Preparation method of amorphous quinoline carboxamide derivative
An amorphous, methanesulfonic acid technology, applied in the field of medicinal chemistry, can solve the problems of easy agglomeration of amorphous form, unfavorable drug safety and quality control, and unsuitable for mass scale-up production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
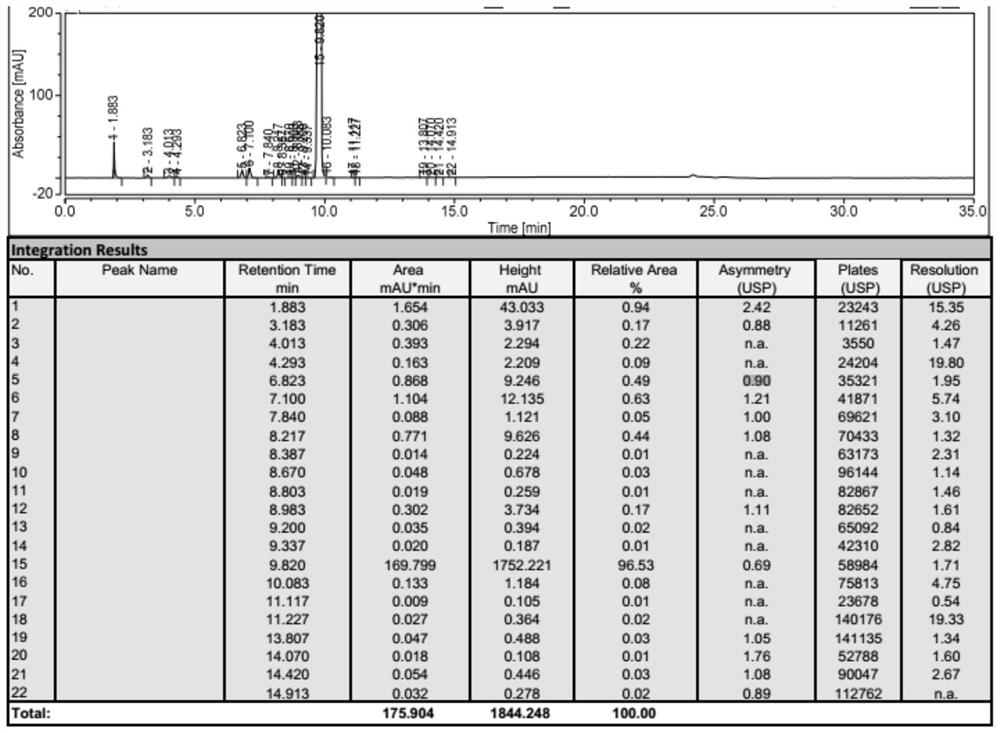
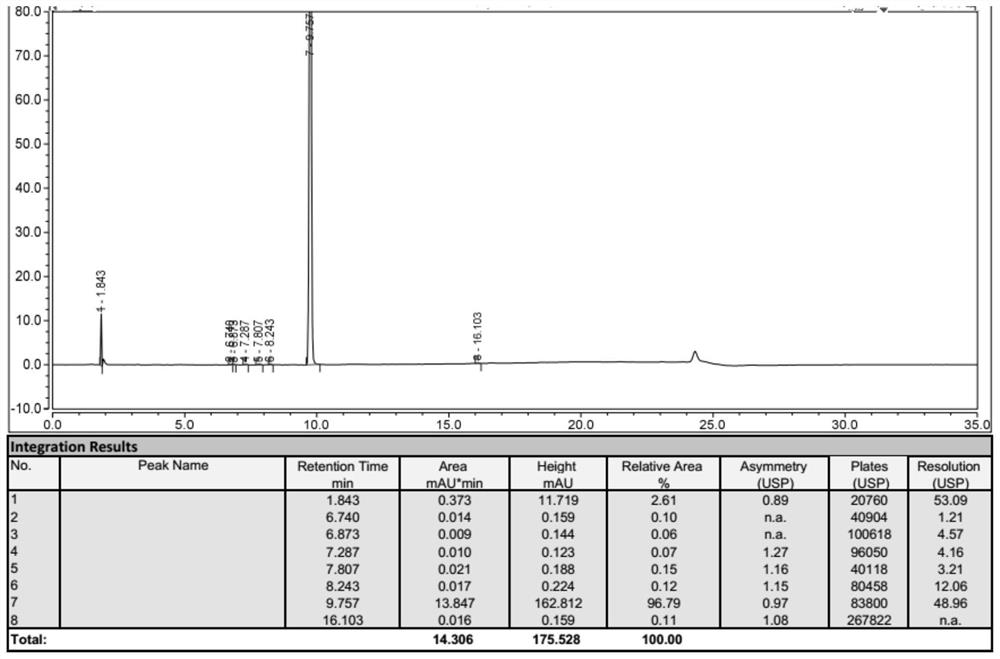
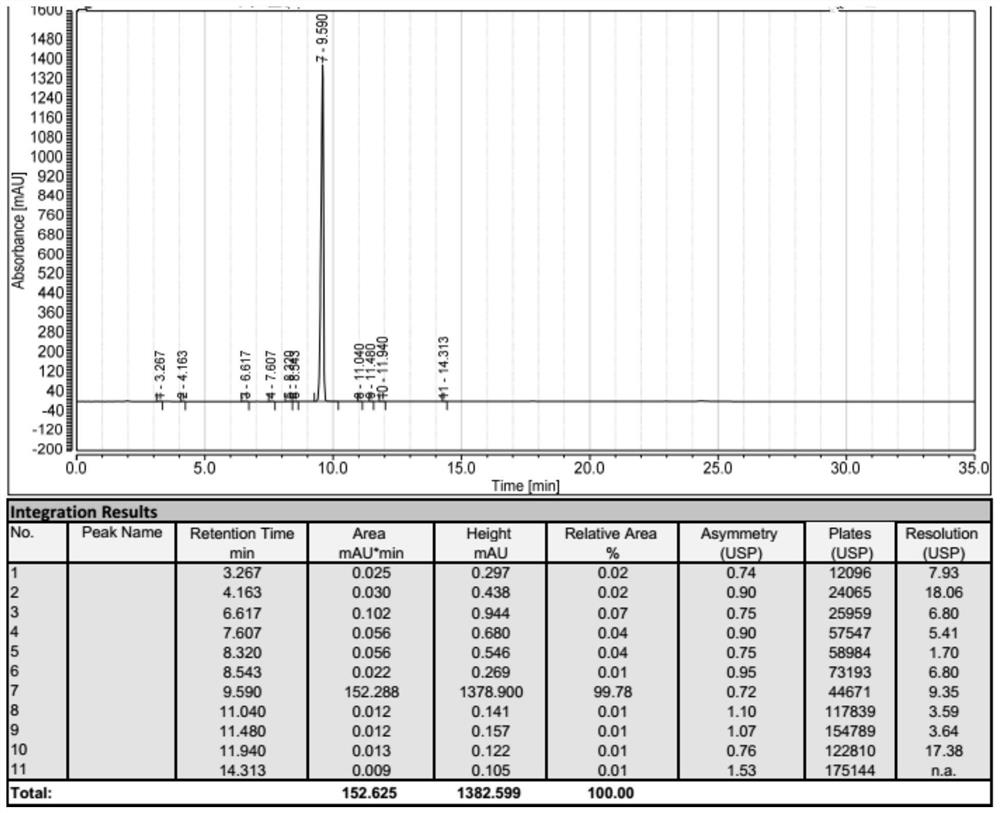
Image
Examples
Embodiment 1
[0051] The investigation of embodiment 1 freeze-drying solvent
[0052] Dissolve lenvatinib mesylate crystal form C (5g) in different solvent systems, stir and dissolve at 20±5°C, filter, collect the filtrate and freeze-dry, the specific freeze-drying method is as follows:
[0053] 1) Pre-freezing
[0054] The temperature of the heat transfer oil reaches -40°C in 1.5h and keeps for 4h;
[0055] 2) Once dry
[0056] In the first stage, the temperature of the heat transfer oil reaches -10°C in 4 hours, the vacuum degree is 10 Pa, and it is kept for 24 hours;
[0057]In the second stage, the temperature of the heat transfer oil reaches 10°C in 2 hours, and the vacuum degree is 10 Pa, and it is kept for 24 hours;
[0058] 3) Analytical drying
[0059] In the first stage, the temperature of the heat transfer oil reaches 20°C in 2 hours, and the vacuum degree is 10 Pa, and it is kept for 4 hours;
[0060] In the second stage, the temperature of the heat transfer oil reaches 35°...
Embodiment 2
[0065] Embodiment 2 tert-butanol / water system investigation
