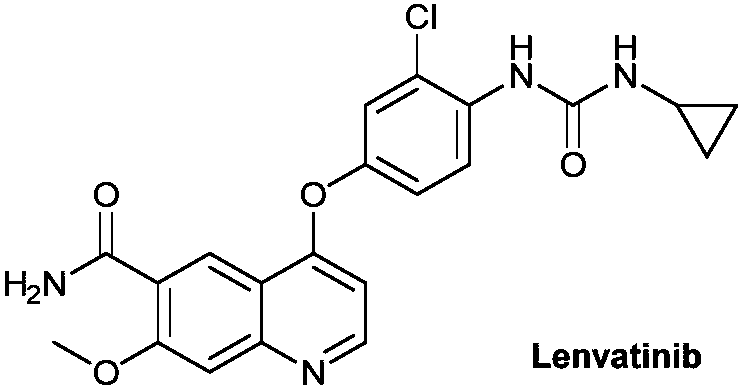
Method for synthesizing lenvatinib
A synthesis method and technology of lenvatinib are applied in the field of medicinal chemical synthesis to achieve the effects of reducing the use of chlorinating agents and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
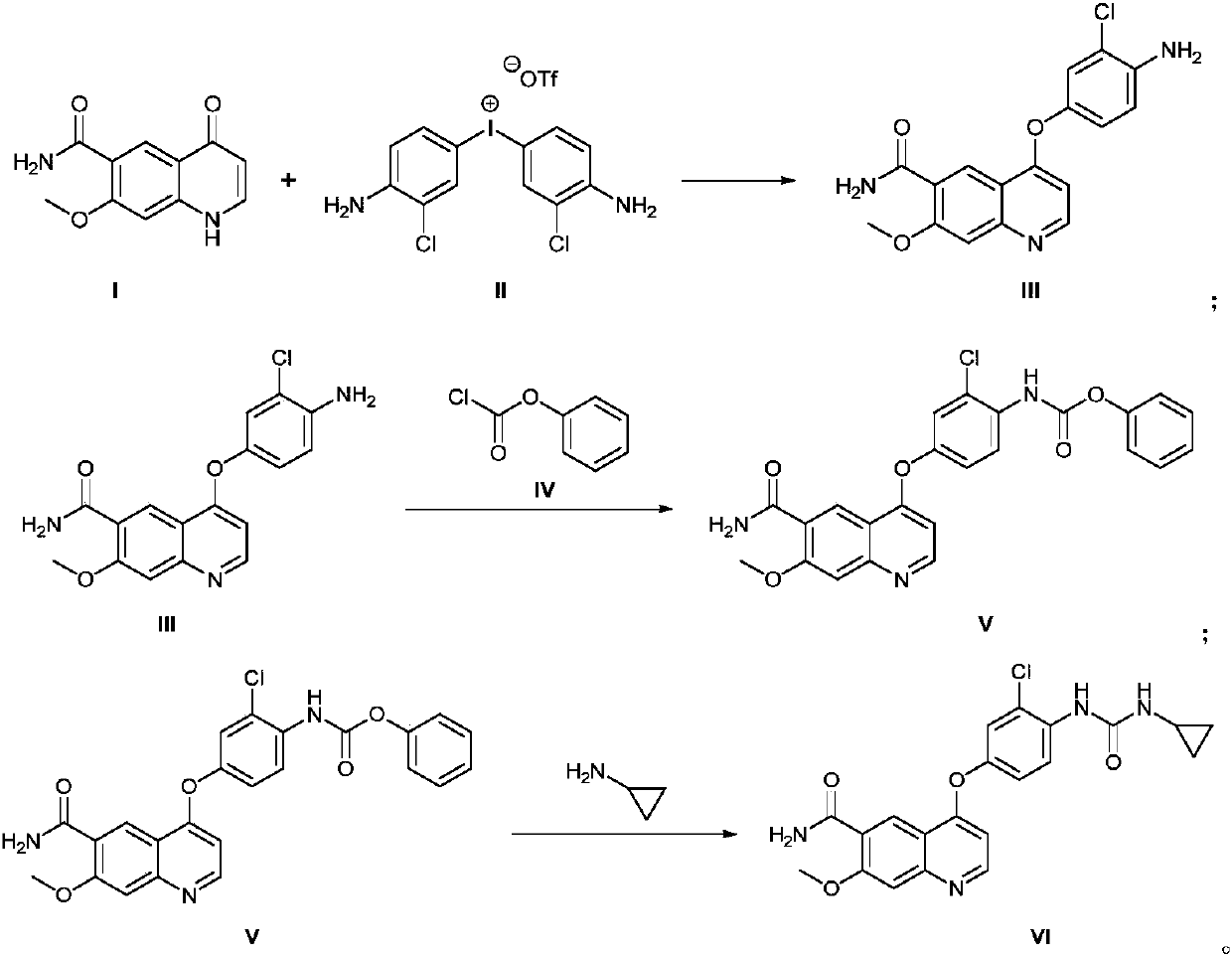
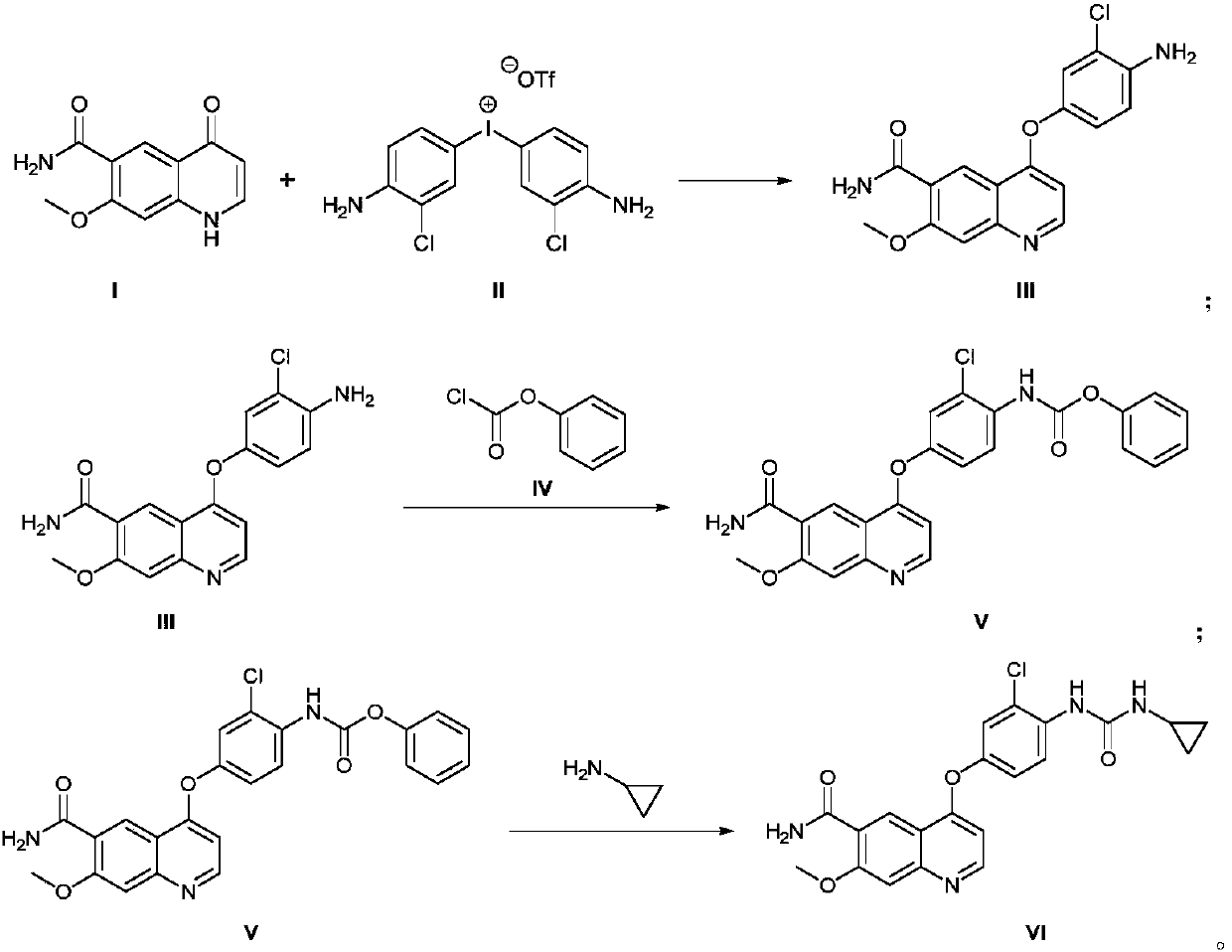
[0030] (1) Synthesis of 4-(4-amino-3-chloro-phenoxy)-7-methoxy-6-formamide (compound III):
[0031] 7-Methoxy-4-oxo-1,4-dihydroquinoline-6-carboxamide (10.9g, 0.05mol) and bis(3-chloro-4-amino)phenyltrifluoromethanesulfonic acid Iodine (31.7g, 0.06mol) was dissolved in dimethyl sulfoxide (80mL, 0.9mol), and cesium carbonate (48.8g, 0.15mol) was added, that is, compound I, compound II, base (cesium carbonate), organic solvent (di The molar ratio between methyl sulfoxide) is (1.0:1.2:3.0:18). The reaction mixture was heated by microwave at 80°C for 60 minutes in a CEM microwave synthesizer (100W), and the reaction was confirmed by TLC spotting. After adding water (200mL) and ethyl acetate (200mL x 3) for separation and extraction, the organic layer was