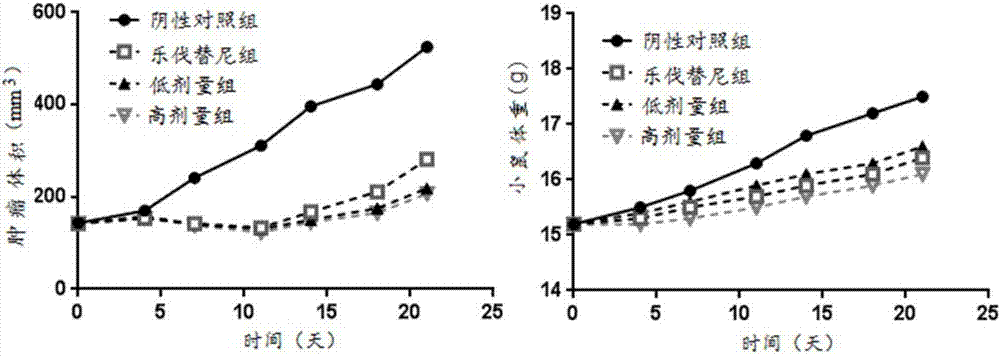
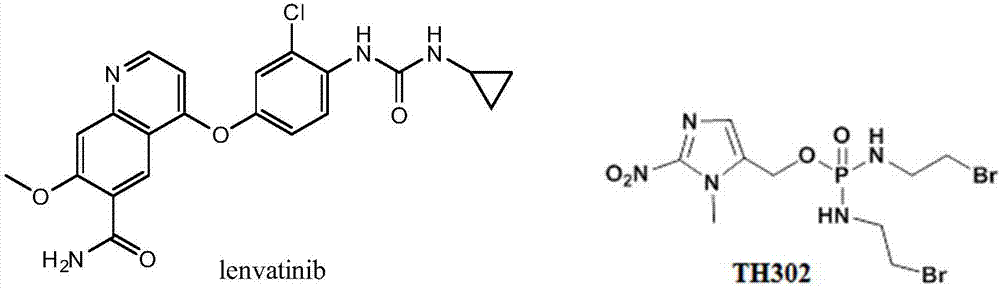
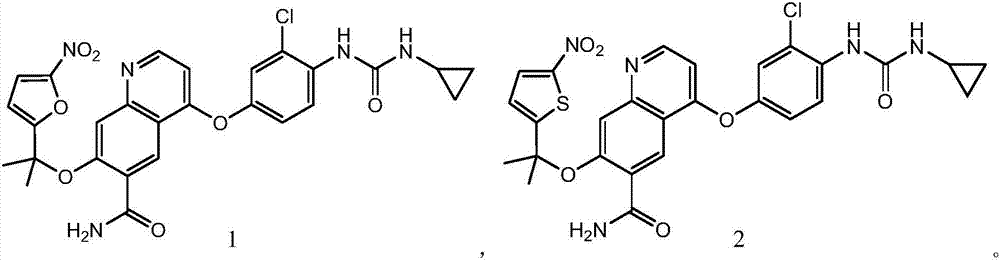
Hypoxia-activated prodrug of lenvatinib and application of hypoxia activated prodrug
A technology of lenvatinib and oxygen activation, applied in the field of hypoxia-activated prodrugs, can solve problems such as limited tumor effect, and achieve the effects of reduced toxic side effects, low inhibitory activity, and reduced possibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the synthesis of target compound:
[0020] Synthesis of compound 1
[0021]
[0022] 0.412g (1.00mmol) O-desmethyllenvatinib (3), 0.28g (3.00mmol) 2-methyl-2(5-nitrofuran)ethanol were dissolved in dry dichloromethane (50mL). Cool to 0°C, add 0.752 mL of tributylphosphine (3.06 mmol) dropwise, and stir at room temperature for 48 hours. The solvent was recovered under reduced pressure, and the title compound (0.035 g) was obtained by silica gel column chromatography. 1 H NMR(DMSO-d6,δ,ppm,300MHz):8.66(d,2H),8.28(d,1H),7.96(d,1H),7.86(s,1H),7.73(s,1H),7.51 (s,1H), 7.48(d,1H), 7.42(d,1H), 7.24(dd,1H), 7.19(d,1H), 6.71(d,1H), 6.53(t,1H), 2.66( s, 1H), 1.77 (s, 6H), 0.66 (q, 2H), 0.41 (s, 2H).
[0023] Synthesis of compound 2:
[0024]
[0025] Referring to the method of target compound 1, it was synthesized with 2-methyl-2(5-nitrothiophene)ethanol instead of 2-methyl-2(5-nitrofuran). 1 H NMR(DMSO-d6,δ,ppm,300MHz):8.62(d,2H),8.23(d,1H),7.92(d,1H),7.8...
Embodiment 2
[0026] Example 2: Inhibitory activity screening of target compounds on tyrosine kinases in vitro
[0027] A series of gradient concentrations of the test compound were incubated with a specific concentration of enzyme solution at room temperature for 5 minutes, and then an appropriate amount of enzyme reaction substrate and ATP was added to start the enzyme reaction process. After 30 minutes, an appropriate amount was added to the enzyme reaction system. After incubation for 1 h, measure the enzyme activity at a specific compound concentration on a multifunctional microplate reader, and calculate the inhibitory activity of different concentrations of compounds on the enzyme activity, and then according to the four-parameter equation, for different The inhibitory activity of the enzyme activity at the concentration of the compound was fitted, and the IC50 value was calculated.
[0028] Table 1 Inhibitory activity of target compounds on tyrosine kinases in vitro (IC50, nM)
[0...
Embodiment 3
[0031] Example 3: Stability investigation of target compound in liver homogenate
[0032] Preparation of NADPH start-up system: Precisely weigh NADPNa 2 , G-6-P-Na, G-6-PDH and MgCl 2 Appropriate amount, add water to dissolve and make to volume, the system contains 2mmol L -1 NADPNa 2 , 40 mmol L -1 G-6-P-Na, 4U L -1 G-6-PDH, 40 mmol L - 1 MgCl 2 , stored at -20°C.
[0033] Sample preparation: First, add an appropriate amount of sample methanol solution to the EP tube, evaporate the solvent in a water bath, add Tris buffer solution, rat liver homogenate, and vortex to mix. Pre-incubate at 37°C for 5 minutes in a constant temperature shaking water tank. Add 200 μL of NADPH priming system and vortex to mix well to initiate the reaction. The final volume of the reaction is 400 μL, containing 1.0 mmol L -1 1NADPNa 2 , 20 mmol L -1 G-6-P-Na, 2U L -1 G-6-PDH, 20 mmol L -1 MgCl 2 , the concentration of liver homogenate protein is 2.0mg mL -1 , with a final substrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com