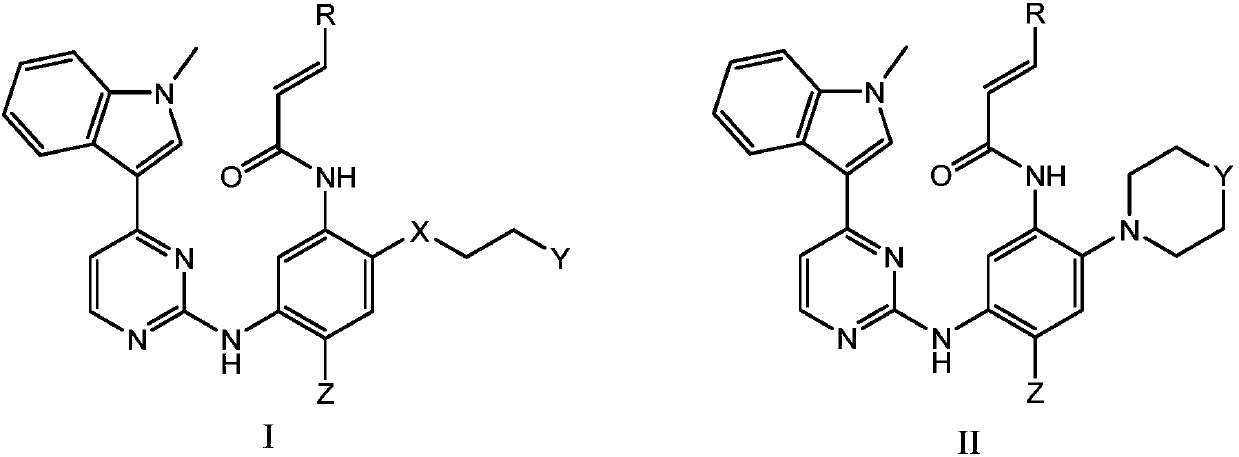
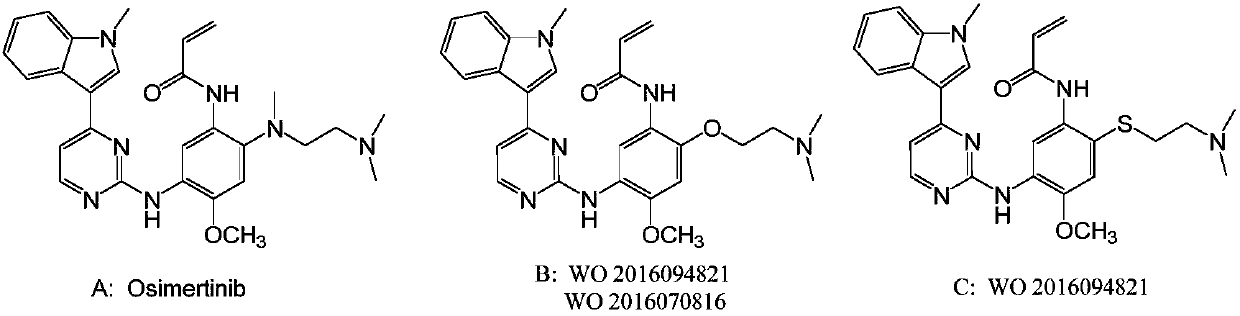
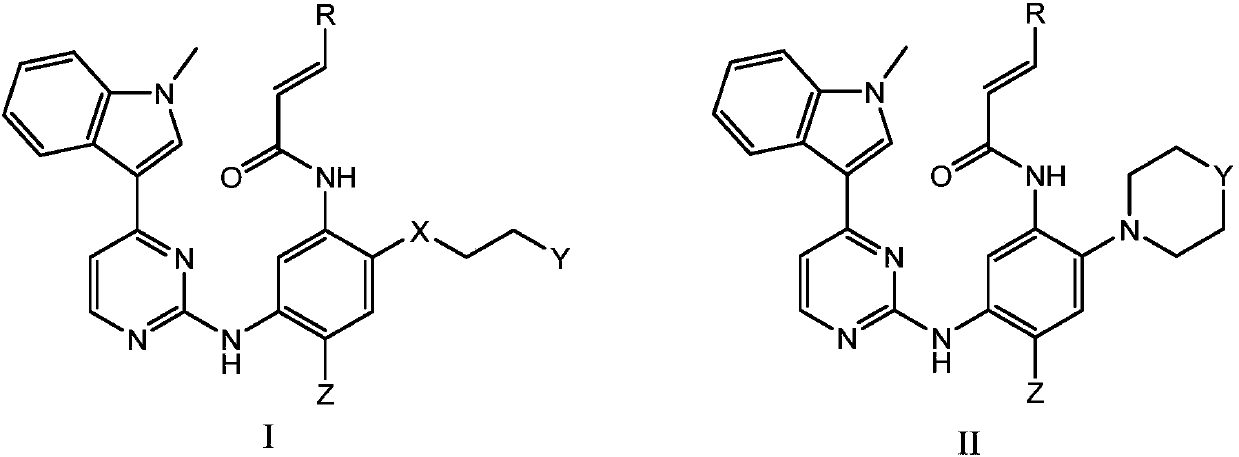
Novel N-phenyl-2-pyrimidinaminederivative and application thereof to preparation of anti-tumor medicine
A technology of anilinopyrimidine and derivatives, which is applied to novel 2-anilinopyrimidine derivatives and its application in the preparation of antitumor drugs, can solve problems such as drug resistance, and achieve improved solubility and antitumor activity. , Good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the synthesis of target product 1 (structural formula 1).
[0040] The synthesis of intermediate 13-16 was carried out with reference to the method in the literature (J.Med.Chem.2014, 57, 8249-8267).
[0041] Preparation of Intermediate 17: Dissolve Intermediate 16 (1.0mmol) in N,N-dimethylformamide (DMF) (10mL), add sodium methyl mercaptide (1.5mmol) and triethylamine (1.5mL) dropwise in sequence mmol), reacted at 50°C; after the reaction was monitored by TLC, the reaction system was poured into ice water, resulting in a large amount of precipitation. The resulting precipitate was filtered off, washed three times with water, and dried in vacuo to afford intermediate 17. Yield: 65%.
[0042] Preparation of Intermediate 18: Intermediate 17 (1.0 mmol) was dissolved in CH 2 Cl 2 (10mL), the reaction system was lowered to 0°C, and m-CPBA (1.0mmol) was added in batches; after the reaction was monitored by TLC, saturated sodium bicarbonate solution was added t...