[0066] In order to dissolve more effectively, the influence of different proportions of tert-butanol / water system on the solubility was investigated, and the experimental data are shown in Table 2.
[0067] Table 2 The influence of different proportions of tert-butanol / water system on solubility
[0068] Numbering Solvent ratio (volume ratio) melting temperature dissolved volume dissolution phenomenon 1. tert-butanol / water=2:1 20±5℃ 18 times form a transparent gel 2. tert-butanol / water=1:1 20±5℃ 45 times Forms a low viscosity clear solution 3. tert-butanol / water=1:2 20±5℃ 70 times Forms a low viscosity clear solution 4. tert-butanol / water=1:3 20±5℃ 129 times Forms a low viscosity clear solution
[0069] Conclusion: The ratio of tert-butanol / water in the range of 1:1 to 1:3 can form a low-viscosity transparent solution with uniform dissolution. Conside...
Embodiment 3
[0070] Embodiment 3 lyophilization curve optimization
[0071] For more effective freeze-drying, a freeze-drying solvent of tert-butanol / water with a volume ratio of 1:1 was selected, and the temperature and time of the pre-freezing stage, sublimation stage, and desorption stage were investigated, and the freeze-drying curve was studied. The experimental results As shown in Table 3, the vacuum degree is 10 Pa.
[0072] Table 3 freeze-drying curve optimization experimental results
[0073]
[0074]
[0075] Conclusion: The tert-butanol / water system lyophilization adopts slow temperature rise and step-by-step analysis drying in the first drying stage, which can ensure the appearance of the drug is fluffy and not collapsed, the sample has low moisture content and low residual solvent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com