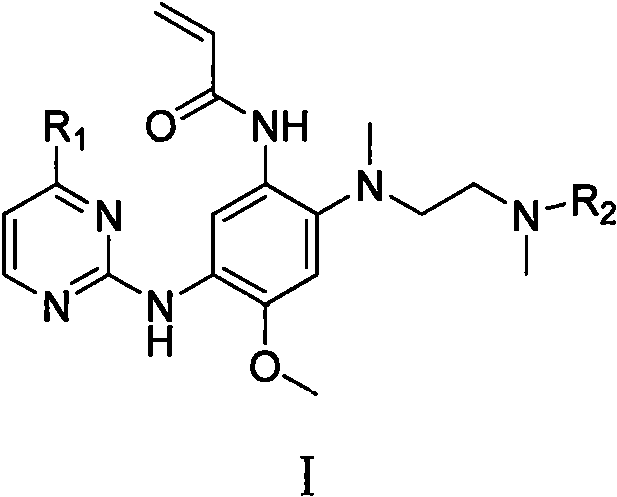
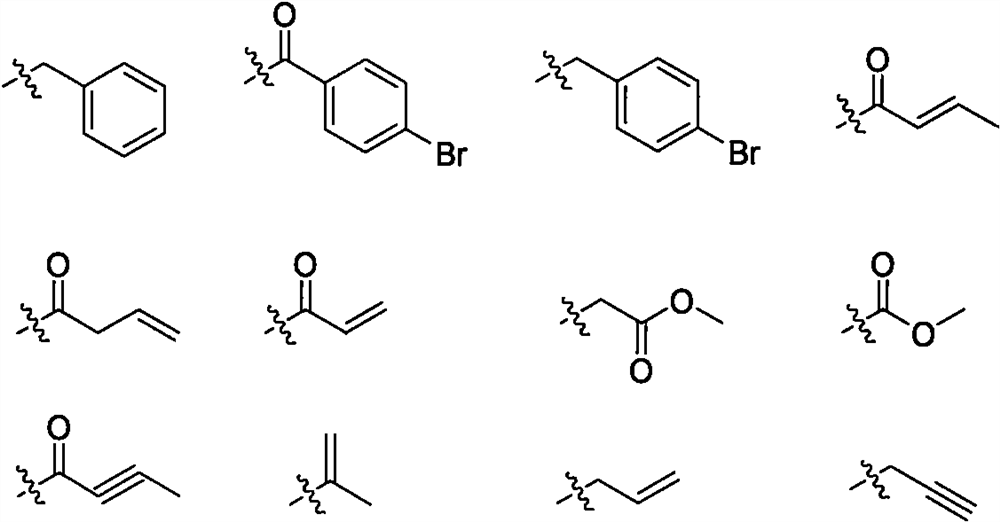
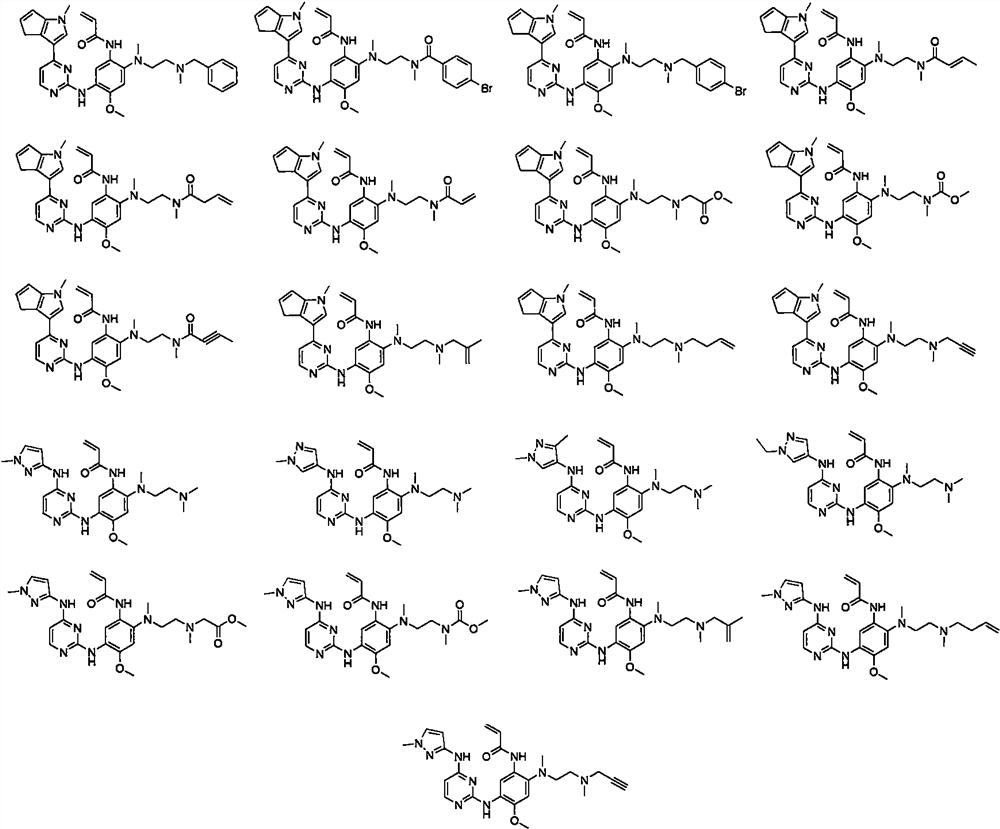
Aminopyrimidine derivative and application as EGFR tyrosine kinase inhibitor
A drug, methyl technology, applied in the field of medicine, can solve problems such as adverse reactions of patients, and achieve the effect of high inhibitory activity, high selectivity, and low inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Methyl N- (2 - (2-amino-5-methoxy-4 - (4- (1-methyl-1H-indole-3-yl) pyrimidine-2-yl) amino group ) Synthesis of phenyl) (meth) amino) N-methyl glycine ethyl ester (ZJT-4-M)
[0051] Synthesis of A.3- (2-pooprid-4-yl) -1-methyl-1H-indole (ZJT-1)
[0052]
[0053] 2,4-dichloronil (120.0 g, 0.81 mol) was added to a 2L reaction bottle, 880 ml 1, 4-Dioxane, stirred and dissolved, batching anhydrous Alcl 3 (120.0g, 0.90mol), stir until AlCl 3 It is uniformly dispersed in the emulsion, and then 1-methyl hydrazine (110.0 g, 0.84 mol) into the reaction liquid, warmed to 80 ° C insulation reaction for 3 hours, TLC detection reaction, 1-methylindole disappeared, The agent is petroleum ether: ethyl acetate = 1: 1. The reaction liquid was slowly poured into 2.5 L of purified water, stirred vigorously, precipitated the solid, and stirred after 1 hour, filtrate, purified with 1 l. The solid was dried in vacuo to dry overnight at 50 ° C to give 210.0 g of a crude product. The c...
Embodiment 2
[0066] Example 2 Methyl N- (2 - (2-Acrylamide-5-Methoxy-4 - (4- (1-methyl-1H-indole-3-yl) pyrimidine-2-yl ) Synthesis of amino) phenyl) (meth) amino) -N-methyl glycine ethyl alkine (CSJ-IM)
[0067]