Embodiment 2
[0045] Embodiment 2: the synthesis of target product 2 (structural formula 2)
[0046]
[0047] Intermediate 20 was prepared in the same way as Intermediate 19, yield: 63%.
[0048] The preparation of target product 2 is the same as that of target product 1. Yield: 72%. 1H NMR (400MHz, Chloroform-d) δ9.79(s,1H),9.28(s,1H),9.00(s,1H),8.36(d,J=5.3Hz, 1H),8.12–7.97(m, 1H),7.68(s,1H),7.43–7.34(m,1H),7.23–7.15(m,1H), 6.72(s,1H),6.47–6.36(m,2H),5.72–5.60(m, 1H), 3.95(s, 3H), 3.86(s, 3H), 3.65(t, J=5.0Hz, 2H), 2.93(t, J=5.0Hz, 2H), 2.69(s, 3H).MS: [M+H] + =473.2.
Embodiment 3
[0049] Embodiment 3: the synthesis of target product 3 (structural formula 3).
[0050]
[0051] Intermediate 21 was prepared in the same way as Intermediate 19, yield: 65%.
[0052] The preparation method of the target product 3 is the same as that of the target product 1. Yield: 82%. 1H NMR (400MHz, Chloroform-d) δ9.89(d, J=3.4Hz, 1H), 9.10(d, J=22.6Hz, 2H), 8.38(s, 1H), 8.06(t, J=5.2Hz ,1H),7.76(s,1H),7.40(d,J=8.1Hz,1H),7.22(d,J=4.6Hz,1H),6.76(d,J=3.5Hz,1H),6.52–6.31 (m,2H),5.74(d,J=9.6Hz,1H), 3.99(d,J=3.5Hz,3H),3.89(d,J=3.3Hz,3H),3.58(q,J=4.9Hz ,2H),3.18 (q,J=5.0Hz,2H),2.71(d,J=3.5Hz,3H).MS: [M+H] + =491.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com