Lenvatinib impurity and preparation method thereof
A technology for lenvatinib and impurities, which is applied in the field of lenvatinib impurities and its preparation, can solve the problems of difficult separation, low content of impurity compounds, and difficulty in quality control of lenvatinib intermediates and APIs, and achieves The effect of stable purity and yield, simple preparation method, and improved product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation method of compound III
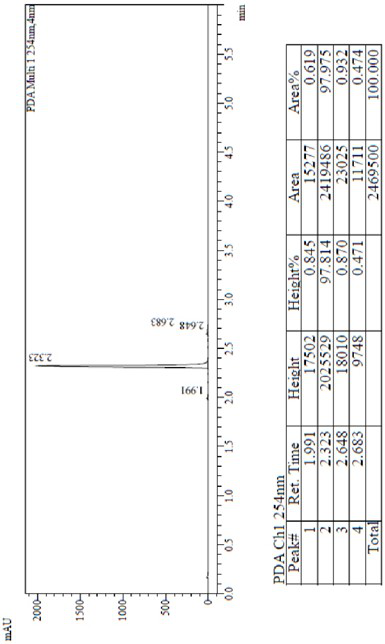
[0031] Add 12.71g of compound I, 23.66g of compound II, 500mL of DMSO, 6.0g of sodium hydroxide into a 1L reactor, raise the temperature to 100°C and react for 2h, disperse the reaction solution in the water phase and ethyl acetate phase, and separate the water layer , the organic phase was concentrated to dryness under reduced pressure to obtain 31 g of a purple solid with a purity of 96%.
[0032] (2) Preparation method of compound V
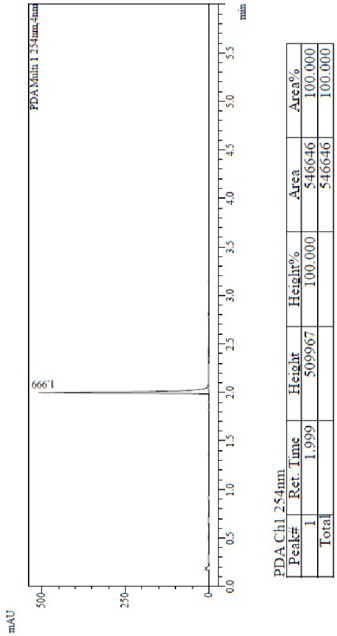
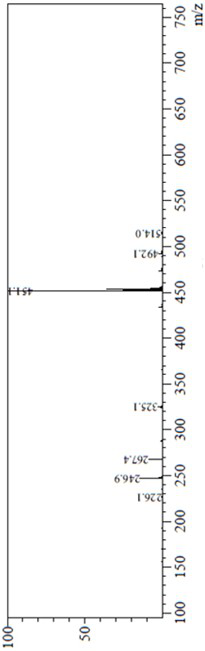
[0033] Add 28.4g of compound of formula III, 14.57g of compound IV, 800mL of DMSO, 6.0g of sodium hydroxide into a 1L reactor, heat up to 100°C for 2h, and purify the reaction solution by column chromatography to obtain 41.4g of a yellow solid with a purity of 100%. , HPLC test results see figure 1 . 1 H-NMR (DMSO-D6, 400MHz) δ ppm 8.61~8.62(d, 2H), 7.96(s, 1H), 7.82(s,1H), 7.47(s, 1H), 6.95~6.94(d, H) , 6.89(s, 1H), 6.87~6.76(m, 3H), 6.58~6.57(d, 1H), 6.46~6.44(d, 1H), 5.12(s, 2H),5.05(s, 2H...
Embodiment 2
[0037] (1) Preparation method of compound III
[0038] Add 12.71g of Compound I, 23.66g of Compound II, 500mL of DMF, and 12.6g of Potassium Hydroxide into a 1L reactor, raise the temperature to 120°C for 2 hours, disperse the reaction solution in the water phase and ethyl acetate phase, and separate the water layer , the organic phase was concentrated to dryness under reduced pressure to obtain 30.3 g of a purple solid with a purity of 95.8%.
[0039] (2) Preparation method of compound V
[0040] Add 28.4g of compound III, 14.57g of compound IV, 800mL of DMF, and 12.6g of potassium hydroxide into a 1L reactor, heat up to 80°C for 3h, and purify the reaction solution by column chromatography to obtain 40.8g of a yellow solid with a purity of 99.5%.
[0041] (3) Preparation method of compound VI
[0042] Add 10g of compound V, 100mL of DMF, 10.96g of isocyanatocyclopropane, and 10g of pyridine into a 500mL reactor, and react at a temperature of 20-30°C for 3h. Water was adde...
Embodiment 3
[0044] (1) Preparation method of compound III
[0045] Add 12.71g of compound I, 23.66g of compound II, 500mL of DMF, and 73.3g of cesium carbonate into a 1L reactor, heat up to 80°C for 2 hours, disperse the reaction solution in the water phase and ethyl acetate phase, and separate the water layer. The organic phase was concentrated to dryness under reduced pressure to obtain 30.2 g of a purple solid with a purity of 96.5%.
[0046] (2) Preparation method of compound V
[0047] Add 28.4g of compound III, 14.57g of compound IV, 800mL of DMF, and 73.3g of cesium carbonate into a 1L reactor, heat up to 120°C for 2 hours, and purify the reaction solution by column chromatography to obtain 40g of a yellow solid with a purity of 99.9%.
[0048] (3) Preparation method of compound VI
[0049]Add 10g of Compound V, 100mL of THF, 10.96g of isocyanatocyclopropane, and 10g of pyridine into a 500mL reactor, and react at a temperature of 20-30°C for 3h. Water was added dropwise to the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com