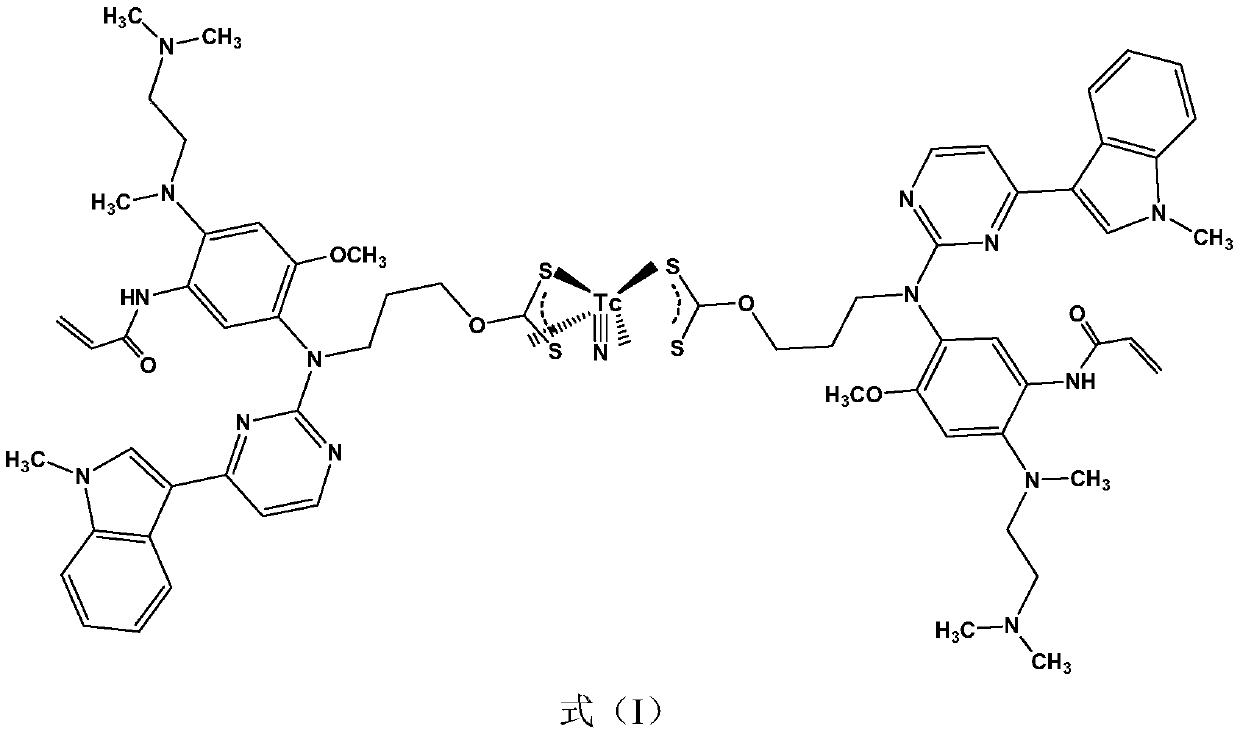
<99m>Tc-labeled complex and application thereof in diagnosis of non-small cell lung cancer
A complex and labeling technology, which is applied to compounds of group 7/17 elements of the periodic table, organic compounds of group 7/17 without C-metal bonds, pharmaceutical formulations, etc., to achieve good retention, simple preparation, and high intake Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
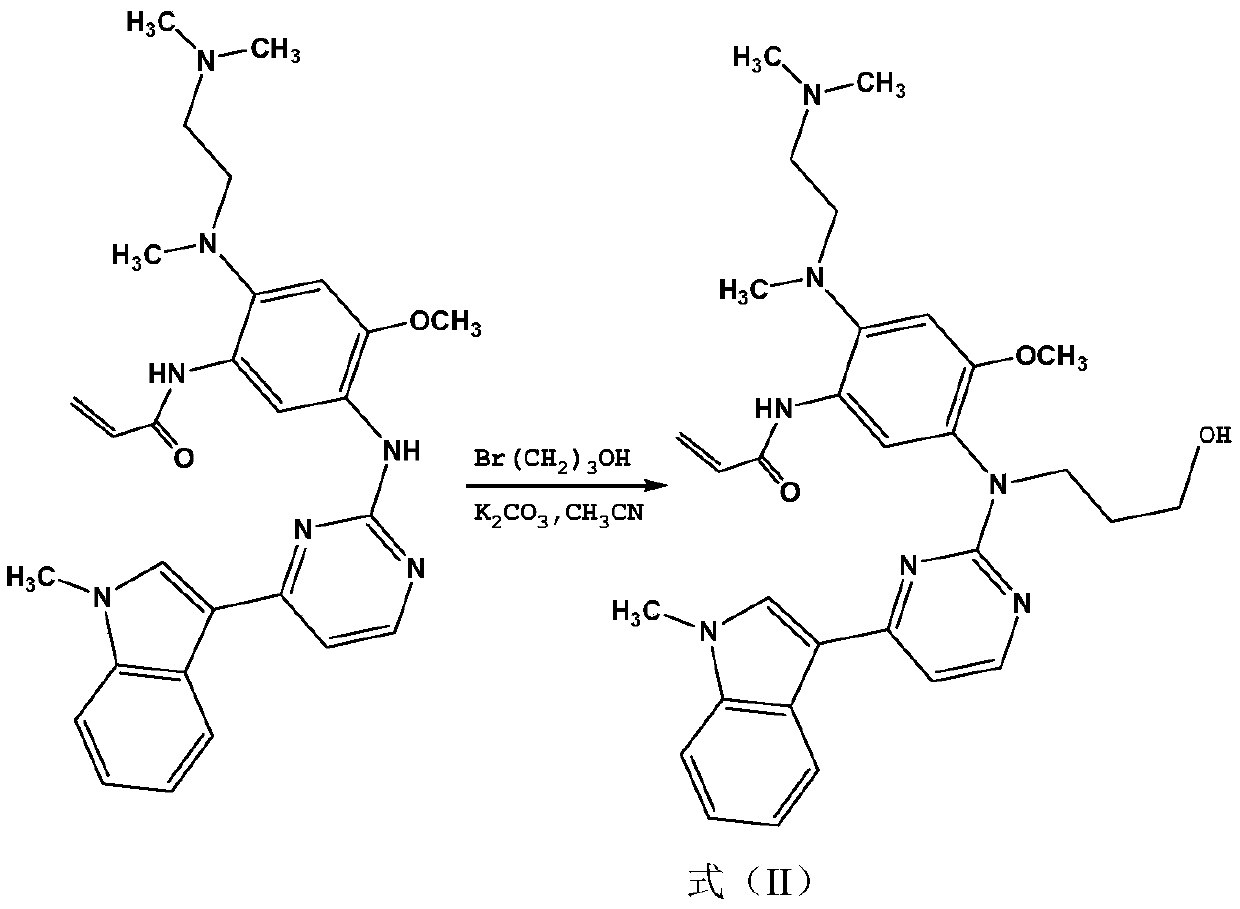
[0019] Embodiment 1: the preparation of formula (II) compound
[0020]
[0021] In a 500 mL round bottom flask, dissolve 50 g of osimertinib in 250 mL of acetonitrile, add 27 g of anhydrous K 2 CO 3 Stir for 0.5 hours, then add 15.2g of 1-bromopropanol and heat to reflux for 12 hours. After the reaction is completed, add deionized water to quench, extract with dichloromethane, and purify by column chromatography to obtain 44.1g of the compound of formula (II), with a yield of 78.9% , ESI / MS: 558.31[M+H + ].
