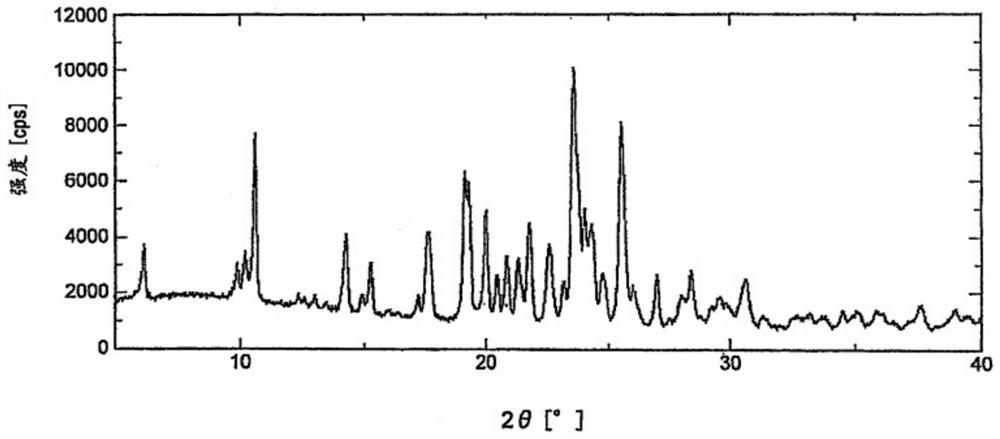
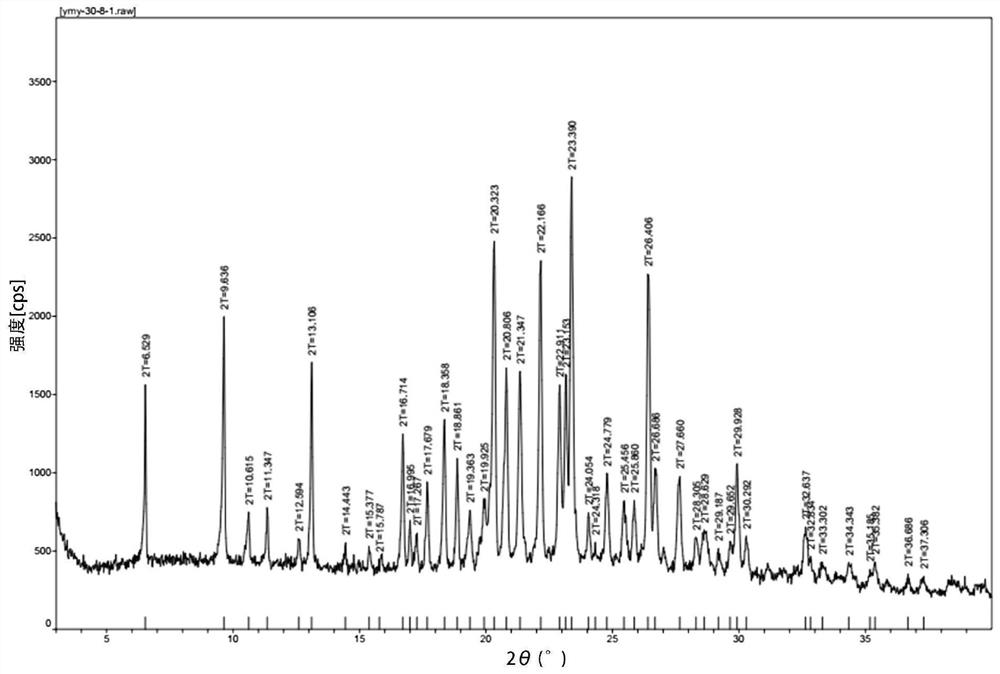
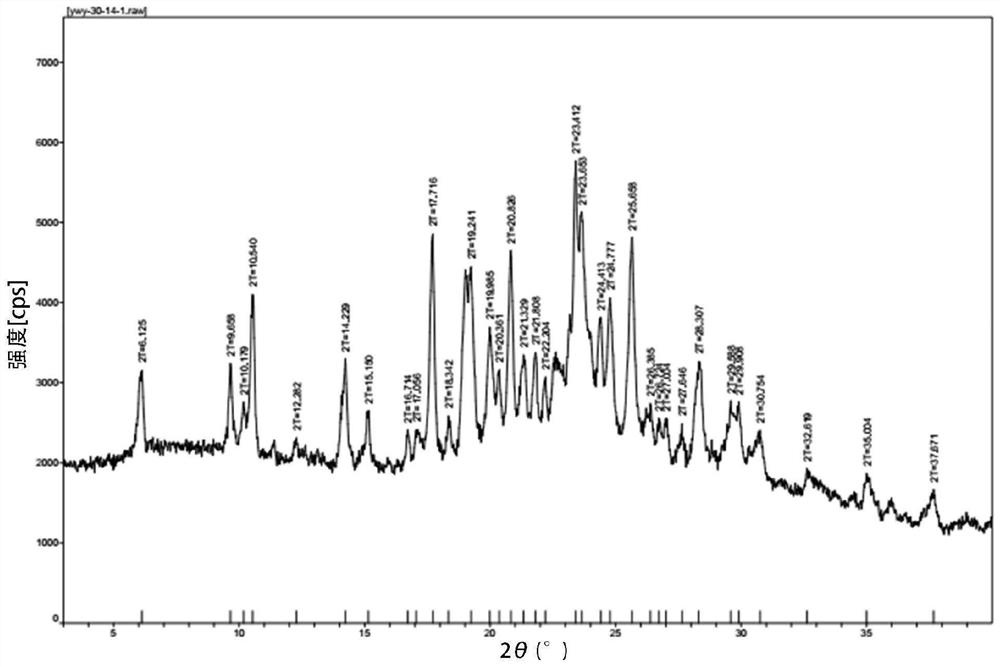
Preparation method of high-purity crystal
A technology of crystallization and concentration, applied in the field of preparation of high-purity crystallization, can solve problems such as unfavorable commercial production, cumbersome operation, generation of genotoxic impurities, etc., to avoid genotoxic impurities, simple operation, and reduce impurity A and impurity B. produced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the preparation of lenvatinib mesylate crude product
[0053]
[0054] Add lenvatinib free base (1 g, 2.34 mmol) to N,N-dimethylformamide (10 mL), and heat to 90° C. to dissolve. After cooling to -20°C, a solution of methanesulfonic acid (0.23 g, 2.34 mmol) in methyl tert-butyl ether (10 mL) was added dropwise. After the addition, keep stirring for 2 hours. Filtration, the filter cake was washed with methyl tert-butyl ether, dried to obtain lenvatinib mesylate crude product (1.16g), the yield was 94.6%, the purity was 99.8%, impurity A: 0.08%, impurity B: 0.05 %.
[0055] H-NMR (600MHz, DMSO-d6): δMSO-(d, 1H), 8.73(s, 1H), 8.38(d, 1H), 8.09(s, 1H), 7.96(d, 2H), 7.66(s ,1H), 7.65(d,1H), 7.37(dd,1H), 7.27(m,1H), 6.96(d,1H), 4.09(s,3H), 2.69(m,1H), 2.36(s, 3H), 0.68 (m, 2H), 0.44 (s, 2H).
