Lervatinib-gallic acid eutectic crystal form and application thereof
A technology of crystal form and compound, applied in the field of lenvatinib-gallic acid co-crystal form, can solve the problem of less research on lenvatinib co-crystal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] LVB-GA·H 2 O crystal form I preparation method: 500mg lenvatinib, 220mg gallic acid monohydrate (molar ratio 1:1), add 8ml methanol:dichloromethane (1:1), stir for 48 hours, filter, and dry at room temperature.
[0088] LVB-GA·H 2 Preparation method of O crystal form II: take the above-prepared LVB-GA·H 2 O crystal form I was stirred under the condition of appropriate amount of methanol / dichloro / water (5 / 5 / 0.5) for 48 hours to obtain crystal form II.
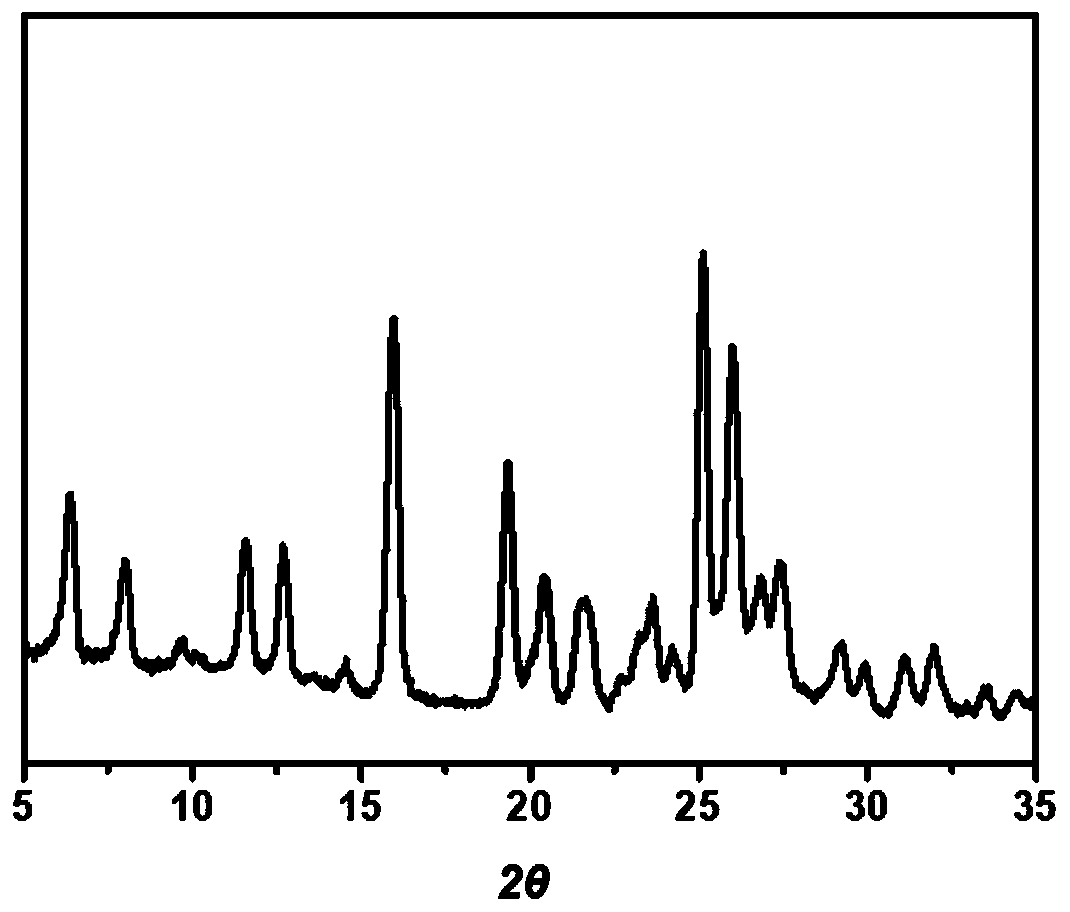
[0089] Characterization of co-crystals by PXRD
[0090] The prepared co-crystal and bulk drug were analyzed by powder X-ray (PXRD) as follows: Figure 5 As shown, it can be seen from the results that the newly prepared LVB-GA·H 2 O co-crystal form I and form II with raw materials lenvatinib (LVB), gallic acid monohydrate (GA·H 2 O) presents a distinctly different diffraction pattern, indicating that a new co-crystal was successfully prepared.