[0068] Place the ZJT-4-M crude product in a 100ml flask, add 20 ml of DCM to solve the product, add K 2 CO 3 (0.45 g, 3.24 mmol), the reaction solution was lowered to 0 ° C for 20 min, and acryloyl chloride (0.20 g, 2.16 mmol) was slowly dripped, and the insulation reaction was carried out under low temperature conditions, and the TLC showed the ZJT-4-M reaction (DCM :: Meoh :nh 3 · H 2 O = 50:5:1). Reaction liquid filtration, remove K 2 CO 3 The solid, the mother liquor was added to 40 mL of methanol in 1 hour, and the solvent was evaporated under reduced pressure. The total yield of the step reaction is 29.91%. 1 H-NMR (400 MHz, Chloroform-D) ΔPPM 9.87 (S, 1H), 9.66 (S, 1H), 9.12 (S, 1H), 8.09-8.04 (M, "8.09-8.04 (M, 1H), 7.77 (S, 1H), 7.42-7.39 (m, 1H), 7.29-7.27 (m, 1H), 7.26 (...
Embodiment 3
[0069] Example 3 N- (2- (2- (benzyl (meth) amino) ethyl) (meth) amino) -4-methoxy-5 - (4- (1-methyl-1h) - Synthesis of hydrazine-3-yl) pyrimidin-2-yl) amino) phenyl) acrylamide (CSJ-IB)
[0070]
[0071] The synthesis method was used as CSJ-I-M, the final product was 0.11g, the light yellow solid, and the total yield of three-step reaction was 17.74%. 1 H-NMR (400 MHz, ChlorOform-D) ΔPPM 9.89 (S, 1H), 9.84 (S, 1H), 9.11 (S, 1H), 8.39 (D, J = 5.3 Hz, 1H), 8.07 (DD, J = 7.5, 1.8 Hz, 1H), 7.72 (S, 1H), 7.40 (D, J = 2.3 Hz, 1H), 7.38 (D, J = 1.2 Hz, 1H), 7.36 (S, 1H), 7.35 (D, J = 1.0 Hz, 1H), 7.33 (D, J = 0.9 Hz, 1H), 7.32-7.30 (m, 1H), 7.29-7.27 (m, 1H), 7.26 (S, 1H), 7.21 (D, J = 5.3Hz, 1H), 6.77 (S, 1H), 6.46-6.37 (m, 1H), 6.20 (S, 1H), 5.57 (D, J = 10.2 Hz, 1H), 3.98 (S, 3H), 3.87 (S, 3H), 3.58 (S, 2H), 2.60 (S, 3H), 2.40 (S, 2H), 2.25 (S, 3H). ESI-MS M / Z: 576.31 [M + H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com