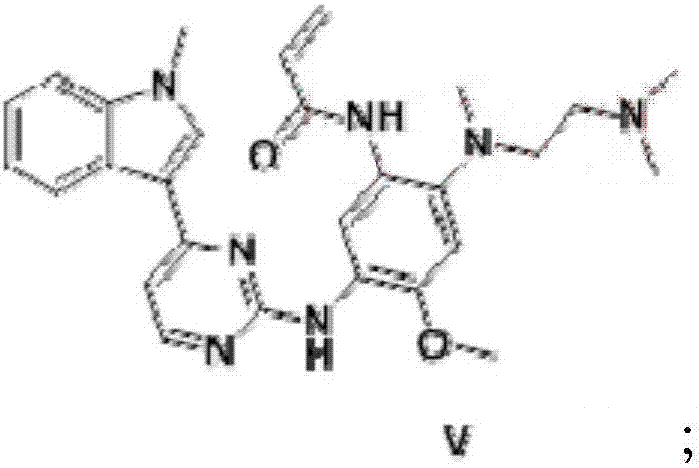
Method for preparing osimertinib mesylate
A technology of meritinib and methanesulfonic acid, applied in the field of medicinal chemistry, can solve the problems of being unsuitable for industrial production, unfavorable to protect the environment, and large adsorption of iron powder, so as to reduce the risk of genotoxic impurities and reduce costs , the effect of small adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
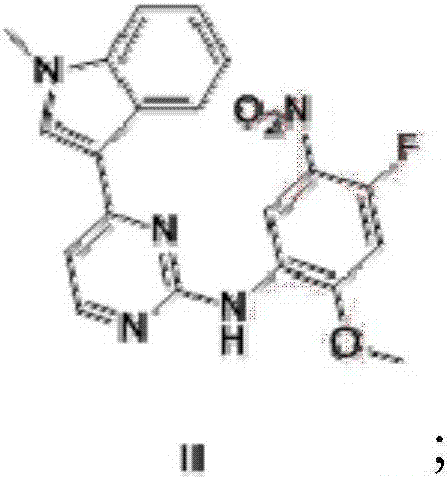
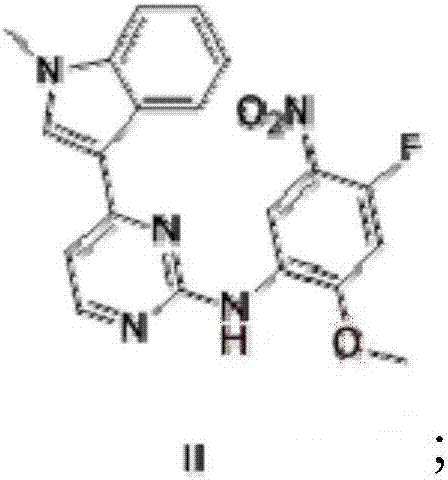
[0062] (1) compound shown in preparation formula (II)
[0063] At room temperature, add 1000 ml of 2-pentanol to a 2L reaction flask, add 50 g of 3-(2-chloro-4-pyrimidinyl)-1-methyl-1H-indole, 42 g of 4-fluoro- 2-methoxyl-5-nitroaniline, 3.54 g of p-toluenesulfonic acid, the mixture was heated to 80°C, and reacted for 6 hours. The mixture was cooled to 25°C-28°C, filtered with suction, and the filter cake was washed with 50 ml of 2-pentanol. After the filter cake was drained, it was dried in a vacuum drying oven to obtain 80.1 g of a yellow solid, with a yield of 99.0%. 1 HNMR (400MHz, DMSO) δ3.92(3H, s), 4.00(3H, s), 7.14(1H, dd), 7.30-7.33(1H, m), 7.44-7.52(2H, m), 7.60(1H , d), 8.22 (1H, m), 8.35 (1H, d), 8.68 (1H, s), 8.79 (1H, d), 9.76 (1H, s). m / z(M+H) + = 394.20.
Embodiment 2
[0065] Compound shown in the preparation formula (III)
[0066] At room temperature, 801 milliliters of N,N-dimethylacetamide was added to a 2L reaction flask, 80.1 grams of the compound shown in formula (II), 20.6 grams of N,N,N'-trimethylethylenediamine were added, 28.1 grams of potassium carbonate. The mixture was heated at 110°C and reacted for 7 hours. Slowly lower the temperature to 25°C-28°C, slowly add 801 ml of water, stir and crystallize for 1 hour. Suction filtration, the filter cake was washed with 160 ml of water. The filter cake was dried in a vacuum drying oven to obtain 92.8 g of orange-red solid, yield: 95.9%. 1 HNMR (400MHz, DMSO) δ2.16 (6H, s), 2.48 (2H, t), 2.86 (3H, s), 3.27 (2H, t), 3.87 (3H, s), 3.95 (3H, s), 6.84(1H,s), 7.12(1H,t), 7.22(1H,d), 7.25(1H,t), 7.52(1H,d), 8.11(1H,s), 8.31(1H,d), 8.32 (1H,s), 8.36(1H,d), 8.64(1H,s). m / z(M+H) + = 476.20.
Embodiment 3
[0068] Compound shown in the preparation formula (IV)
[0069] Under nitrogen protection, 928 milliliters of methanol was added to a 2L four-necked flask, then 92.8 grams of the compound shown in formula (III) was added, and under stirring, 18.6 g of Raney nickel (water-containing wet product) was added, and the hydrogenation reduction was carried out at 8 atmospheric pressure. , the reaction temperature was 25°C, and the reaction was carried out for 6 hours. Filtration and spinning to obtain 82.6 g of gray solid, yield: 95.0%. 1 HNMR(400MHz,DMSO)δ2.18(6H,s),2.37(2H,t),2.63(3H,s),2.89(2H,t),3.74(3H,s),3.88(3H,s), 4.62(2H,s), 6.76(1H,brs), 7.14-7.19(2H,m), 7.23-7.26(1H,m), 7.48(1H,s), 7.52(1H,d), 7.81(1H, s), 8.27(1H,d), 8.31(1H,s), 8.43(1H,d). m / z(M+H) + = 446.30.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com