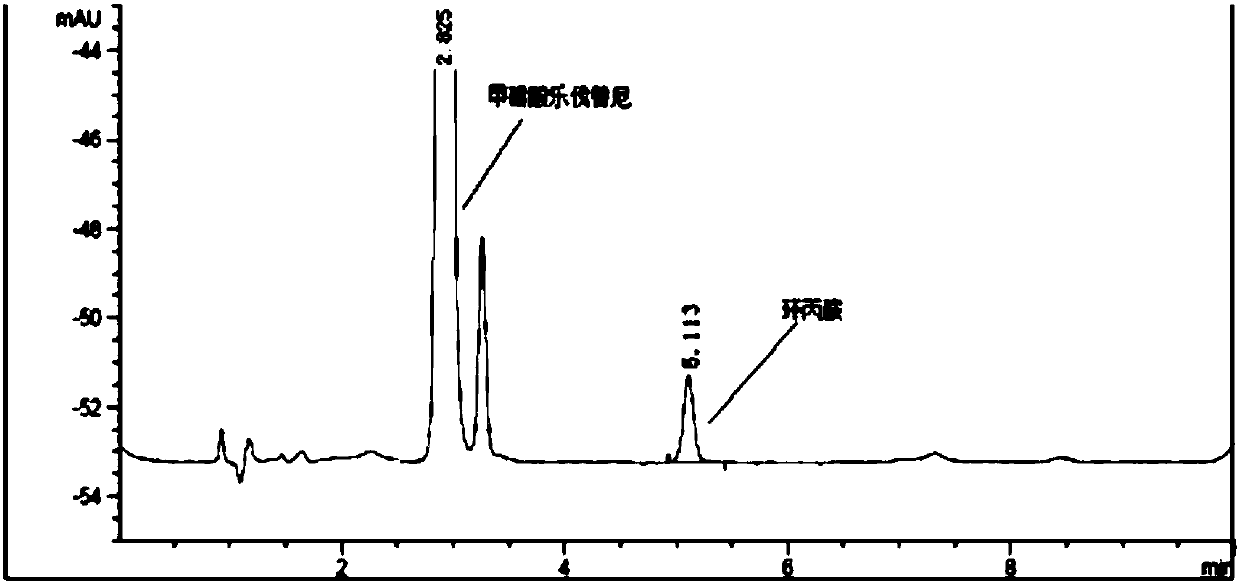
[0056] Accurately weigh an appropriate amount of cyproteramine reference substance, add solvent acetonitrile-water (80:20) and quantitatively dilute to make a solution containing about 7 μg per 1ml as the reference substance solution, take 2ml of the reference substance stock solution and put it in a 20ml headspace bottle, add derivative Chemical reagent 2,4-dinitrochlorobenzene solution (1mg / ml) 0.1ml or 1ml, investigate 40 ℃, 60 ℃ an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com