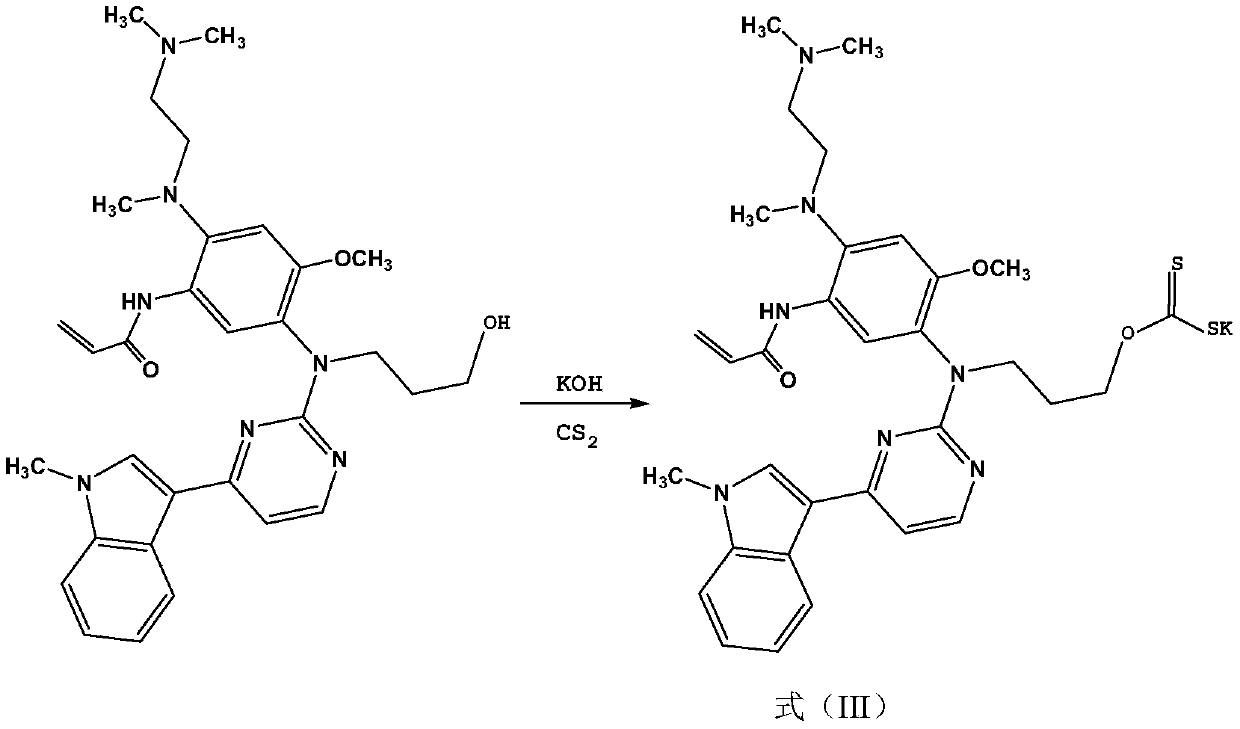
[0022] Step (2): preparation of formula (III) compound
[0023]
[0024] In a 500mL round-bottomed flask, dissolve 55.7g of the compound of formula (II) in 200mL of DMSO (dimethyl sulfoxide), add 25g of KOH, heat to 100°C for 1 hour, and then dropwise add 10mL of carbon disulfide (CS 2 ), after the TLC detection reaction was completed, the solvent was removed, and ethanol / ether recrystallized to obtain 37.8g of compound of formula (III), yield 56.3%, ESI / MS: 672...
Embodiment 2
[0028] Embodiment 2: adopt HPLC method to identify formula (I) complex
[0029] Kromasil C18 analytical column (4.6×250mm), the mobile phase is water containing 0.1% TFA (trifluoroacetic acid) (phase A) and methanol containing 0.1% TFA (phase B), the flow rate is 1mL / min; t=0, A / B =5 / 5; t=15min, A / B=1 / 9; t=20min, A / B=1 / 9; t=25min, A / B=5 / 5. The retention time (Rt) of the complex of formula (I) is 11.5 min.
[0030] The results of HPLC analysis show that the radiochemical purity of the compound of formula (I) prepared by the above method is greater than 90%.
Embodiment 3
[0031] Embodiment 3: formula (I) complex stability analysis
[0032] The radiochemical purity of the labeled complex of formula (I) was measured after placing it in mouse serum at room temperature and at 37°C for different times (1, 2, 3, 4, 5, 6, 8, 10 hours), Experimental results show that the radiochemical purity of the complex is greater than 90% at room temperature and in mouse serum at 37°C for 10 hours, indicating that it has good stability in vitro.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com