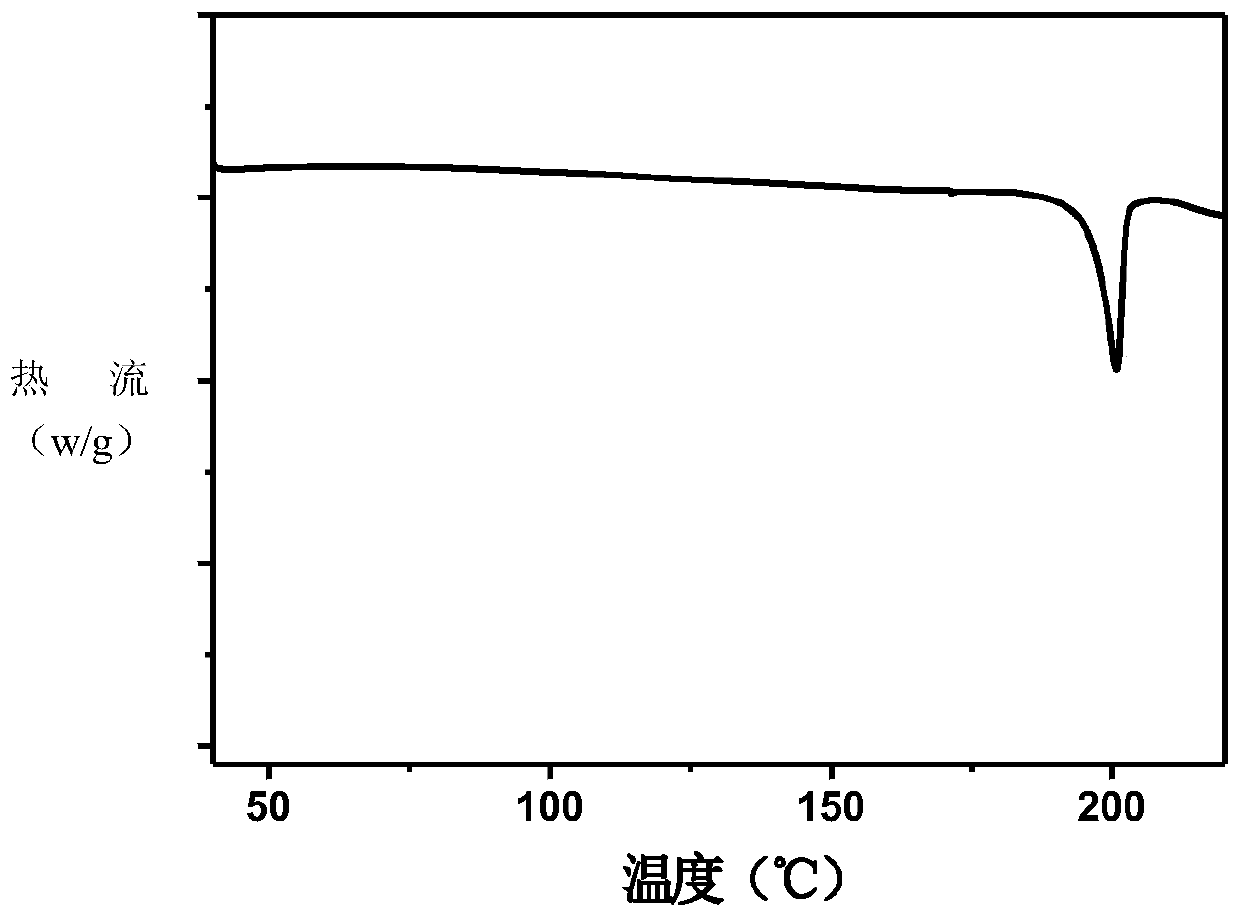
[0091] Characterization of co-crystals by TGA and DSC
[0092] Thermogr...
