Kit for quantitatively detecting EGFR mutation
A kit and chain reaction technology, which is applied in the determination/testing of microorganisms, DNA/RNA fragments, and the introduction of foreign genetic material using vectors, etc., which can solve problems such as inability to quantify
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Human fresh tumor tissue, paraffin-embedded tissue, peripheral blood, pleural effusion, human Genomic DNA Extraction from Cell Lines
[0030] The human cancer cell lines we tested include non-small cell lung cancer (NSCLC) (A549, H460, H838 and H1703), breast cancer (MCF-7, BT474 and HuL100), malignant multiple mesothelioma cell lines (H513, H2052, H290, MS-1 and H28), colon cancer cell lines (SW480), head and neck cancer cell lines (U87), cervical cancer (Hela), sarcoma cell lines (Mes-SA, Saos-2 and A204).
[0031] Human fresh tumor tissues, peripheral blood, and paraffin-embedded tissues that we tested included NSCLC, mesothelioma, colon cancer, malignant melanoma, renal cancer, esophageal cancer, thyroid cancer, malignant tumor, and ovarian cancer.
[0032] Specimen DNA Extraction
[0033] Can use the DNA extraction kit of Qiagen Company, Promega Company, Roche Company to extract sample genomic DNA, use Gene Company Nanodrop ND1000 type nucleic acid ...
Embodiment 2
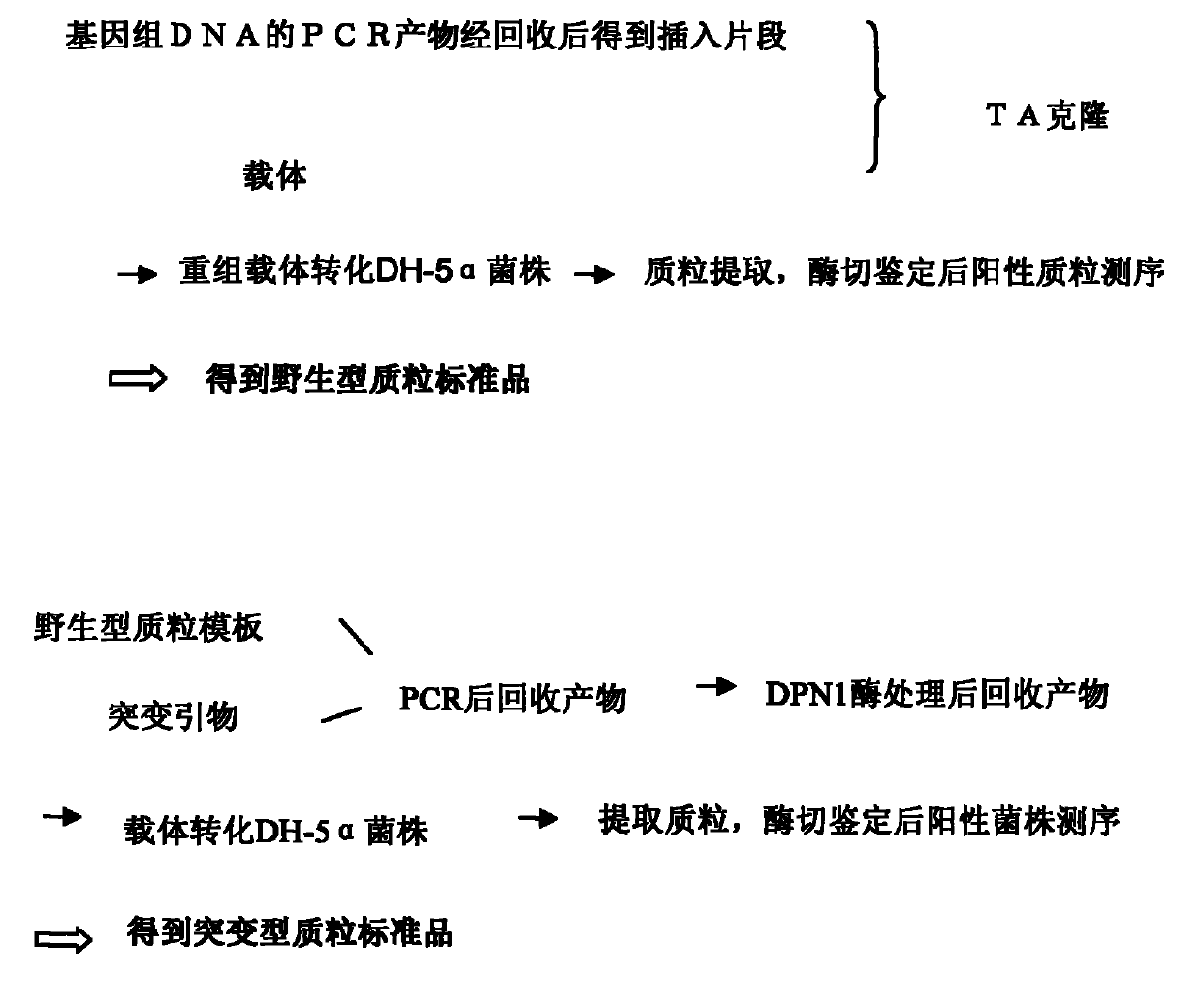
[0088] Example 2: Preparation of plasmid standards containing mutant and wild-type detection sequences
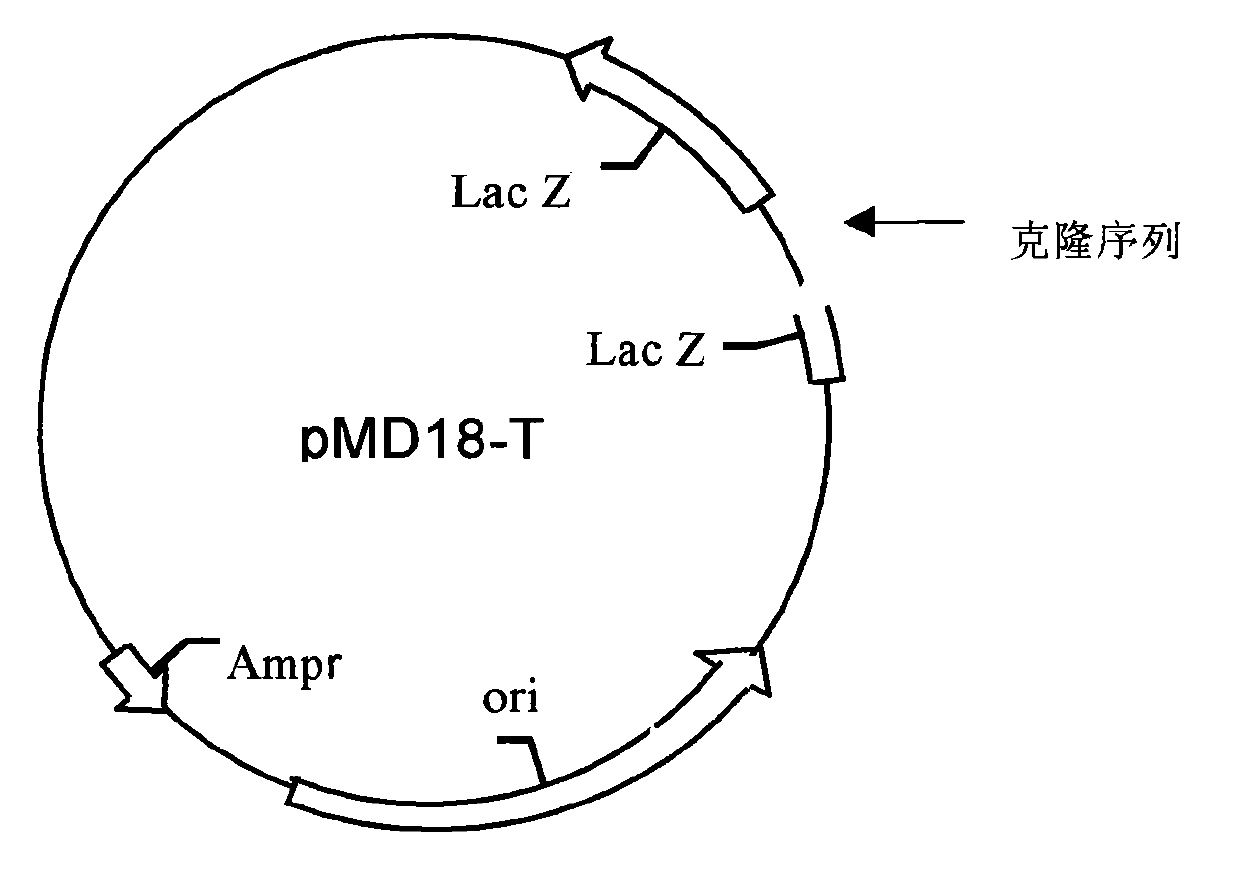
[0089] 1. Wild-type plasmid construction ( figure 1 , figure 2 )
[0090] 1.1 Preparation of the carrier
[0091] The TA cloning vector pMD18-T was purchased from TAKARA Company.
[0092] 1.2 Preparation of insert fragments
