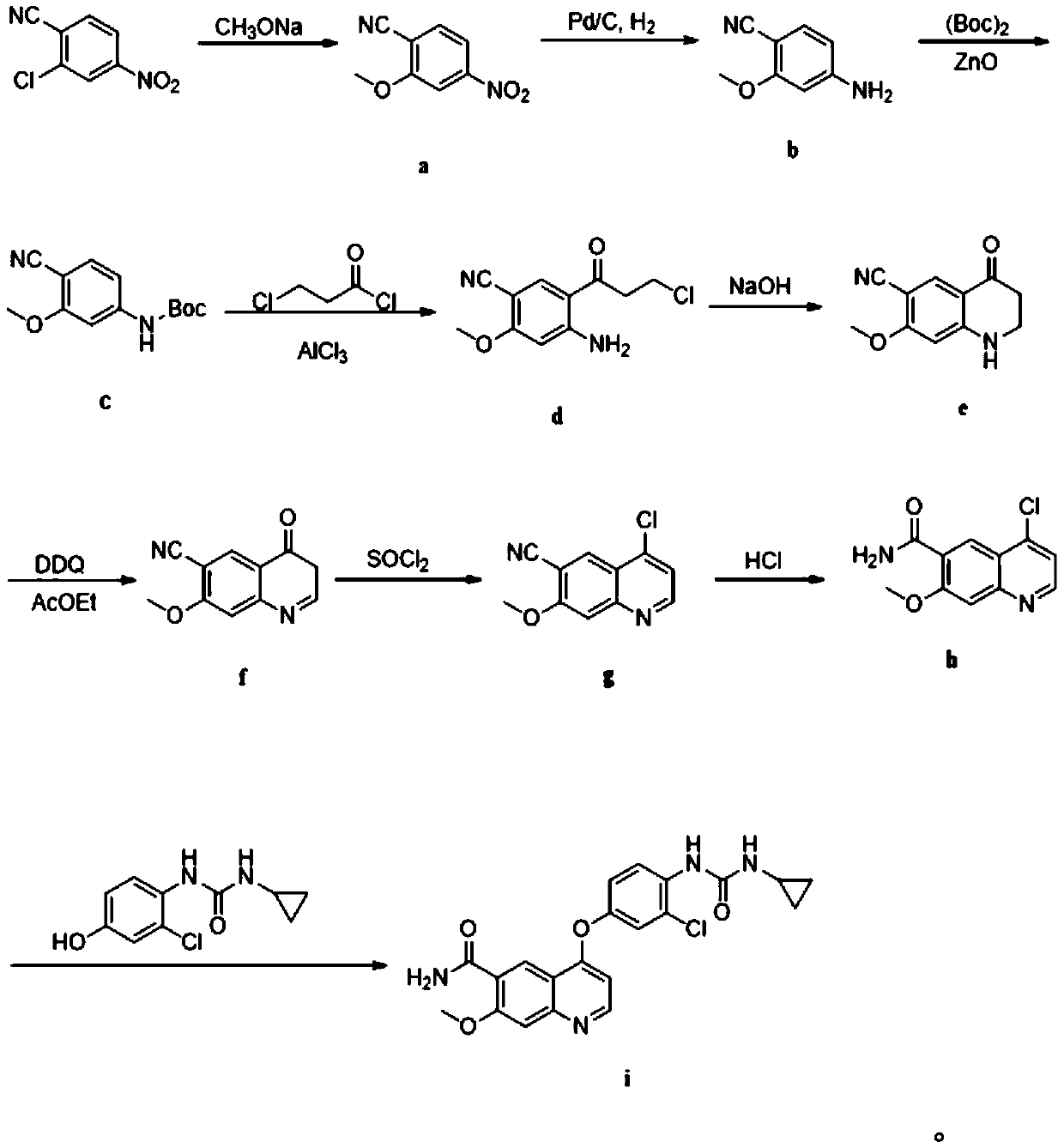
Preparation method of lenvatinib
A technology for lenvatinib and synthetic products, which is applied in the field of new preparation of oral tyrosine kinase inhibitor lenvatinib, can solve problems such as harsh reaction conditions, achieve low reaction temperature, novel synthetic route, and no dangerous steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
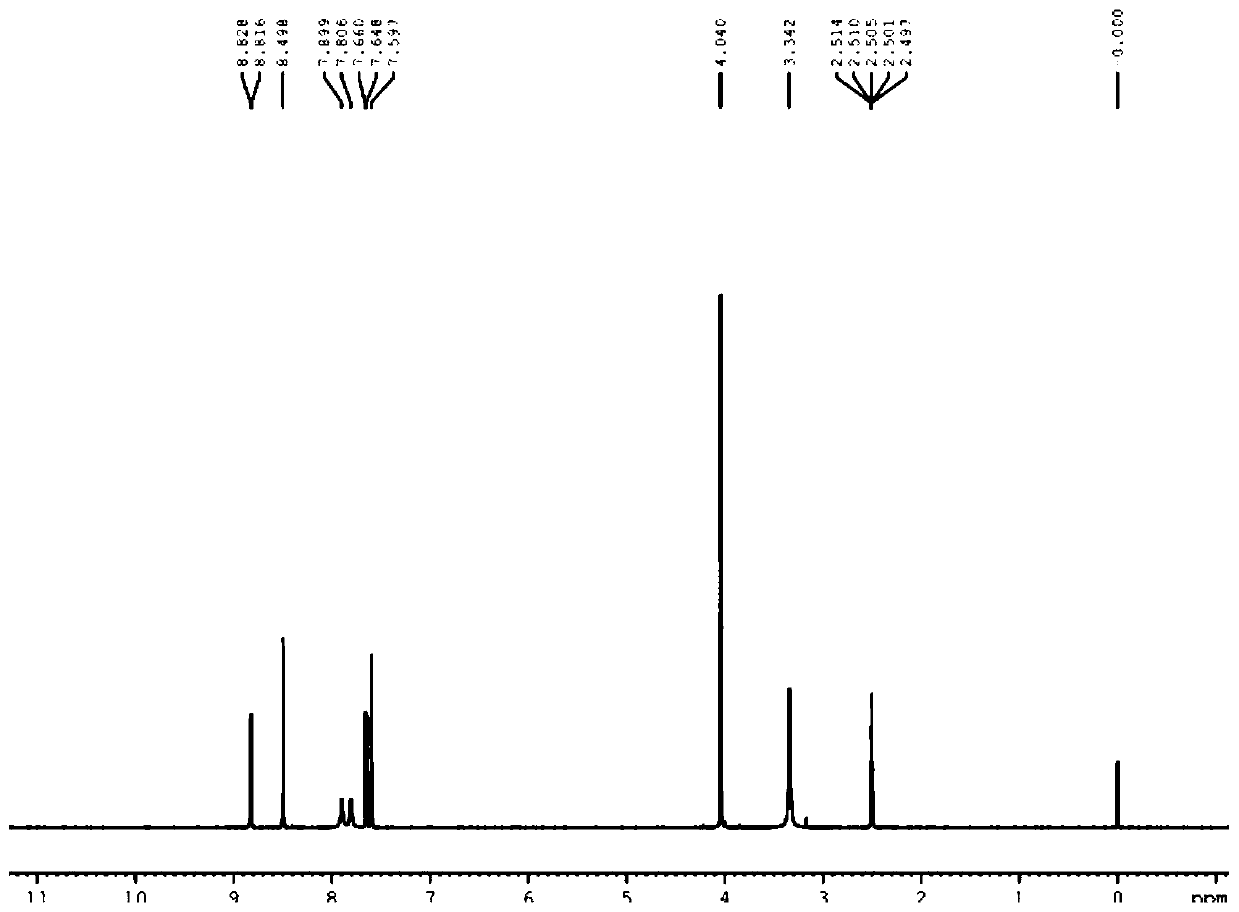
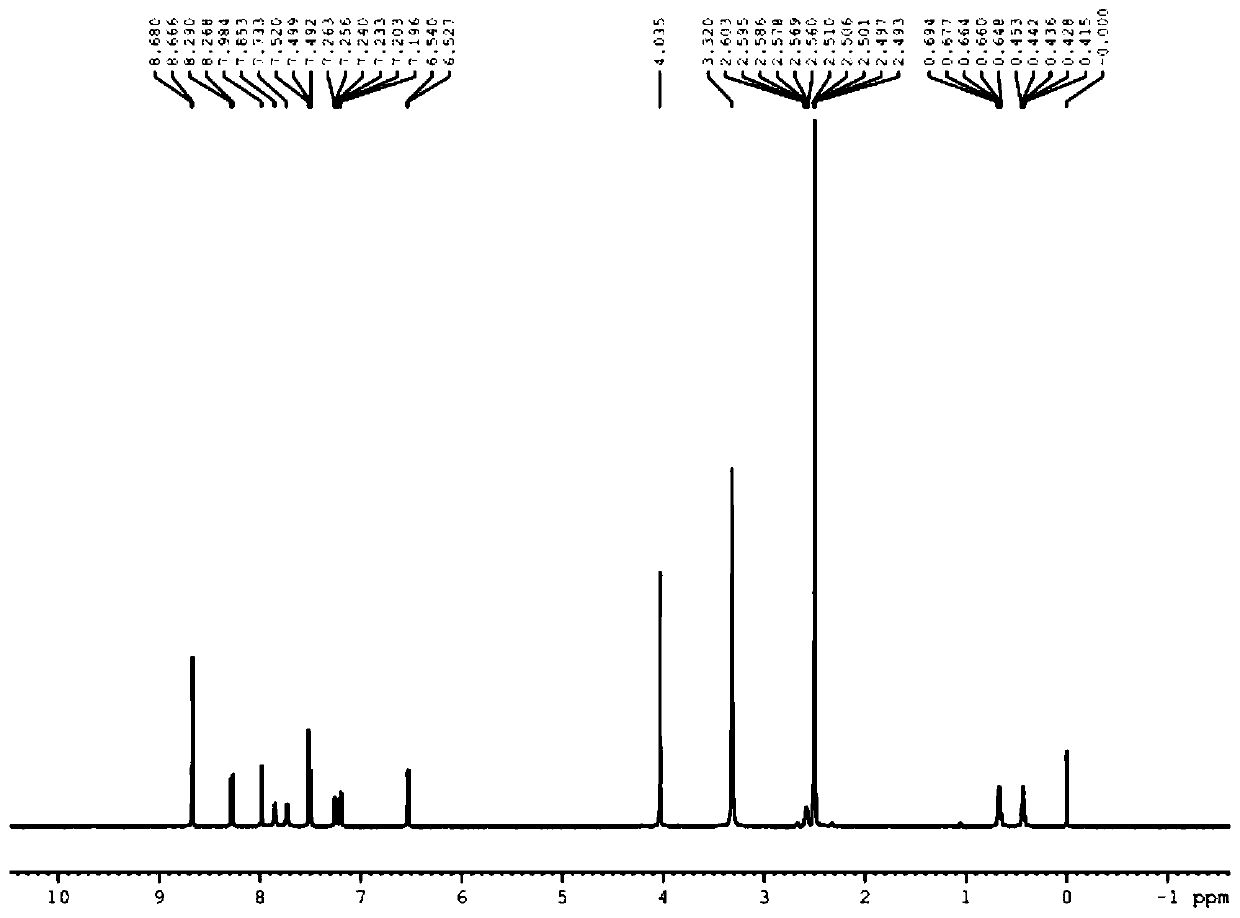
[0049] Synthesis of S1, 4-nitro-2-methoxybenzonitrile (a)
[0050] Take 181g of 4-nitro-2-chlorobenzonitrile, dissolve it in 300g of methanol, heat and reflux to dissolve, add 300g of 30% sodium methoxide solution dropwise to the system, and drop it for 1 hour. After the drop, keep the reaction system for 12 hours. After the reaction, most of the methanol was recovered under reduced pressure, the residue was poured into ice water, a large amount of yellow solid was precipitated, filtered, the filter cake was washed with water until neutral, and the filter cake was recrystallized with isopropanol to obtain 147 g of yellow solid with a yield of 83%.
[0051] Synthesis of S2, 4-cyano-2-methoxyaniline (b)
[0052]Take 17.8 g of 4-nitro-2-methoxybenzonitrile, dissolve it in 50 ml of ethanol, first replace the air in the system with nitrogen for three times, then pass through the reaction system for hydrogen replacement three times, add 1.5 g of Pd / C, and the system Raise the tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com