Embodiment 2
[0056] Embodiment 2: the preparation of lenvatinib mesylate crude product
[0057] Add lenvatinib (1 g, 2.34 mmol) into dimethyl sulfoxide (10 mL), and heat to 70° C. to dissolve. After cooling to 20°C, a solution of methanesulfonic acid (0.21 g, 2.22 mmol) in ethyl acetate (10 mL) was added dropwise. After the addition, keep stirring for 2 hours. After filtering, the filter cake was washed with ethyl acetate and dried to obtain lenvatinib mesylate crude product (1.10 g), with a yield of 90.1%, a purity of 99.9%, impurity A: 0.05%, and impurity B: 0.05%.
Embodiment 3
[0058] Embodiment 3: the preparation of lenvatinib mesylate crude product
[0059] Add lenvatinib (1 g, 2.34 mmol) into N-methylpyrrolidone (10 mL), and heat to 80° C. to dissolve. After cooling to 0°C, a solution of methanesulfonic acid (0.21 g, 2.22 mmol) in n-butyl acetate (10 mL) was added dropwise. After the addition, keep stirring for 2 hours. After filtering, the filter cake was washed with ethyl acetate and dried to obtain the crude product of lenvatinib mesylate (1.14 g), with a yield of 93.0%, a purity of 99.8%, impurity A: 0.05%, and impurity B: 0.04%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com