Method for refining Lenvatinib mesylate
A technology of lenvatinib mesylate and a refining method, which is applied in the refining field of lenvatinib mesylate, can solve the problems of low refining yield, high impurity content and high production cost, and achieves high refining yield and high production cost. The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
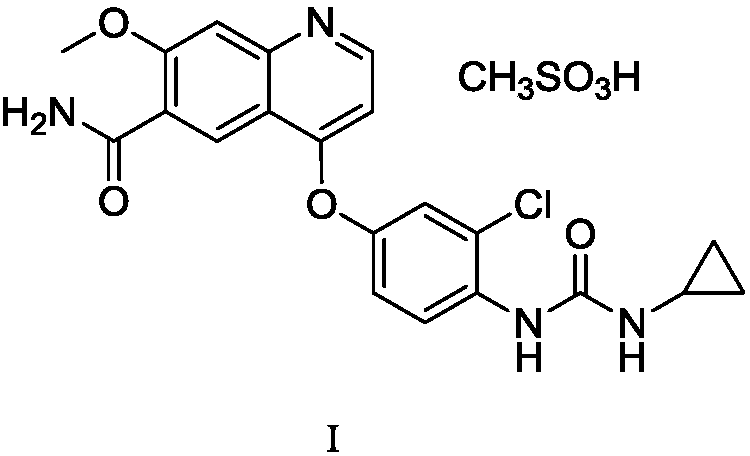
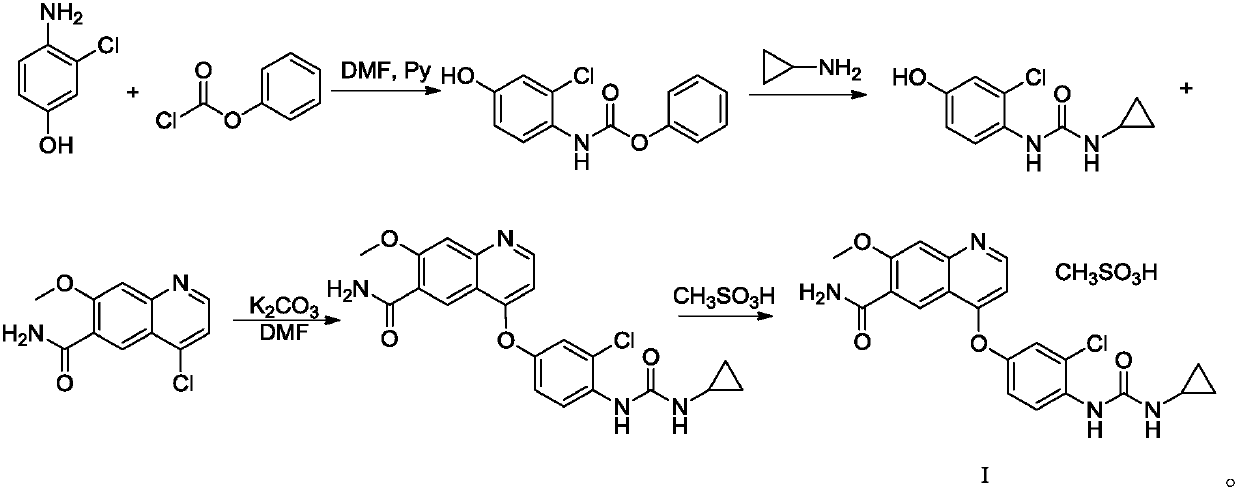
[0033] Embodiment 1: the preparation of lenvatinib mesylate I crude product (referring to the method of CN200480036184.1)
[0034]
[0035]Under the protection of nitrogen, add 37.9g (0.264mol) of 4-amino-3-chlorophenol to 160mL of N,N-dimethylformamide, cool to 0-5°C, add 36.6g (0.475mol) of pyridine, dropwise 42.9 g (0.274 mol) of phenyl chloroformate was raised to room temperature and stirred for 1 hour. Add water and ethyl acetate, stir, stand still, and separate liquids; separate the organic phase, and extract the aqueous phase with ethyl acetate once more. Organic phase merges, and with mass concentration successively be 7% sodium bicarbonate aqueous solution (the described mass concentration refers to the percentage that the quality of sodium bicarbonate accounts for the total mass of sodium bicarbonate aqueous solution), mass concentration is 15% sodium chloride aqueous solution (the stated mass concentration The mass concentration mentioned refers to the quality...
Embodiment 2
[0036] Embodiment 2: the preparation of lenvatinib mesylate I crude product (referring to the method of CN200480036184.1)
[0037] Under the protection of nitrogen, add 2.37Kg (16.5mol) of 4-amino-3-chlorophenol to 10L of N,N-dimethylformamide, cool to 0-5°C, add 2.29Kg (29.7mol) of pyridine, dropwise Phenyl chloroformate 2.68 Kg (17.1 mol), raised to room temperature and stirred for 1 hour. Add water and ethyl acetate, stir, stand still, and separate liquids; separate the organic phase, and extract the aqueous phase with ethyl acetate once more. Organic phase merges, and with mass concentration successively be 7% sodium bicarbonate aqueous solution (the described mass concentration refers to the percentage that the quality of sodium bicarbonate accounts for the total mass of sodium bicarbonate aqueous solution), mass concentration is 15% sodium chloride aqueous solution (the stated mass concentration The mass concentration mentioned refers to the quality of sodium chloride a...
Embodiment 3
[0038] Embodiment 3: the preparation of lenvatinib mesylate I crude product (referring to the method of CN200480036184.1)
[0039] Under the protection of nitrogen, add 11.7g (0.081mol) of 4-amino-3-chlorophenol to 50mL of N,N-dimethylformamide, cool to 0-5°C, add 11.4g (0.148mol) of pyridine, dropwise 13.4 g (0.085 mol) of phenyl chloroformate was raised to room temperature and stirred for 1 hour. Add water and ethyl acetate, stir, stand still, and separate liquids; separate the organic phase, and extract the aqueous phase with ethyl acetate once more. Organic phase merges, and with mass concentration successively be 7% sodium bicarbonate aqueous solution (the described mass concentration refers to the percentage that the quality of sodium bicarbonate accounts for the total mass of sodium bicarbonate aqueous solution), mass concentration is 15% sodium chloride aqueous solution (the stated mass concentration The mass concentration mentioned refers to the quality of sodium chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com