Method for detecting methanesulfonate genotoxic impurities in lenvatinib mesylate by gas chromatography
A technology of gas chromatography and methyl methanesulfonate, applied in the field of pharmaceutical analysis, can solve the problems of low accuracy, inability to accurately control mesylate substances, low recovery rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] 1) Test solution preparation:
[0063] (1) Solvent: Acetonitrile and water are mixed in a volume ratio of 60:40;
[0064] (2) Derivatization solution: Weigh 20 g of sodium iodide and 20 mg of vitamin C, weigh them accurately, add water to dissolve and dilute to 20 mL, and obtain a derivatization solution containing about 1 g of sodium iodide and 1 mg of vitamin C per 1 ml.
[0065] (3) Blank solution: Weigh 10g of sodium iodide and 10mg of vitamin C, weigh them accurately, add water to dissolve and dilute to 10ml, and obtain a blank solution containing 1g of sodium iodide and 1mg of vitamin C per 1ml of solution.
[0066] (4) Reference substance stock solution: Weigh 25 mg of methyl methanesulfonate, ethyl methanesulfonate and isopropyl methanesulfonate respectively, weigh them accurately, add 100 mL of solvent to quantitatively dilute to make about 1 ml of methanesulfonic acid A solution of 250 μg each of methyl ester, ethyl methanesulfonate and isopropyl methanesulfo...
Embodiment 2
[0093] Embodiment 2 external standard method comparative verification
[0094] Under the preparation of the solution, solvent, 100% reference substance solution, 50% limit solution of the test product, 100% limit solution of the test product, and 150% limit solution of the test product are chromatographed according to specificity Conditional headspace injection was performed, and the recovery rate was determined by the peak area external standard method.
[0095] Table 2 External standard method recovery rate result
[0096]
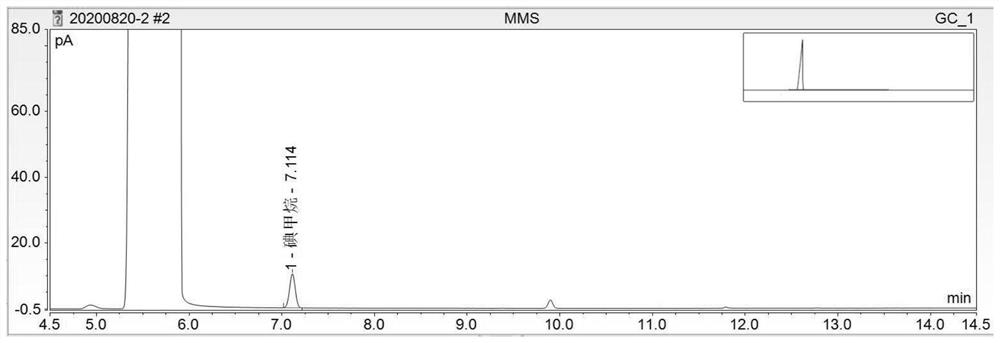
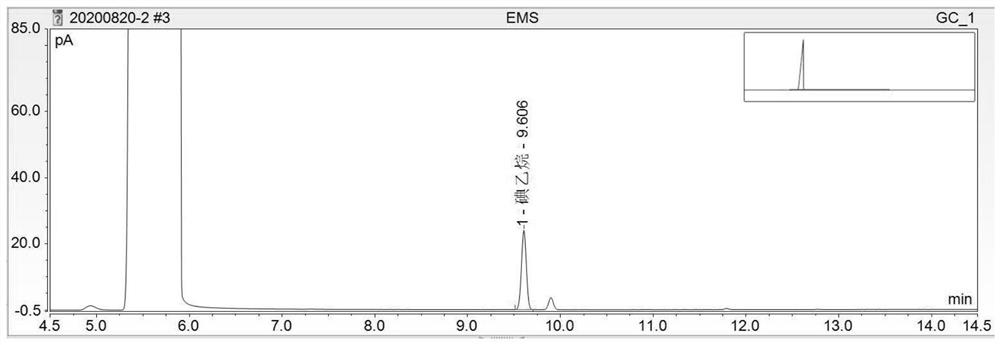
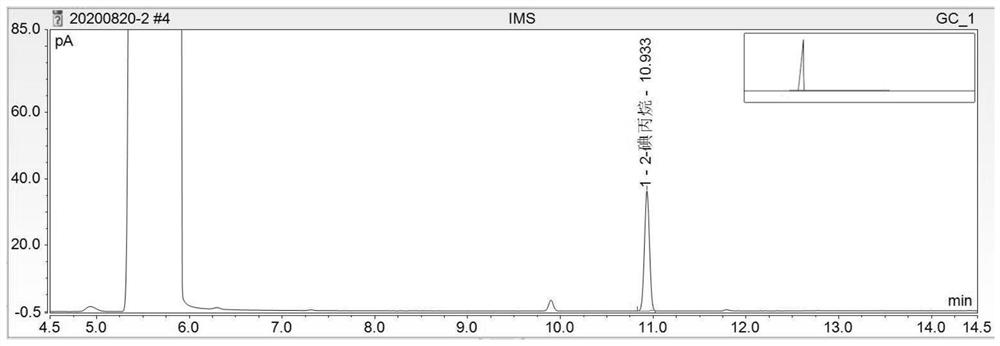
[0097] In conclusion, under the chromatographic conditions, the components of methyl methanesulfonate, ethyl methanesulfonate and isopropyl methanesulfonate were derivatized to generate methyl iodide, ethyl iodide and isopropane iodide respectively, and the recoveries of each component were average. If it is not within the range of 80% to 120%, the accuracy of the method does not meet the requirements. It is necessary to improve the optimization met...
Embodiment 3
[0098] Embodiment 3 standard addition method verification
[0099] Need testing solution, need testing solution spiked 50% limit solution, need testing substance spike 100% limit solution, need testing sample spike 150% limit solution headspace sample record chromatogram respectively, as Figure 4 to Figure 7 , according to each chromatogram, calculate the content of mesylate impurities by the standard addition method. In the chromatogram of the test product spiked solution, methyl iodide (methyl methanesulfonate), ethyl iodide (ethyl methanesulfonate), and isopropane iodide (isopropyl methanesulfonate) go out successively. The degree of separation is greater than 1.5. Taking the added content (μg / ml) of each mesylate reference substance as the X-axis, and taking the peak area as the Y-axis, draw a standard curve. Calculate the content of each mesylate in the test product by the following formula.
[0100] Methyl methanesulfonate or ethyl methanesulfonate or isopropyl metha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com