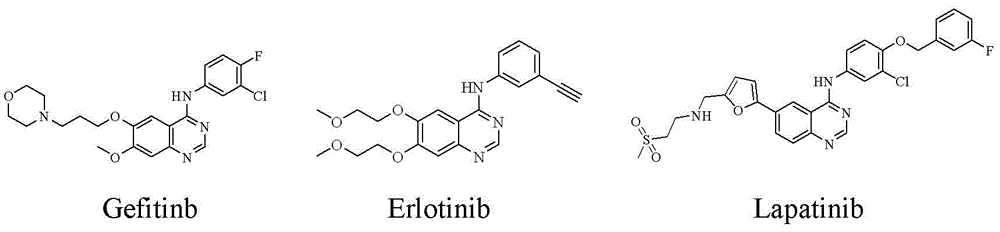
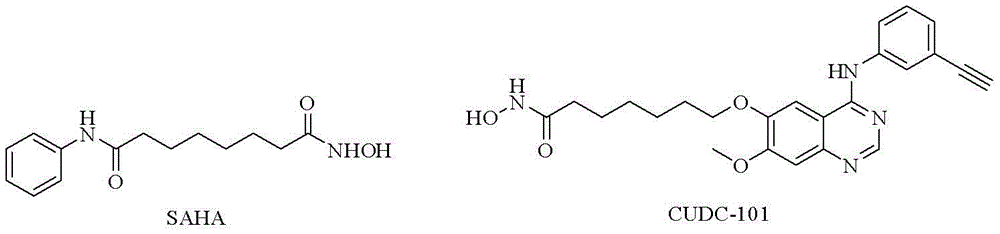
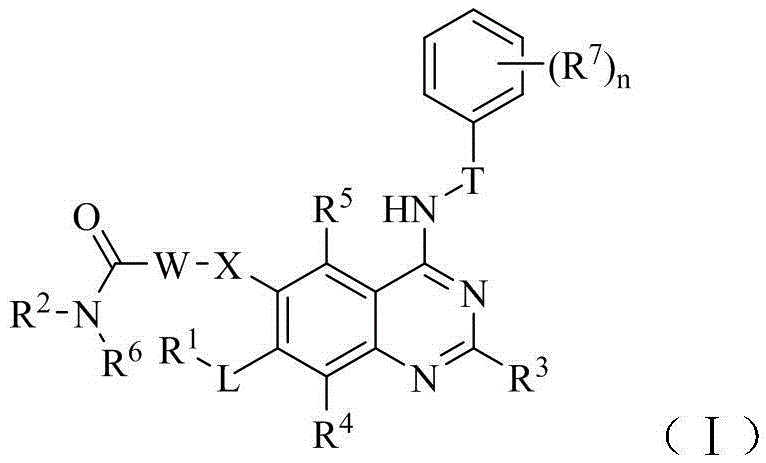
Quinazoline-based egfr tyrosine kinase inhibitor containing a zinc-binding group
A technology of alkyl and hydroxyl, applied in the field of medicine, can solve the problem of drug resistance of tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] The preparation of embodiment 17-(4-(3-chloro-4-fluoroanilino)-7-(tetrahydrofuran-3-yloxy)quinazolin-6-yloxy)-N-hydroxyheptanamide (compound 1)
[0172]
[0173] (1) Preparation of 3-iodotetrahydrofuran
[0174]
[0175] Add triphenylphosphine (52.4g, 0.2mol), imidazole (13.6g, 0.2mol) and iodine (50.7g, 0.2 mol), the reaction solution in N 2 Reflux overnight under protection with 0.2M Na 2 S 2 o 3 (30mL) to quench the reaction, the organic layer was separated, the aqueous phase was extracted three times with dichloromethane and the combined organic phase was washed with anhydrous MgSO 4 After drying, filtering and concentrating, a wet, yellow solid was obtained. The solid was added to pentane (100 mL) and stirred for 2 h. The insoluble solid was filtered and the filtrate was concentrated to obtain the target product (18.6 g, yield 94%).
[0176] (2) Preparation of methyl 3-hydroxy-4-(tetrahydrofuran-3-yloxy)benzoate
[0177]
[0178] 3,4-Dihydroxybenzoic ...
Embodiment 2
[0208] Example 2N 1 -(4-(3-Chloro-4-fluorophenylamino)-7-(tetrahydrofuran-3-yloxy)quinazolin-6-yl)-N 8 Preparation of -Hydroxysuberamide (compound 3)
[0209]
[0210] (1) Preparation of N-(3-chloro-4-fluorophenyl)-6-nitro-7-(tetrahydrofuran-3-yloxy)quinazolin-4-amine
[0211]
[0212]3-Hydroxytetrahydrofuran (0.88g, 10mmol) was dissolved in tetrahydrofuran (50mL), under ice-cooling conditions, sodium hydride (0.6g, 15mmol) was stirred for 1 hour, and then 7-fluoro-4-(3 -Chloro-4-fluoroaniline)-6-nitroquinazoline (3.36g, 10mmol) was dissolved in tetrahydrofuran (50mL) and added to the above reaction solution, refluxed for 24 hours, and purified by column to obtain 1.01g of the product. The rate is 25%.
[0213] (2) Preparation of N-(3-chloro-4-fluorophenyl)-6-amino-7-(tetrahydrofuran-3-yloxy)quinazolin-4-amine
[0214]
[0215] N-(3-chloro-4-fluorophenyl)-6-nitro-7-(tetrahydrofuran-3-yloxy)quinazolin-4-amine (2.02g, 5mmol) and Raney nickel (RaneyNi ) (0.2g) was a...
Embodiment 3
[0227] Example 3(S)-N 1 -(4-(3-Chloro-4-fluorophenylamino)-7-(tetrahydrofuran-3-yloxy)quinazolin-6-yl)-N 8 Preparation of -Hydroxysuberamide (Compound 4)
[0228]
[0229] (1) Preparation of (S)-N-(3-chloro-4-fluorophenyl)-6-nitro-7-(tetrahydrofuran-3-yloxy)quinazolin-4-amine
[0230]
[0231] (S)-3-Hydroxytetrahydrofuran (0.88g, 10mmol) was dissolved in tetrahydrofuran (50mL), under ice-cooling conditions, sodium hydride (0.6g, 15mmol) was stirred for 1 hour, and then 7-fluoro- 4-(3-Chloro-4-fluoroaniline)-6-nitroquinazoline (3.36g, 10mmol) was dissolved in tetrahydrofuran (50mL) and added to the above reaction solution, refluxed for 24 hours, and purified by column to obtain the product 1.30 g, 32% yield.
[0232] (2) Preparation of (S)-N-(3-chloro-4-fluorophenyl)-6-amino-7-(tetrahydrofuran-3-yloxy)quinazolin-4-amine
[0233]
[0234] (S)-N-(3-Chloro-4-fluorophenyl)-6-nitro-7-(tetrahydrofuran-3-yloxy)quinazolin-4-amine (2.02g, 5mmol) and Ra RaneyNi (0.2g) was a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com