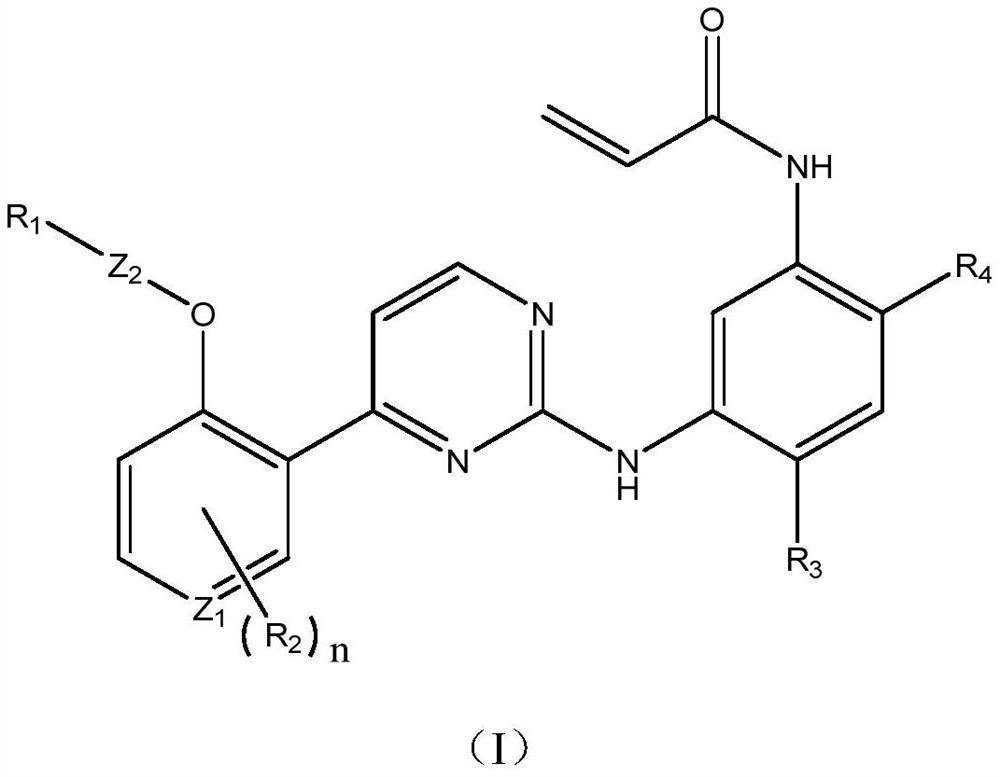
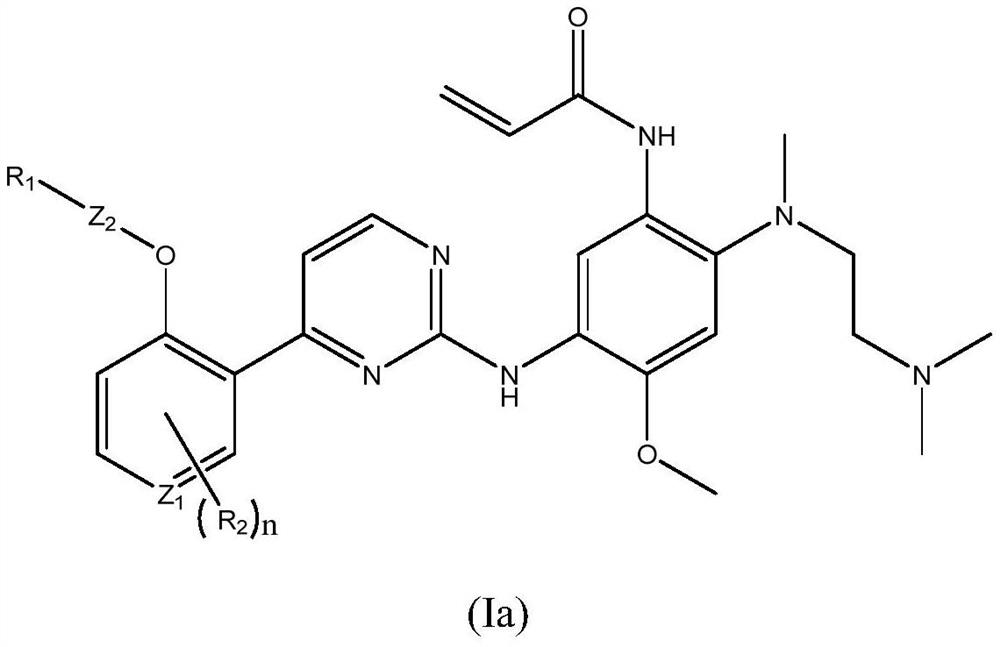
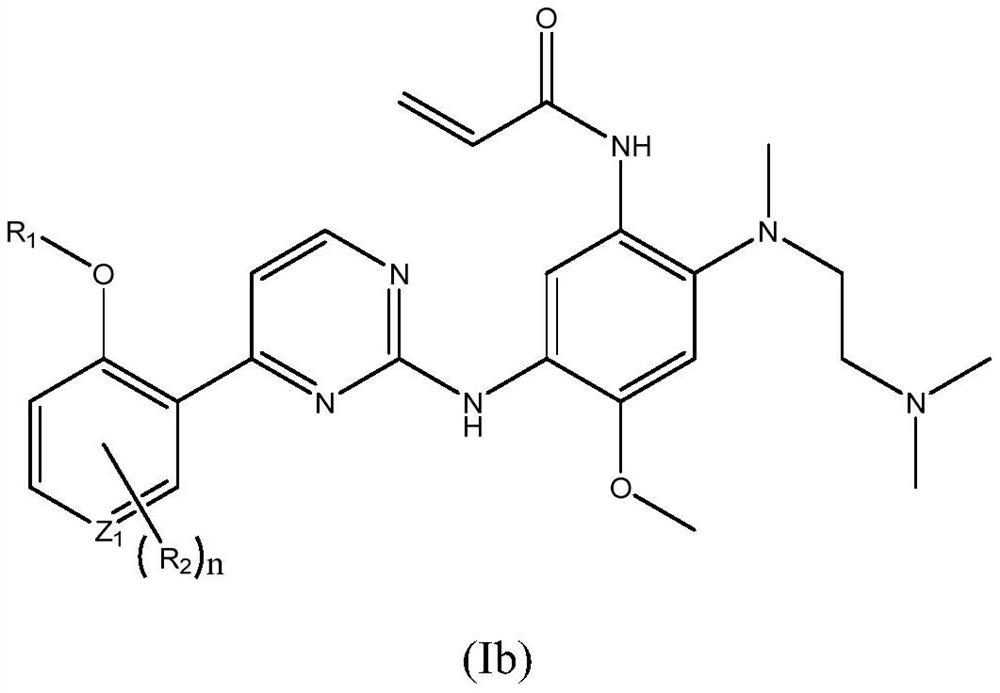
EGFR tyrosine kinase inhibitor and application thereof
A technology of amino compounds, applied in the field of chemical medicine, can solve the problems of dose-dependent toxicity, no further confirmation of clinical effect, poor effect of drug-resistant T790M mutant, etc., achieve good inhibitory effect, good prognosis effect, and improve curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-5-((4-(2-phenoxyphenyl)pyrimidin-2-yl)amino ) phenyl) acrylamide
[0052]
[0053] step one:
[0054]
[0055] Compound 1.1 (1g, 6.71mmol, 1eq.), compound 1.2 (1g, 4.67mmol, 0.69eq.), tetrakistriphenylphosphine palladium (775.28mg, 670.91umol, 0.1eq.), potassium carbonate (2.78g, 20.14mmol, 3eq.) was dissolved in dioxane (20mL) and water (4mL), replaced with nitrogen three times, heated to 75°C, and reacted for 5 hours. Add EA (50mL) to the reaction solution for extraction, separate the organic phase, dry over anhydrous magnesium sulfate, spin dry, column chromatography (n-heptane: ethyl acetate = 10:1), to obtain light yellow oil (1.20g , yield: 63.23%).
[0056] Step two:
[0057]
[0058] Compound 1.3 (1.20g, 4.24mmol, 1eq.), compound 1.4 (993.92mg, 6.37mmol, 1.5eq.), cesium carbonate (4.15g, 12.73mmol, 3eq.), xphos (404.68mg, 848.88umol, 0.2 eq.), Pd 2 (dba) 3 (388.67mg, 424.44umol, 0.1eq.), diox...
