Therapeutic combination of a third generation EGFR tyrosine kinase inhibitor and a cyclin d kinase inhibitor
A tyrosine kinase, EGFRT790M technology, applied in the field of cancer treatment of human subjects), can solve the problem of NSCLC can not be cured
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
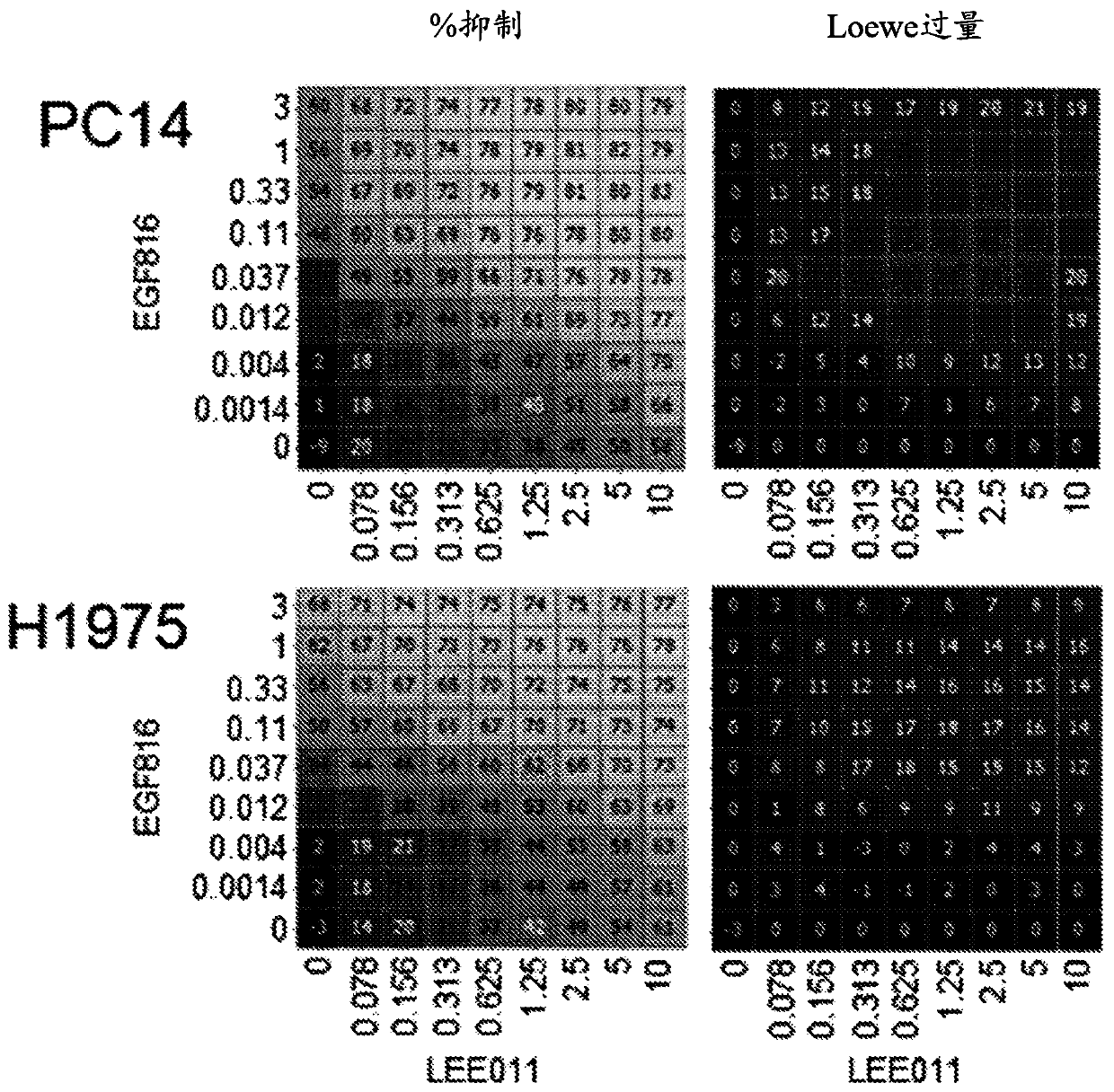
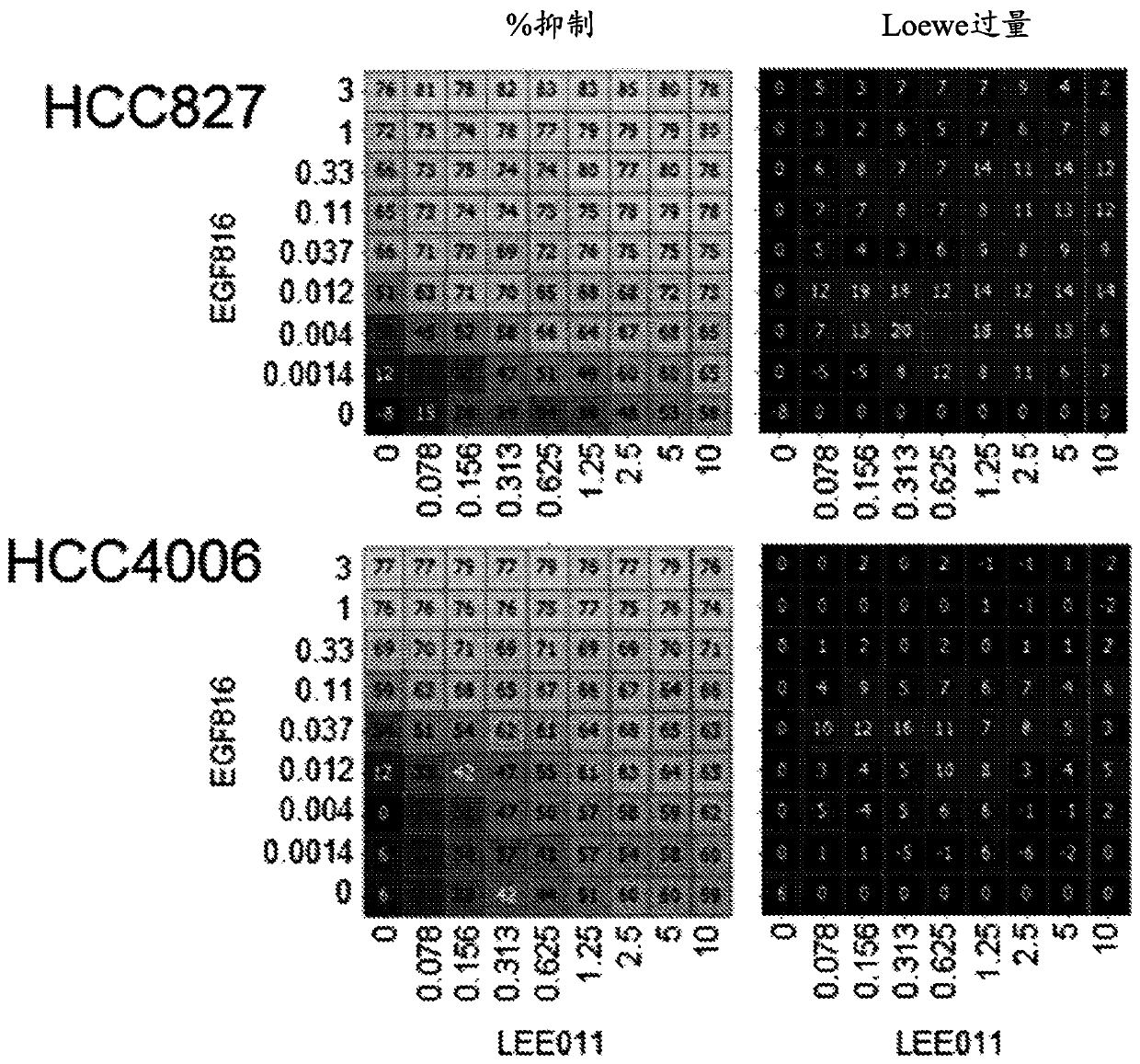
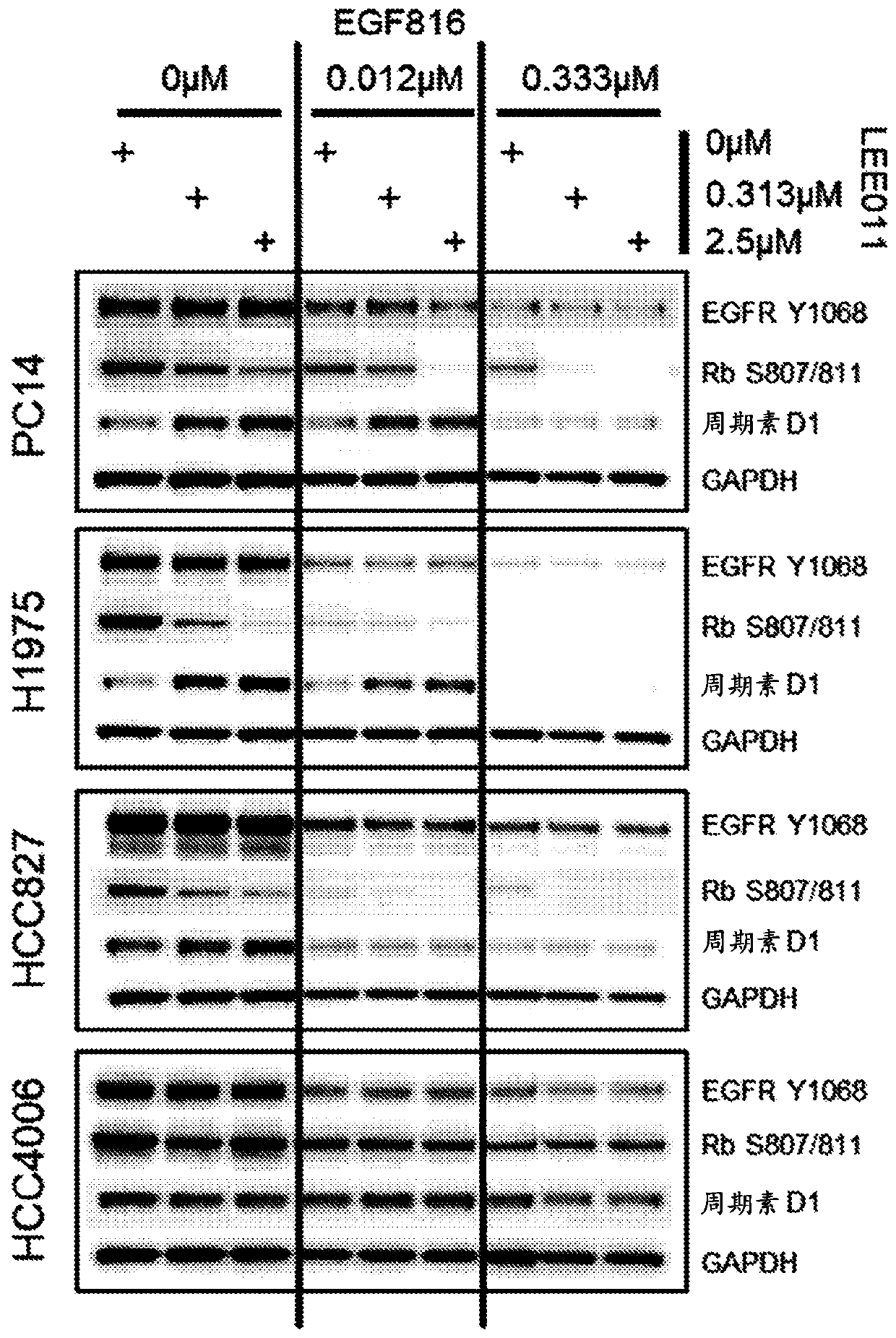
[0146] Example 1: Mechanistic Combination Studies to Demonstrate Target Activity and Combinatorial Effects of Compounds on Proximal Biomarkers.
[0147] This experiment was performed to test the combined effect of Compound A (EGF816) and Compound B (LEE011) on EGFR mutant NSCLC cell lines and to demonstrate that the observed anti-proliferative synergy was determined by mechanistic analysis of proximal readouts. Target efficacy driven.
[0148] Several EGFR mutant NSCLC cell lines were treated with combinations of EGF816 and LEE011 at different concentrations as follows for 72 hours. NCI-H1975 (with mutation L858R, T790M), PC-14 (with mutation ex 19del or exon 19 deletion), HCC827 (with mutation ex19del) and HCC4006 (with mutation ex19del) cells in RPMI-1640 growth medium ( ATCC, catalog number 20-2001), and suspended with 10% fetal bovine serum (GIBCO, catalog number F4135) in a humidified 5% CO2 incubator at 37°C. For mechanistic studies, for HCC827, NCI-H1975, HCC4006, a...
example 2
[0160] Example 2: Long-term viability study: Combination of EGFRi plus CDK4 / 6i slows down the viability of EGFR-mutant NSCLC cells regrowth.
[0161] PC9 (3,000 / well), HCC827 (10,000 / well), HCC4006 (5000 / well) and MGH707 (5000 / well) cells were seeded into 96-well plates, and treated with EGF816 (300nM) alone or with LEE011 (1000nM) combined treatment for four weeks. Drugs are updated twice a week. Cell confluency was used as a surrogate indicator of cell number and was measured by incucyte zoom (Essen Biosciences) at the beginning of treatment and twice weekly thereafter (2x).
[0162] Single-agent treatment with EGF816 resulted in varying degrees of apoptosis and cell cycle arrest in the four EGFR-mutant NSCLC cell lines tested (PC9, HCC827, HCC4006, and MGH707), although in all cases, by the end of the four-week treatment time course These cells were then able to begin to slowly re-grow in the presence of EGFRi. To test whether the regrowth was mediated by residual ac...
example 3
[0163] Example 3: In vivo efficacy studies of Compound A and Compound B alone or in combination
[0164] Combination activity with co-treatment with Compound B and Compound A was observed in vivo in a patient-derived xenograft model harboring EGFR mutations, L858R and T790M, as described below ( Figure 4 ).
[0165] tumor cell culture
[0166] NCI-H1975 cells were grown to mid-log phase in RPMI 1640 medium containing the following: 10% fetal bovine serum, 2 mM glutamine, 100 units / mL penicillin G sodium, 25 μg / mL gentamicin, and 100 μg / mL streptomycin sulfate. The tumor cells were incubated at 37°C in 5% CO 2 and 95% air in tissue culture flasks in a humidified incubator.
[0167] In vivo implantation and tumor growth
[0168] NCI-H1975 cells for implantation into mice were harvested in log phase and resuspended in 50% Matrigel TM (BD Biosciences) in cold PBS. Inject 1 x 107 cells (0.2 mL of cell suspension) subcutaneously into the right flank of each mouse. Whe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com