Application of Lenvatinib to preparation of medicine for preventing and curing macular degeneration
A technology of macular degeneration and lenvatinib, applied in the field of medicine, can solve problems such as poor curative effect and poor patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
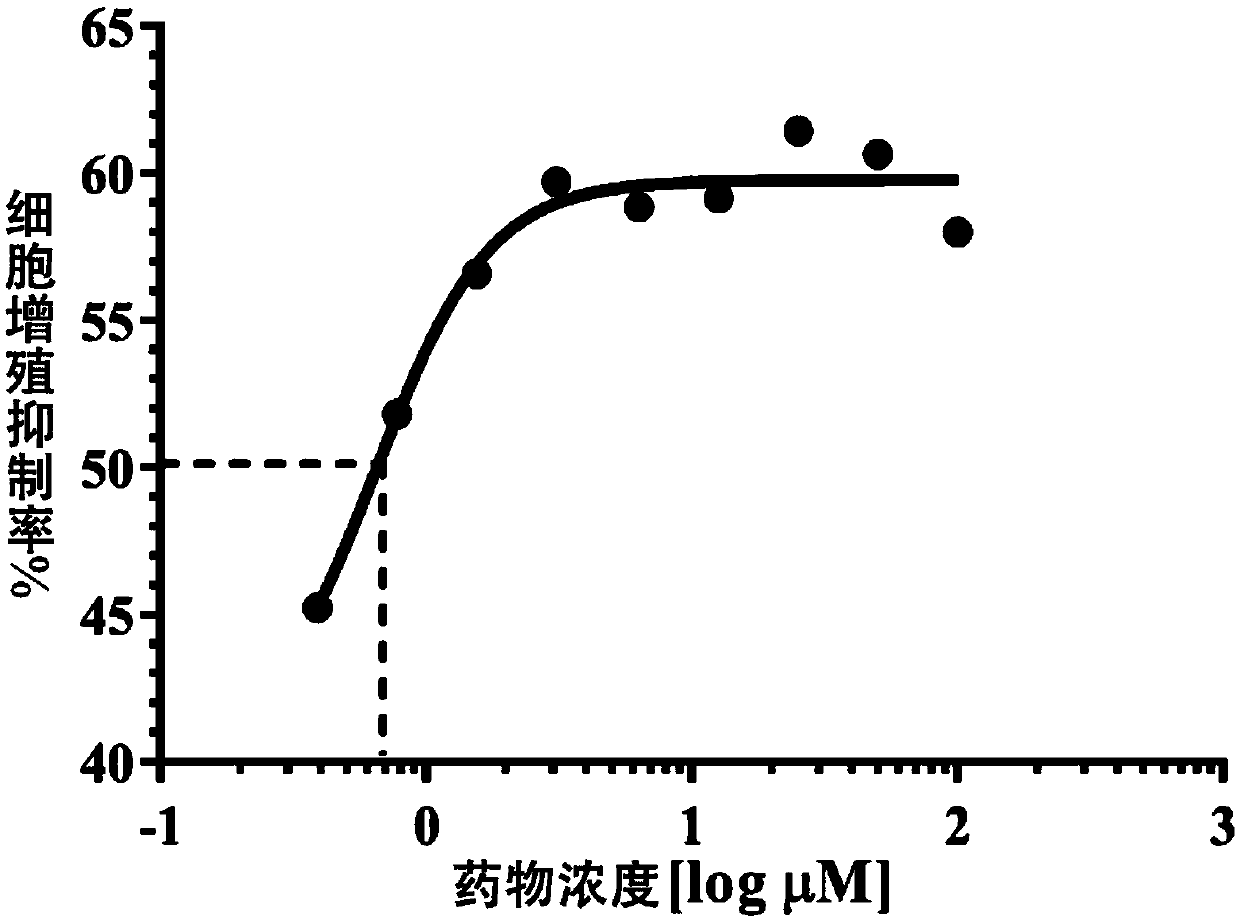
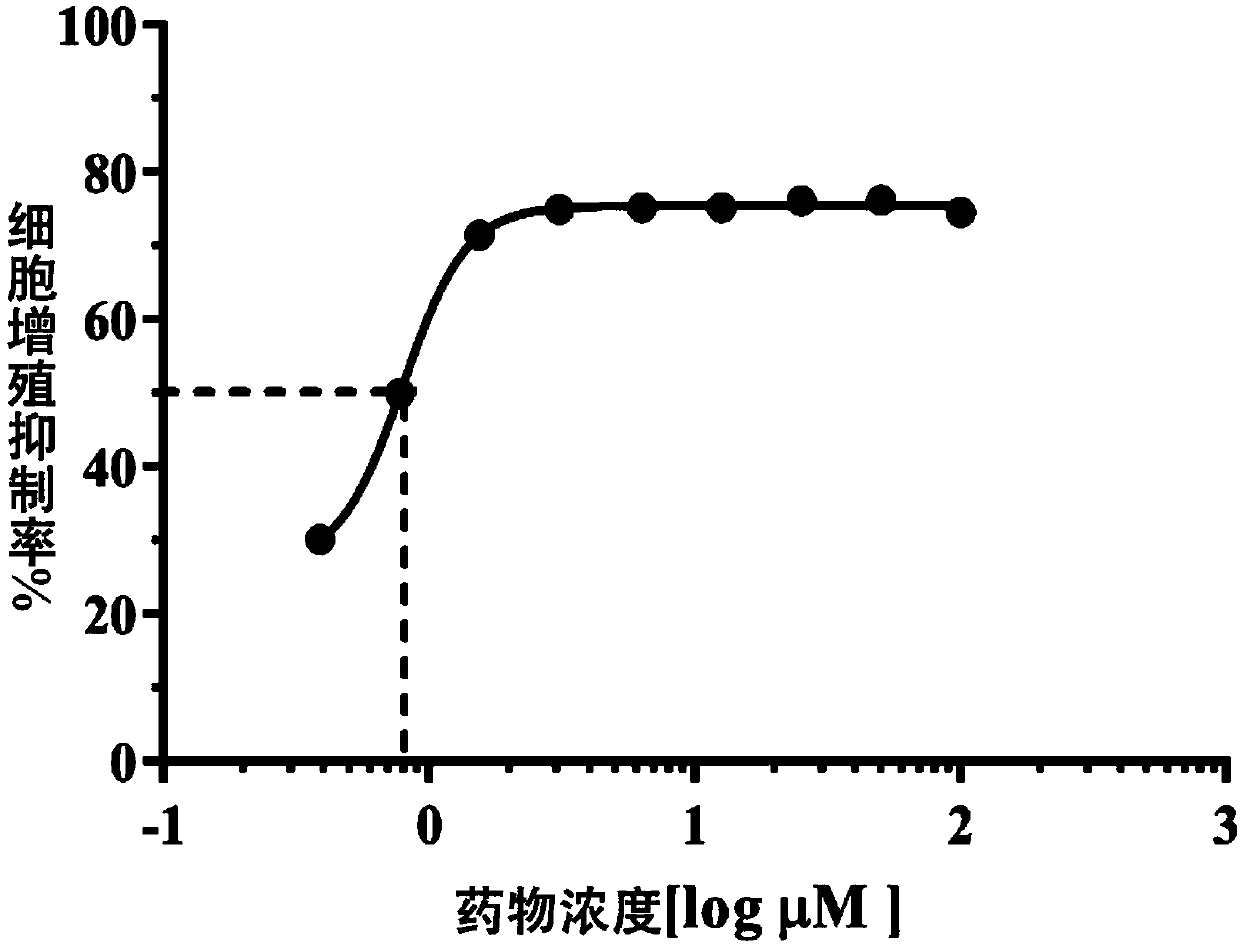
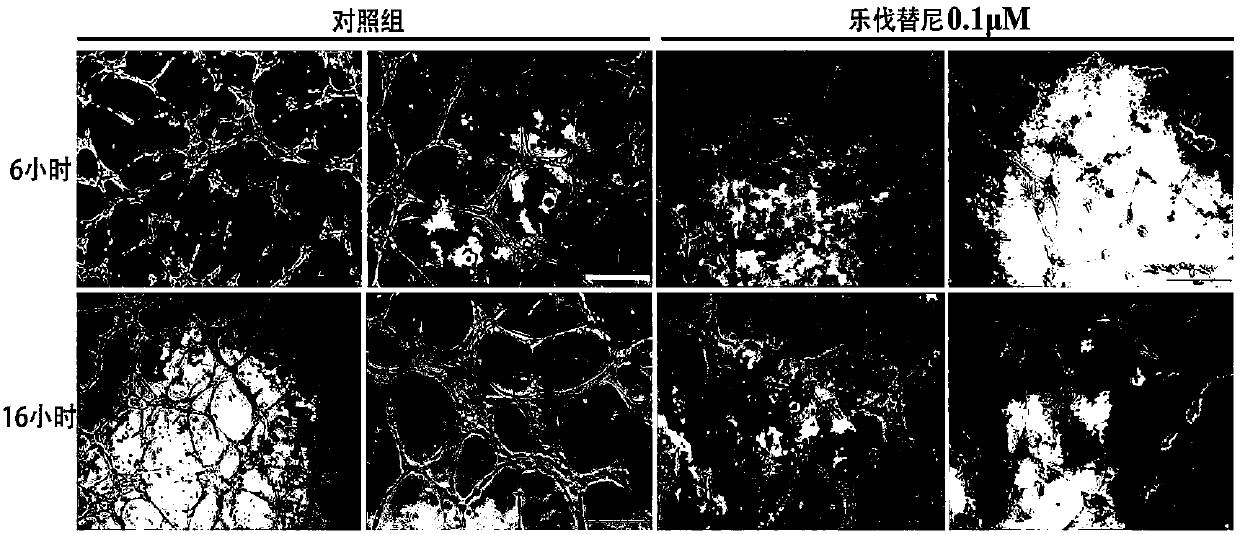
[0029] Example 1 In Vitro Cytological Experiment of Lenvatinib's Anti-Angiogenesis Ability in Fundus
[0030] Human choroidal microvascular endothelial cell HCMEC is the primary cell model for research and evaluation of fundus neovascularization. The proliferation, tube formation and migration ability of HCMEC are the pathological process of fundus neovascularization. The following three aspects prove that lenvatinib has The ability to resist neovascularization in fundus.
[0031] 1. Culture of human choroidal microvascular endothelial cells HCMEC
[0032] Human choroidal microvascular endothelial cells HCMEC are cultured in ECM medium containing 5% FBS and 1% growth factor additive ECGS. In order to ensure that the cells are