Preparation method of osimertinib
An intermediate, nitroanisole technology, applied in the field of organic synthesis technology medicinal chemistry, can solve problems such as being unsuitable for scale-up production, high equipment requirements, very low temperature, etc., and achieves mild reaction conditions, safe operation, and easy post-processing. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
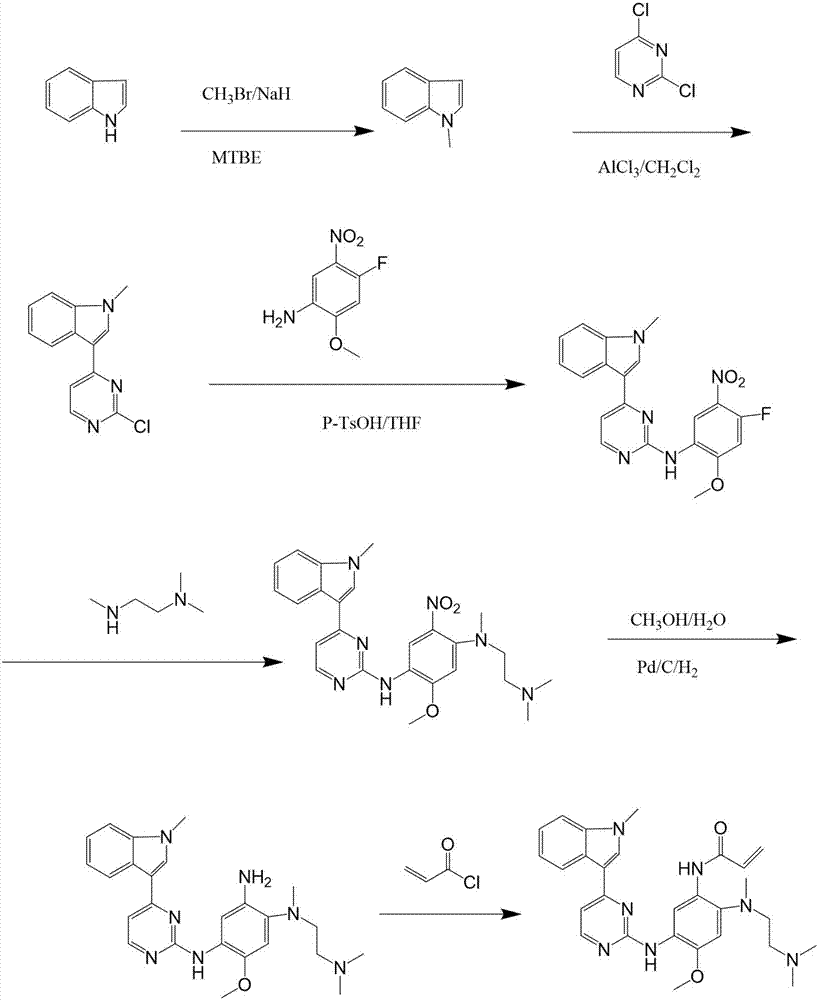
[0021] Preparation of Intermediate 1
[0022] Weigh 50g of 5-fluoro-2-nitroanisole into a three-necked flask equipped with a thermometer, add 300ml of DMF, raise the temperature to 85°C, stir for 10min, add dropwise N,N,N'-trimethylethylenediamine 31.4 g. Stir for 4h. After the reaction was complete, 600ml of water was added dropwise. After stirring for 3 h, intermediate 1 was obtained as a yellow solid by filtration, with a yield of 90%.
[0023] Preparation of intermediate 2
[0024] Weigh 50g of intermediate 1, weigh 1.0g of Pd / C, add 250ml of methanol into a three-necked flask, and inject H 2 Keep 0.02MPa and react at 35°C for 5h. After the reaction was completed, Pd / C was filtered out, and the feed solution was concentrated to obtain a white solid with a yield of 85%.
[0025] Preparation of intermediate 3
[0026] Weigh 50g of intermediate 2, put it in a three-neck flask with 250ml of methanol, stir evenly, cool down to -10°C, add HNO dropwise 3 The amount was 14...
Embodiment 2
[0036]Preparation of Intermediate 1
[0037] Weigh 50g of 5-fluoro-2-nitroanisole into a three-necked flask equipped with a thermometer, add 300ml of DMF, raise the temperature to 85°C, stir for 20min, add dropwise N,N,N'-trimethylethylenediamine 32.8 g. Stir for 5h. After the reaction was complete, 600ml of water was added dropwise. After stirring for 3 h, the intermediate 1 was obtained as a yellow solid by filtration, with a yield of 95%.
[0038] Preparation of Intermediate 2
[0039] Weigh 50g of intermediate 1, weigh 0.5g~1.0g of RancyNi, add 250ml of methanol into a three-necked flask, and pour in H 2 Keep 0.02MPa and react at 35°C for 5h. After the reaction was completed, RancyNi was filtered out, and the feed liquid was concentrated to obtain a white solid with a yield of 70%.
[0040] Preparation of Intermediate 3
[0041] Weigh intermediate 2 as 50g, place 250mlH 2 SO 4 In a three-neck flask, stir evenly, cool down to -10°C, add HNO dropwise 3 The amount w...
Embodiment 3
[0051] Preparation of Intermediate 1
[0052] Weigh 50g of 5-fluoro-2-nitroanisole into a three-necked flask equipped with a thermometer, add 250ml of DMF, raise the temperature to 85°C, stir for 20min, add dropwise N,N,N'-trimethylethylenediamine 32.8 g. Stir for 5h. After the reaction was complete, 600ml of water was added dropwise. After stirring for 3 h, the intermediate 1 was obtained as a yellow solid by filtration, with a yield of 95%.
[0053] Preparation of Intermediate 2
[0054] Weigh 50g of intermediate 1, add 250ml of methanol and 100ml of water into a three-neck flask, stir, and add 25g of NH 4 Cl was reacted at 80°C for 6h. Fe powder was filtered out after the reaction was completed, and the feed solution was concentrated to obtain a white solid with a yield of 70%.
[0055] Preparation of Intermediate 3
[0056] Weigh intermediate 2 as 50g, place 250mlH 2 SO 4 In a three-neck flask, stir evenly, cool down to -10°C, weigh KNO 3 The amount is 26.0g, dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com