[0093] Use the PCR method to prepare the insert fragment. The template for PCR is the sample genomic DNA extracted in step 1. The reaction system and amplification conditions are as follows (Table 1, Table 2, Table 3):
[0094] Table 1: PCR reaction system (50μl)
[0095]
[0096] To prepare a plasmid containing the wild-type sequence at position 2155 of exon 18 of the EGFR gene, E18-F-1 (SEQ ID NO: 5) or E18-F-2 (SEQ ID NO: 6) and E18-R-1 (SEQ ID NO: 7) or E18-R-2 (SEQ ID NO: 8) primer sequence; if the preparation contains EGFR gene exon 19 2235-2249, 2236-2250, 2254- For the wild-type plasmid at position 2277, E19-F-1 (SEQ ID NO: 9) or E1...
Embodiment 3
[0116] Example 3: Taking lung cancer and cervical cancer samples as examples: human cell lines, human fresh tumors Detection of EGFR mutation in genomic DNA of tumor tissue, peripheral blood, and paraffin-embedded tissue
[0117] 1. The fluorescent quantitative PCR reaction template is the genomic DNA of lung cancer and cervical cancer samples extracted in Example 1 and the standard prepared in Example 2, and double distilled water is used as a negative control. In order to make a standard curve, standard dilutions were 1ng / μl, 0.5ng / μl, 0.25ng / μl, 0.125ng / μl, 0.0625ng / μl, 0.03125ng / μl.
[0118] 2. Reaction system and reaction conditions (table 2, table 5, table 6, table 7), wherein labeling probe fluorescent emission group is selected from: FAM, TET, HEX, ROX; Fluorescence quencher group is selected from: BHQ, TAMARA.
[0119] Table 5: Real-time quantitative PCR reaction system (20μl / tube)
[0120]
[0121] To detect the G→A mutation at position 2155 of exon 18 of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com