Biomarkers for combination therapy comprising lenvatinib and everolimus
A technology of lenvatinib and combination therapy, applied in biological testing, biomaterial analysis, active ingredients of heterocyclic compounds, etc., can solve problems that have not been disclosed or indicated, and cannot predict the responsiveness of angiogenesis inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0104] Example 1: Biomarkers for identifying a subgroup of renal cell carcinoma patients requiring combination therapy comprising lenvatinib and everolimus
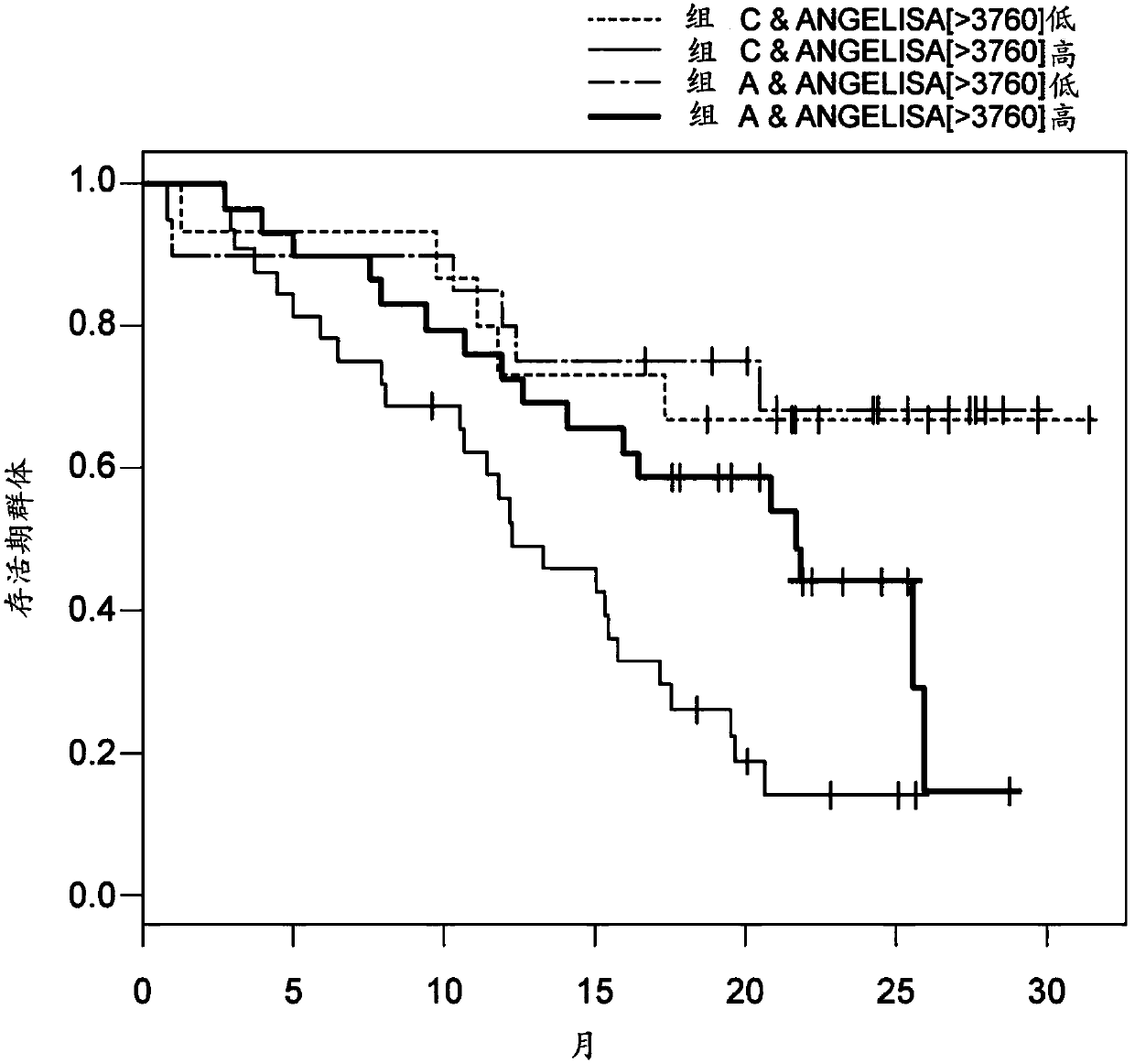
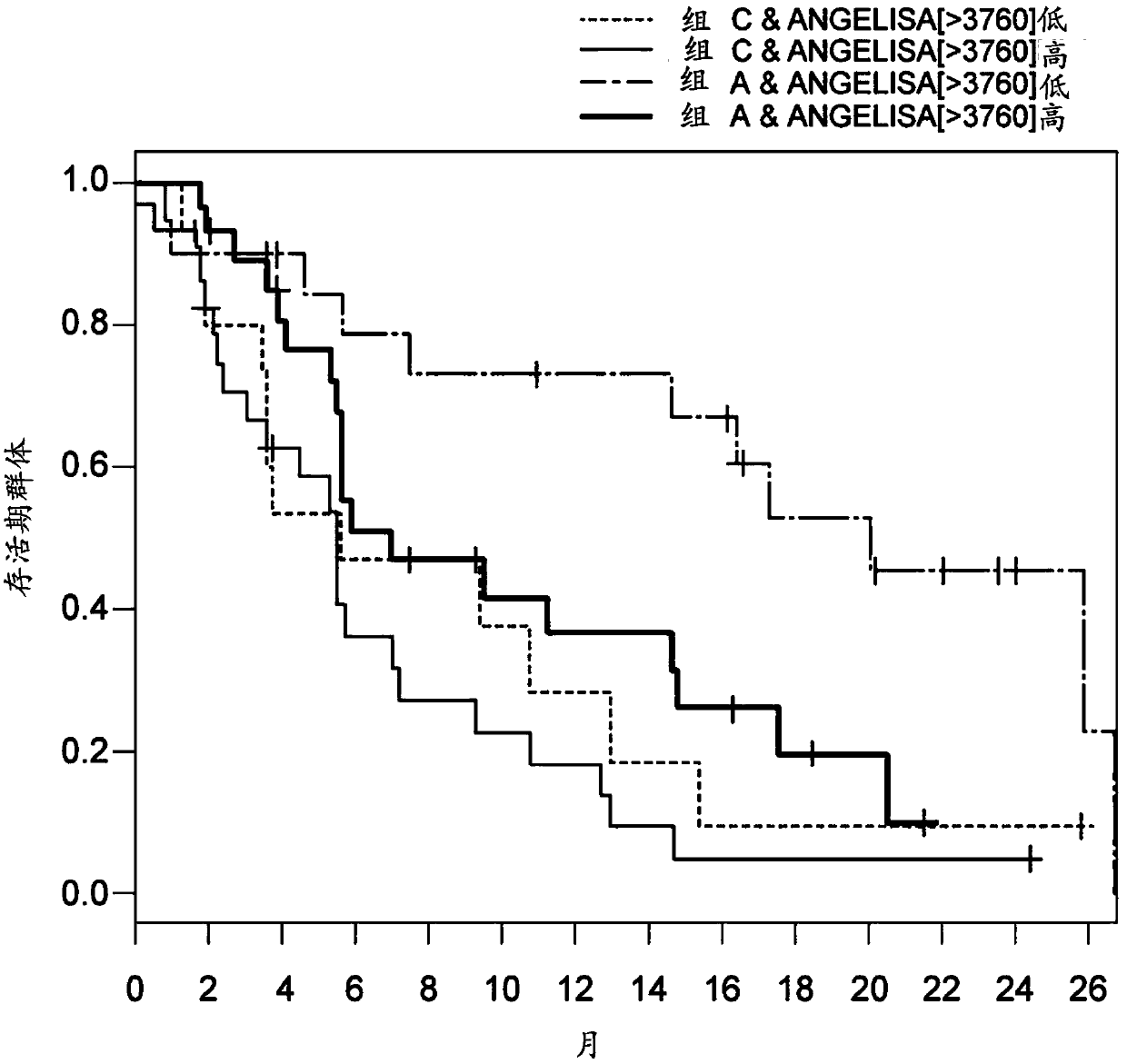
[0105] Objective: A phase 2 study of lenvatinib was conducted in patients with metastatic renal cell carcinoma (RCC) following VEGF-targeted therapy. The importance of angiogenesis in RCC underscores the need to understand the clinical mechanisms behind anti-angiogenic therapies such as everolimus. A clinical biomarker analysis was performed to identify predictive markers of clinical benefit in RCC patients on combination therapy comprising lenvatinib and everolimus compared with everolimus monotherapy.
[0106] The purpose of the assay is to measure Ang2 levels in blood samples (e.g., serum) obtained from patients before and after treatment with lenvatinib, everolimus, or a combination thereof in a clinical trial, and To identify blood biomarkers that can be used to predict whether a patient will need the combination th...
specific Embodiment
[0110] Specific embodiments of the present invention are as follows:
[0111] (1) A method for identifying a human subject suffering from, suspected of suffering from, or at risk of developing renal cell carcinoma in need of a combination therapy comprising lenvatinib or a pharmaceutically acceptable salt thereof and everolimus , the method includes:
[0112] Before administering a combination therapy comprising lenvatinib or a pharmaceutically acceptable salt thereof and everolimus, assaying a biological sample obtained from the human subject and determining angiopoietin-2 in the biological sample (Ang2) protein concentration is high compared to the control; and
[0113] A human subject having a high concentration of Ang2 protein in the biological sample is identified as being in need of a combination therapy comprising lenvatinib or a pharmaceutically acceptable salt thereof and everolimus.
[0114] (2) The method as described in (1), wherein the biological sample is selec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com