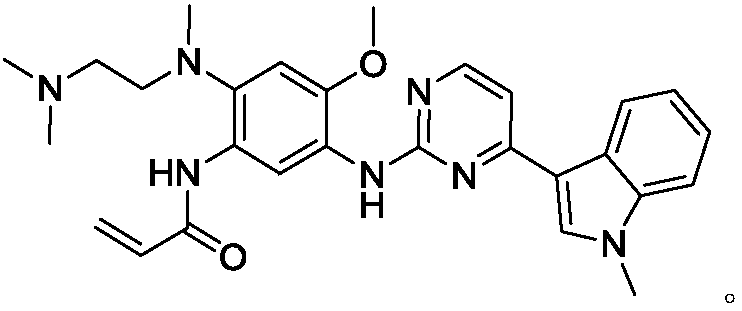
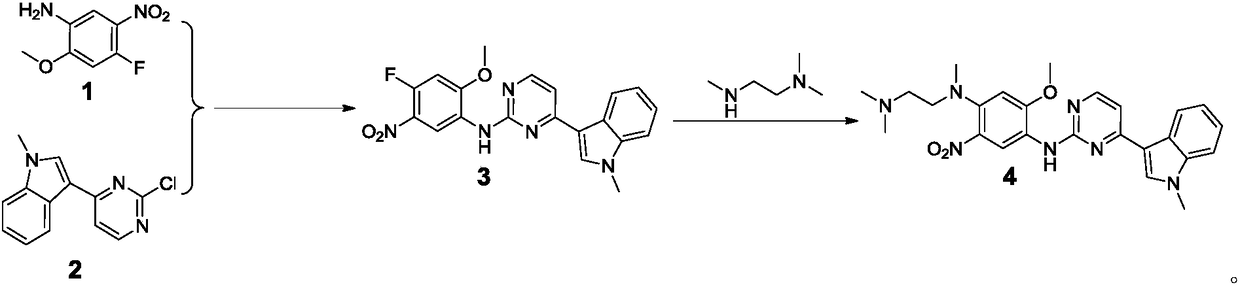
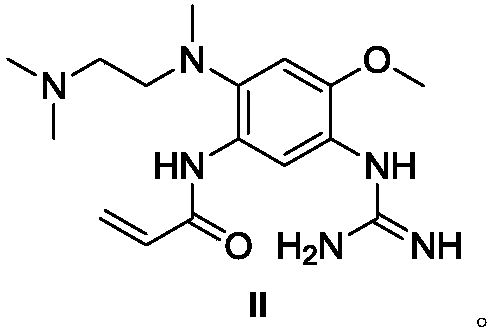
Osimertinib intermediate and preparation method thereof
A technology of intermediates and compounds, which is applied in the field of osimertinib intermediates and its preparation, can solve the problems of increasing the production cost of the final product, high purification cost, and high quality risk, and achieves reduced electron cloud density, high reaction yield, The effect of speeding up the reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] Take a 500ml four-necked flask, put in compound A (20.17g, 108.36mmol), triethylamine (18.57g, 183.52mmol), dissolve in dichloromethane (320ml), stir under nitrogen protection, and cool down to 0°C. A mixed solution of acetyl chloride (12.56g, 161.15mmol) and dichloromethane (30ml) was added dropwise at 0-5°C. After the dropwise addition was completed, the reaction was continued. After the reaction, add water (150ml), stir for 30min, and filter with suction. The filter cake was washed twice with water (20ml×2), and the filter cake was dried to obtain 23.63 g of solids, with a molar yield of 95.58%. 1H NMR (400MHz, DMSO) δ2.13(s, 3H), 3.99(s, 3H), 7.33(d, J=13.2Hz, 2H), 8.87(d, J=8.4Hz, 2H), 9.58(s , 1H). MS(ES+): m / z 229[M+H] +
Embodiment 2
[0046]
[0047] Take a 50ml one-mouth bottle, put in compound B (5.30g, 23.23mmol), N,N,N-trimethylethylenediamine (3.33g, 32.59mmol), sodium carbonate (3.30g, 23.88mmol), add absolute ethanol (30ml), stirred evenly, protected by nitrogen, and heated to 80°C for 1.5 hours. After the reaction was completed, the reaction solution was concentrated to dryness at 40° C. under reduced pressure, and the residue was added with dichloromethane (30 ml) and stirred for 10 minutes, and suction filtered. The filter cake was washed twice with dichloromethane (10ml×2). The filtrate was concentrated to dryness at 40°C under reduced pressure to obtain a blood-red oily liquid (7.20 g, 23.20 mmol), with a molar yield of 99.87%. 1 H NMR (400MHz, DMSO) δ2.07(s, 3H), 2.12(s, 6H), 2.43(t, J=7.6Hz, 2H), 2.80(s, 3H), 3.22(t, J=6.4Hz ,2H), 3.93(s,3H), 6.77(s,1H), 8.47(s,3H), 9.25(s,1H). MS(ES+): m / z 311[M+H]+
Embodiment 3
[0049]
[0050] Take a 100ml autoclave, add absolute ethanol (25.29g), potassium hydroxide (1.37g, 24.42mmol) and water (8.49g), and stir to dissolve. Compound C (4.68g, 15.06mmol) was added and flushed with nitrogen protection. Seal the autoclave and heat up to 120°C for reaction. After the reaction was complete, dichloromethane (30ml) and water (20ml) were added, stirred for 30min, and separated. The organic phase was concentrated to dryness at 55°C under reduced pressure to obtain a blood-red oily liquid (3.84 g, 14.31 mmol), with a molar yield of 95.03%. 1 H NMR (400MHz, DMSO) δ2.10(s, 6H), 2.32(t, J=6.8Hz, 2H), 2.70(s, 3H), 3.03(t, J=6.8Hz, 2H), 3.87(s ,3H), 4.91(s,1H), 6.75(s,1H), 7.11(s,1H). MS(ES+): m / z 269[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com