in the best period of morphology and physiological characteristics, only cells within 1-7 passages are used for experiments; The above cell lines need to be added with 100 U / mL penicillin and streptomycin during the culture and experiment process, and cu...
Embodiment 2
[0045] Embodiment 2 Lenvatinib treats macular degeneration in vivo animal experiment
[0046] Based on the high-energy laser photocoagulation of the retina, the destruction of Bruch's membrane, followed by secondary damage repair reaction, inflammation, choroidal epithelial cells, pericytes, fibroblasts, inflammatory cells, etc. enter the subretinal space, forming choroidal neovascularization, that is, subretinal Based on the principle of neovascularization, a C57 mouse choroidal neovascularization model (CNV) was established to evaluate the new use of lenvatinib in the treatment of wet AMD in vivo.
[0047] Instruments and parameters: Maida 532nm argon laser treatment instrument setting parameters: laser spot diameter 50μm; exposure time: 100ms; excitation energy: 150mw.
[0048] (1) Anesthesia: 1% sodium pentobarbital (containing 20% ethanol), intraperitoneal injection of 1% sodium pentobarbital according to the weight of mice at 50 mg / kg.
[0049] (2) Experiment preparat...
Embodiment 3
[0065] Embodiment 3 Lenvatinib pharmacokinetic characteristics in retina
[0066] 1. Animal and Instrument Preparation:
[0067] 25 female SD rats, weighing about 210 grams, aged 7-8 weeks, Huabukang, Beijing; UPLC / MS / MS: Shimadzu UPLC-MS8050 system; TL2010S medium-throughput tissue grinder (Beijing Dinghaoyuan Technology Co., Ltd. company)
[0068] 2. Preparation of reagent consumables:
[0069] Gastric injection needle, ether cotton ball, foam box, timer, sampling registration form (printing), experimental protocol (printing), 1.5mL EP tube (exygen), anticoagulant tube, cotton swab, dry cotton ball, disposable tablecloth, sterile Normal saline, anatomical surgical instruments, ophthalmic microscopic instruments, 75% alcohol and cotton balls, protective masks, gloves, hats, ice boxes, EP tube racks, BD tube racks, marker pens, plates, filter paper, stereoscopes, plug-in boards, Grinding steel ball 3mm specification 24X5 pieces
[0070] 3. Analysis method:
[0071] LC / MS / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com