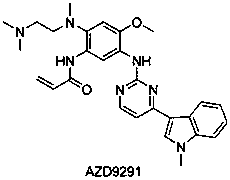
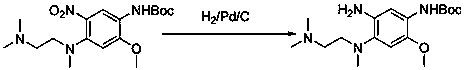Novel method for catalytically synthesizing osimertinib intermediate with a graphene/Pd catalyst
A palladium catalyst and graphene technology, applied in organic chemistry and other fields, can solve problems such as complex conditions, high prices, and inapplicability to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Mix 6.25 mL of 8 mg / mL graphene oxide aqueous slurry with 3 mL of 1 mg / mL palladium chloride aqueous solution, the mass ratio of graphene oxide to palladium chloride is 50:3, ultrasonically disperse, add 15 mg of oil Amine, under stirring, dropwise the 5mg / mL sodium borohydride solution of 2.5mL, the mass ratio of graphene oxide and sodium borohydride is 10:2.5, and the resulting product is centrifuged, washed, dried to obtain the graphene catalyst of supported palladium .
Embodiment 2
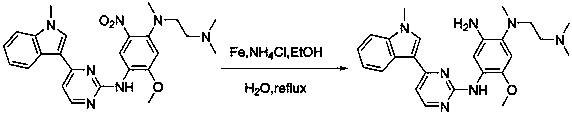
[0019] Example 2: Add N-benzyloxycarbonyl-2-methoxy-4-[2-dimethylaminoethylmethylamine]- 5-Nitroaniline (3.2g, 10mmol), graphene palladium catalyst (0.22g, 1mmol), water (5mL), irradiated with 500nm LED light, reacted for 15min, filtered, extracted with 15mL dichloromethane, and saturated The organic layer was washed with salt water, dried over anhydrous sodium sulfate, filtered, and finally dichloromethane was removed by rotary evaporation to obtain 2.83 g of a foamy off-white solid, with a yield of 96.9%.
Embodiment 3
[0020] Example 3: N-benzyloxycarbonyl-2-methoxy-4-[2-dimethylaminoethylmethylamine]- 5-Nitroaniline (3.2g, 10mmol), graphene palladium catalyst (0.22g, 1mmol), water (5mL), irradiated with 400nm LED light, reacted for 15min, filtered, extracted with 15 mL of dichloromethane, and used The organic layer was washed with saturated brine, dried, filtered, and finally dichloromethane was removed by rotary evaporation to obtain a foamy off-white solid (2.80 g), with a yield of 95.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com