Embodiment 2
[0068] Example 2 N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(2-phenoxyphenyl)pyrimidine -2-yl)amino)phenyl)acrylamide
[0069]
[0070] Using a method similar to Example 1, the compound 1.4 in Step 2 was replaced with 4-fluoro-2-methoxy-5-nitroaniline to obtain the title compound. 1 H NMR(400MHz,DMSO-d6)(ppm):9.85(brs,1H),8.90(s,1H),8.42-8.43(d,1H),8.20-8.30(m,1H),8.09(s,1H ),7.49-7.54(m,1H),7.29-7.40(m,4H),7.11-7.14(t,1H),6.98-7.02(m,4H),6.30-6.35(dd,1H),5.76-5.79 (d,1H), 3.88(s,3H), 3.0-3.29(m,2H), 2.64(m,6H). LC-MS(m / z):538.86[M+H] + .
Embodiment 3
[0071] Example 3 N-(5-((4-(2-(benzyloxy)phenyl)pyrimidin-2-yl)amino)-2-((2-(dimethylamino)ethyl)(methyl )amino)phenylamide
[0072]
[0073] Using a method similar to Example 1, the compound 1.2 in Step 1 was replaced with (2-(benzyloxy)phenyl)boronic acid to obtain the title compound. 1 H NMR(400MHz,DMSO-d6)(ppm):10.21(s,1H),9.55(s,1H),8.76(s,1H),8.42-8.43(d,1H),8.10-8.11(d,1H ),7.44-7.50(m,4H),7.33-7.44(m,4H),7.22-7.27(t,2H),7.10-7.12(t,1H),6.37-6.40(d,1H),6.31-6.32 (d,1H),5.78-5.81(dd,1H),5.26(d,2H),2.80-2.82(t,2H),2.65(s,3H),2.21-2.26(m,2H),2.20(s ,6H). LC-MS(m / z):524.09[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com