Application of aspirin in preparing medicine for treating NSCLC (non-small cell lung cancer)
A technology of aspirin and drugs, applied in drug combinations, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problems of acquired drug resistance, absence of acquired drug resistance of osimertinib, EGFR-TKI drugs, etc., to overcome acquired drug resistance Effects of drug resistance and increased sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 In vitro experiment
[0026] The experimental steps are as follows:
[0027] (1) Amplify the two sensitive cells H1975, PC-9GR and the corresponding resistant cells H1975-OR, PC9GR-OR respectively, collect the cancer cell lines in log phase, digest the cells with trypsin, and centrifuge the cells to suspend the cells. fluid, allowing the cells to settle. Resuspend cells in medium and count;
[0028] (2) Dilute the cell density to 2×10 after counting 4 / ml, add 100 μl of cell suspension to each well of a flat-bottom 96-well plate with a sampler, each well containing 2000 cells; put the seeded 96-well plate into 5% CO 2 , overnight culture in a cell incubator at 37°C and 90% humidity;
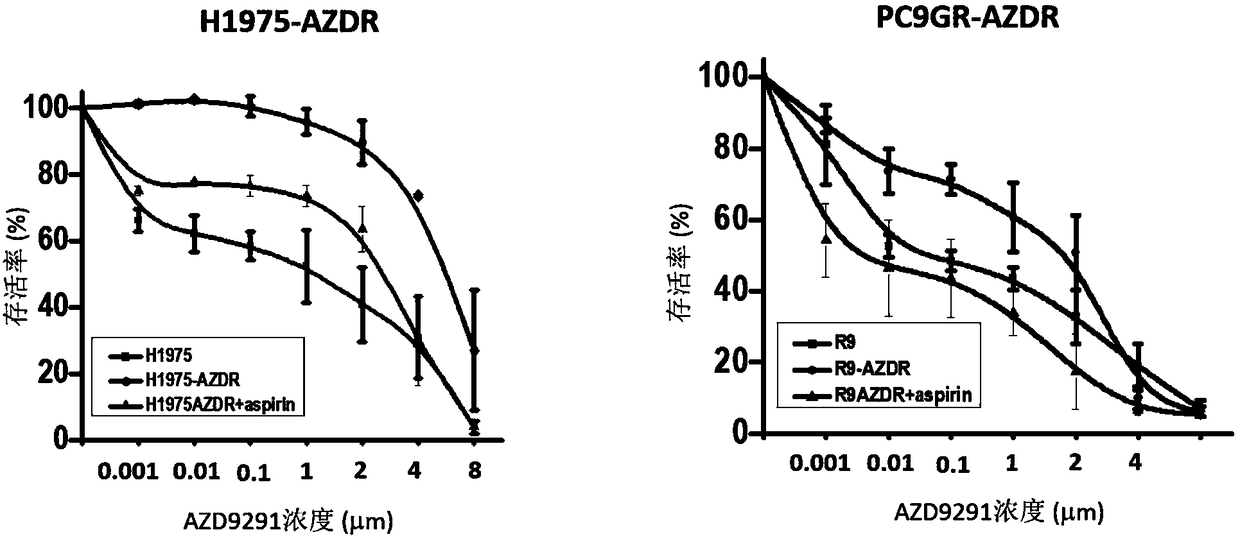
[0029] (3) The next day, 200 mmol / L aspirin was prepared, and a concentration gradient of osimertinib was added. A total of 8 concentrations were set, and 5 replicate wells were set for each concentration. There were three groups of each cell experiment, as follows:
[0030]...
