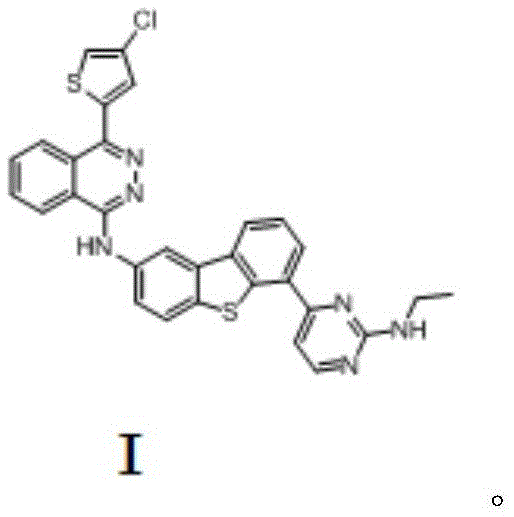
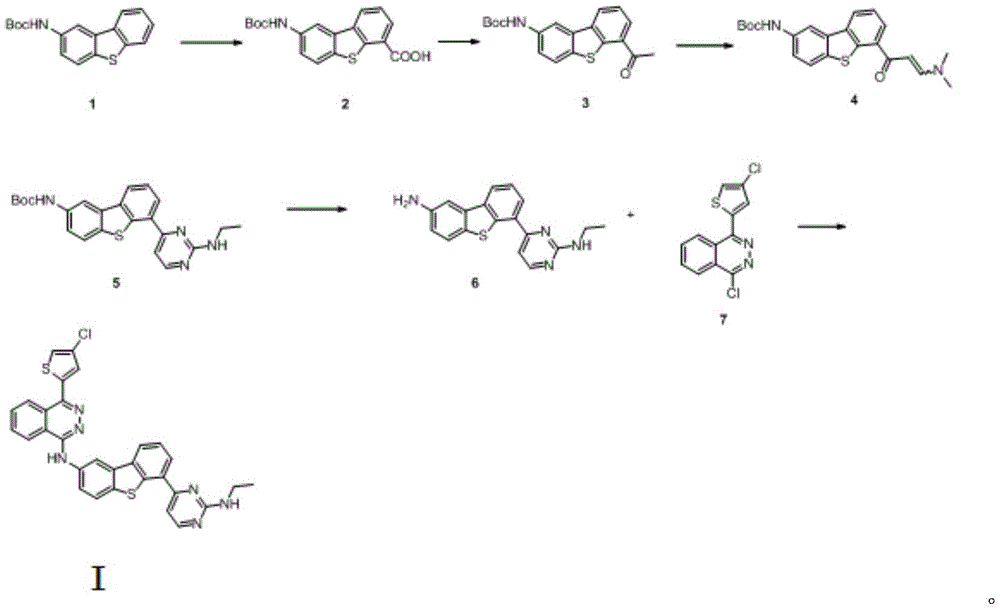
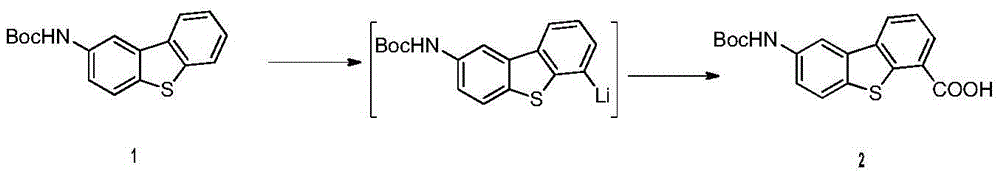
Anticancer compound osimertinib and synthesis method thereof
A technology for omegatinib and its synthesis method, which is applied in the field of anticancer compound omegatinib and its synthesis, can solve the problems of low selectivity, poor kinase inhibitory effect, etc., and achieve good product quality, simple and easy synthesis method, growth inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of compound (2)
[0033] Under the protection of nitrogen, 1.5kg (5mol) of compound (1) was added into 15L of anhydrous tetrahydrofuran into a 50L reactor at -20°C, and 352g (5.5mol) of sec-butyl lithium was slowly added dropwise. After stirring for half an hour, 264 g (6 mol) of dry ice was slowly added. After the reaction was monitored by TLC, the reaction solution was neutralized to pH 6.8 with 5% dilute hydrochloric acid, filtered, and the filter cake was washed with 5 L of tetrahydrofuran. The combined filtrate and washings were concentrated to obtain the compound (3) The crude product was recrystallized from ethyl acetate to obtain 1.65 kg (4.8 mol) of the refined product of compound (3), with a yield of 96%. Purity by HPLC: 99.2%.
[0034] 1 HNMR (400MHz, DMSO-d 6 )δ12.62(b,1H),9.88(s,1H),8.79(dd,J=14.9,3.0Hz,1H),8.20(dd,J=14.9,3.0Hz,1H),8.07(d,J =3.0Hz,1H),7.72(dt,J=14.7,7.4Hz,2H),7.48(dd,J=14.9,3.0Hz,1H),1.49(s,9H).
[0035] ESI+[M+H] + =344. ...
Embodiment 2
[0051] Example 2: Inhibitory effect of compounds of the present invention on aurora kinase
[0052] Aurora Kinase HTRF Assay
[0053] The Aurora-A-TPX2-HTRF assay for Aurora-A was started in the presence of ATP-phosphorylated biotinylated polypeptide PLK. The test was carried out for about 120 minutes, and after 120 minutes, the detection reagent was added to extract it. These detection reagents stop the reaction by diluting the kinase and chelating heavy metal ions with EDTA. Leave it overnight after adding the detection reagent, so that the detection reagent reaches equilibrium.
[0054] The Aurora-A-HTRF assay used 1uL of omeretinib in 100% DMSO solution, 20uL of ATP and biotinylated PLK, and 20uL of Aurora-A-TPX2-KGDGST kinase. The buffer conditions are: 60mM HEPES (4-hydroxyethylpiperazineethanesulfonic acid) pH7.5, 25mM aqueous sodium chloride, 10mM MgCl aqueous solution, 2mM DTT (dithiothreitol), 0.05% BSA (Bovine Serum Albumin).
[0055] The experiment was extract...
Embodiment 3
[0058] Example 3: High Selectivity of Compounds of the Invention to Aurora Kinases
[0059] The line containing Aurora kinase, p38α, Tyk2, JNK2, Met or Tie2 is selected for cultivation, and then the compound obtained by the present invention is added for detection.
[0060] Assay results show that the compounds of the present invention are 28 times, 35 times, 24 times, 38 times and 35 times more selective for Aurora kinase than for p38α, Tyk2, JNK2, Met and Tie2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com