Embodiment 2
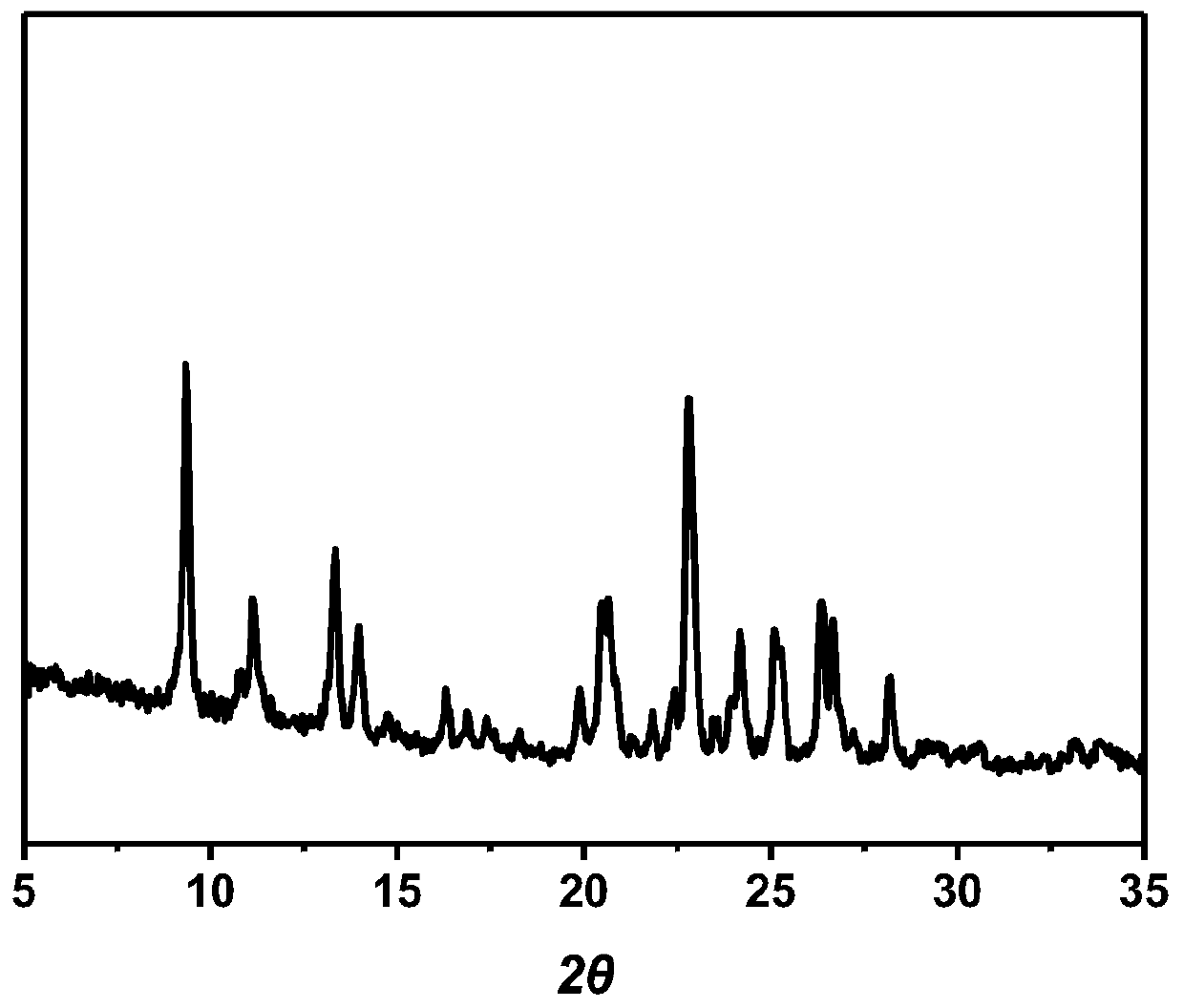
[0100] LVB-GA·H 2 Preliminary Stability Test of O Eutectic Form I
[0101] The prepared LVB-GA·H 2 O eutectic crystal form I was placed at 40 ° C, 10 days under the conditions of 75% RH accelerated stability determination, from DSC, TGA and PXRD (see Figure 10 ) showed no significant change in the characterization results, indicating that LVB-GA·H 2 O eutectic form I has good initial stability.
[0102] LVB-GA·H 2 O cocrystal form I and form II and the inherent dissolution test results of LVB mesylate salt already on the market
[0103] Experiments were carried out with an inherent dissolution device (Fucus Analytical Instrument Co., Ltd., China). LVB-GA·H 2 O eutectic crystal form I, crystal form II or LVB methanesulfonic acid and water-soluble starch are physically mixed in a weight ratio of 7 / 3, and then compressed at 300kgf for 2 minutes to prepare tablets for dissolution. Intrinsic dissolution conditions: 500ml 0.1M HCl (0.1% Tween 80) dissolution medium, rotating...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com