Embodiment 2
[0039] Example 2 In vitro experiment
[0040] The experimental steps are as follows:
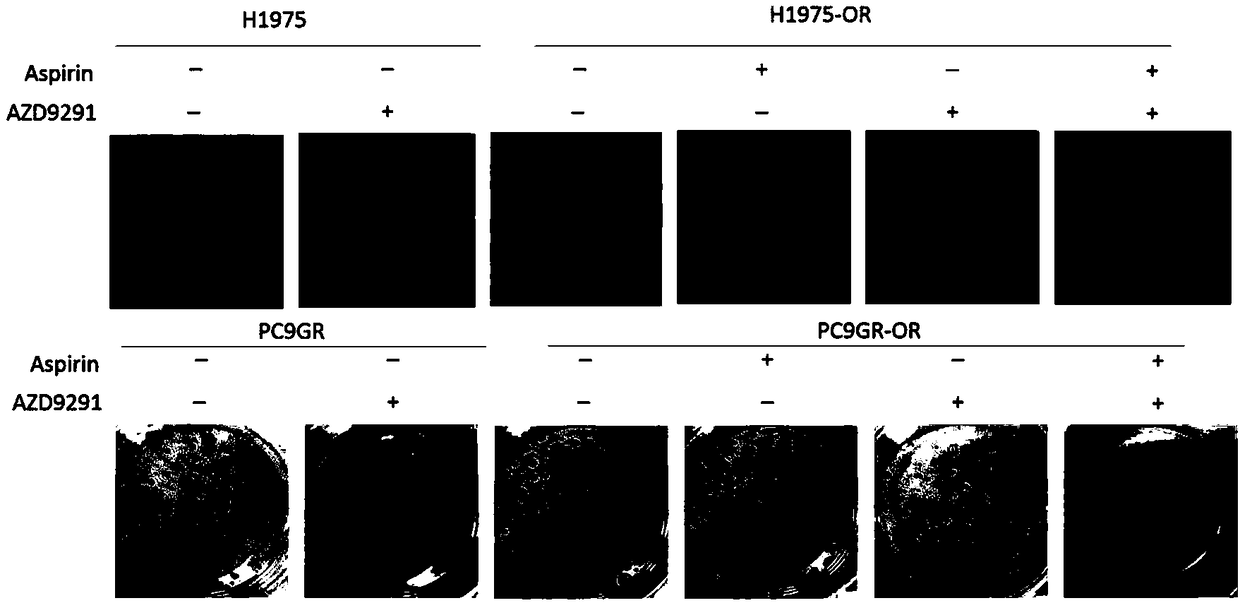
[0041] 1. Clone Formation Experiment
[0042] (1) Sensitive cells and drug-resistant cells were digested and seeded in 6-well plates, with 500 cells per well;
[0043] (2) After culturing for 24 hours, group administration: sensitive cell control group, sensitive cell plus osimertinib group, drug-resistant cell control group, drug-resistant cell plus aspirin group, drug-resistant cell plus osimertinib group, drug-resistant cell plus aspirin group in combination with osimertinib. Aspirin (200 mmol / L), osimertinib (3 mmol / L), the control group was not treated; the culture was continued for 12 d until macroscopic clones were formed, and the medium was discarded.
[0044] (3) Immerse with phosphate buffered saline (PBS) for a short time, fix with 4% paraformaldehyde in PBS solution for 20min, then wash with PBS, stain with 0.1% crystal violet for 20min, then slowly wash away the staining solu...
Embodiment 3
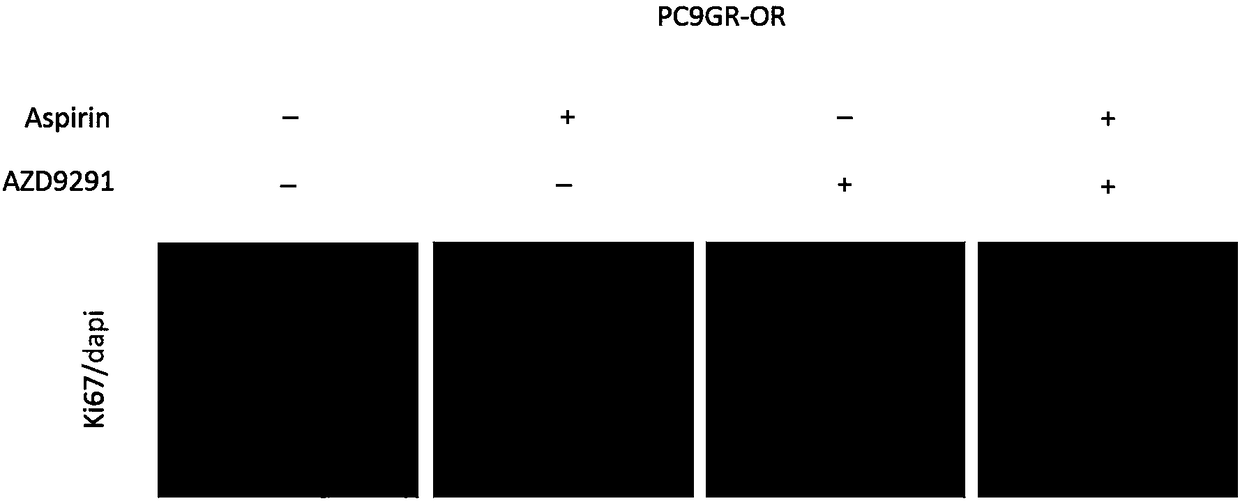
[0058] Example 3 Apoptosis Experiment
[0059] The experimental steps are as follows:
[0060] (1) The H1975-OR drug-resistant cells in logarithmic growth phase were digested with 0.25% trypsin and collected in a 1.5 ml sterile centrifuge tube. The centrifuge frequency was set to 1500 r / min, and the cell solution was centrifuged for 5 min. Resuspend in RPMI 1640 medium containing 10% fetal bovine serum, count and record under a microscope with the help of a counting plate, inoculate 250,000 cells per well in a 6-well culture plate, and place in a constant temperature and humidity cell incubator Overnight, waiting for cells to attach.
[0061] (2) Take out the 6-well plate, replace 4ml of fresh medium containing 3umol / L osimol / L and / or 200umol / L aspirin, and return the 6-well plate to 37°C, 5% CO 2 The cells were incubated in a constant temperature cell incubator for 48 h.
[0062] (3) Take out the 6-well plate, digest the cells with 0.25% trypsin, collect the cells in a 1.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com