separated and collected. The organic layer was washed with saturated aqueous sodium chloride solution (100 mL), and the organic phase was collected and dried over sodium sulfate. The solvent was distilled off under reduced press...
Embodiment 2
[0040] The preparation method is the same as in Example 1, but the mol ratio between compound I, compound II, alkali (cesium carbonate), organic solvent (dimethyl sulfoxide) in step (1) is (1.0:1.0:2.5:10) , microwave heating temperature was 90°C, 1.0 reaction for 40min gave 12.8g light yellow solid 4-(4-amino-3-chloro-phenoxy)-7-methoxy-6-carboxamide (compound III). rate 74.5%;
[0041] In step (2), the molar ratio between compound III, compound IV, base (pyridine), and organic solvent (N,N-dimethylformamide) is (1.0:1.2:3.0:10.0), and the reaction temperature is 60°C , reacted for 4h to obtain 15.2g yellow solid 4-[3-chloro-4-(benzyloxycarbonyl)aminophenoxy]-7-methoxyquinoline-6-carboxamide (compound V), yield 88.2% ;
[0042] In step (3), the molar ratio between compound V, cyclopropylamine, condensing agent (N,N'-carbonyldiimidazole), base (N,N-diisopropylethylamine), and organic solvent (tetrahydrofuran) is ( 1.0:1.0:1.5:4.0:10), the reaction temperature was 80°C, and ...
Embodiment 3
[0044] The preparation method is the same as in Example 1, but the mol ratio between compound I, compound II, alkali (cesium carbonate), organic solvent (dimethyl sulfoxide) in step (1) is (1.0:1.6:4.0:20) , the temperature of microwave heating was 70°C, and 13.08g of light yellow solid 4-(4-amino-3-chloro-phenoxy)-7-methoxy-6-carboxamide (compound III) was obtained after 80 minutes of reaction. The yield 76.1%;
[0045] In step (2), the molar ratio between compound III, compound IV, base (pyridine), and organic solvent (N,N-dimethylformamide) is (1.0:1.7:6.0:20), and the reaction temperature is 30°C , reacted for 12h to obtain 16.2g of yellow solid 4-[3-chloro-4-(benzyloxycarbonyl)aminophenoxy]-7-methoxyquinoline-6-carboxamide (compound V), yield 91.6% ;
[0046] In step (3), the molar ratio between compound V, cyclopropylamine, condensing agent (N,N'-carbonyldiimidazole), base (N,N-diisopropylethylamine), and organic solvent (tetrahydrofuran) is ( 1.0:1.6:1.9:5.5:20), the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com