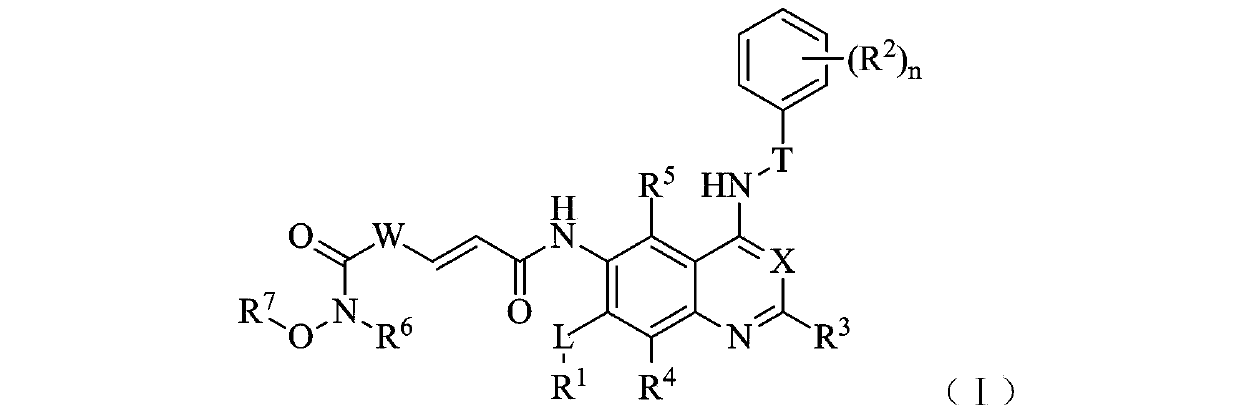
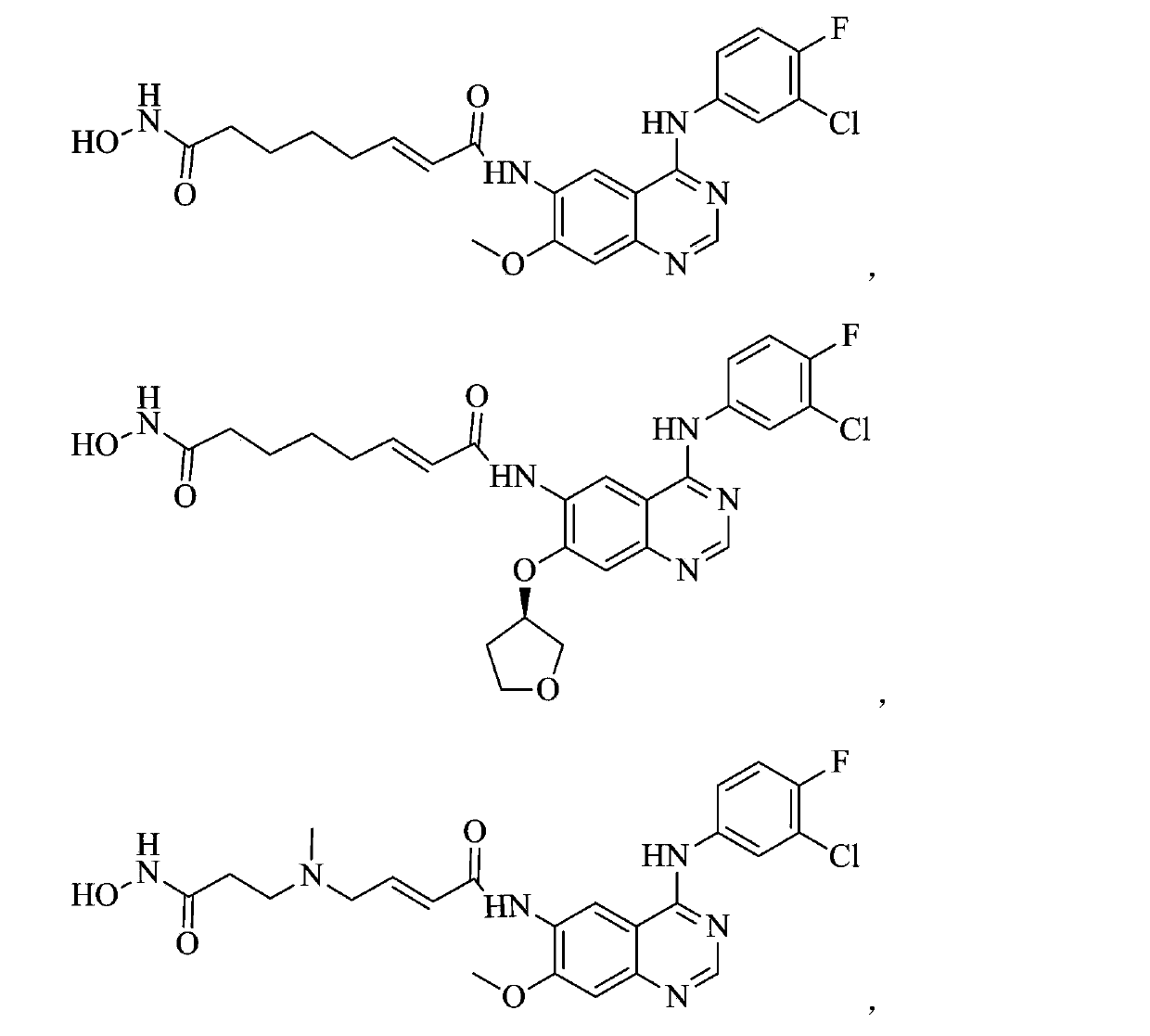
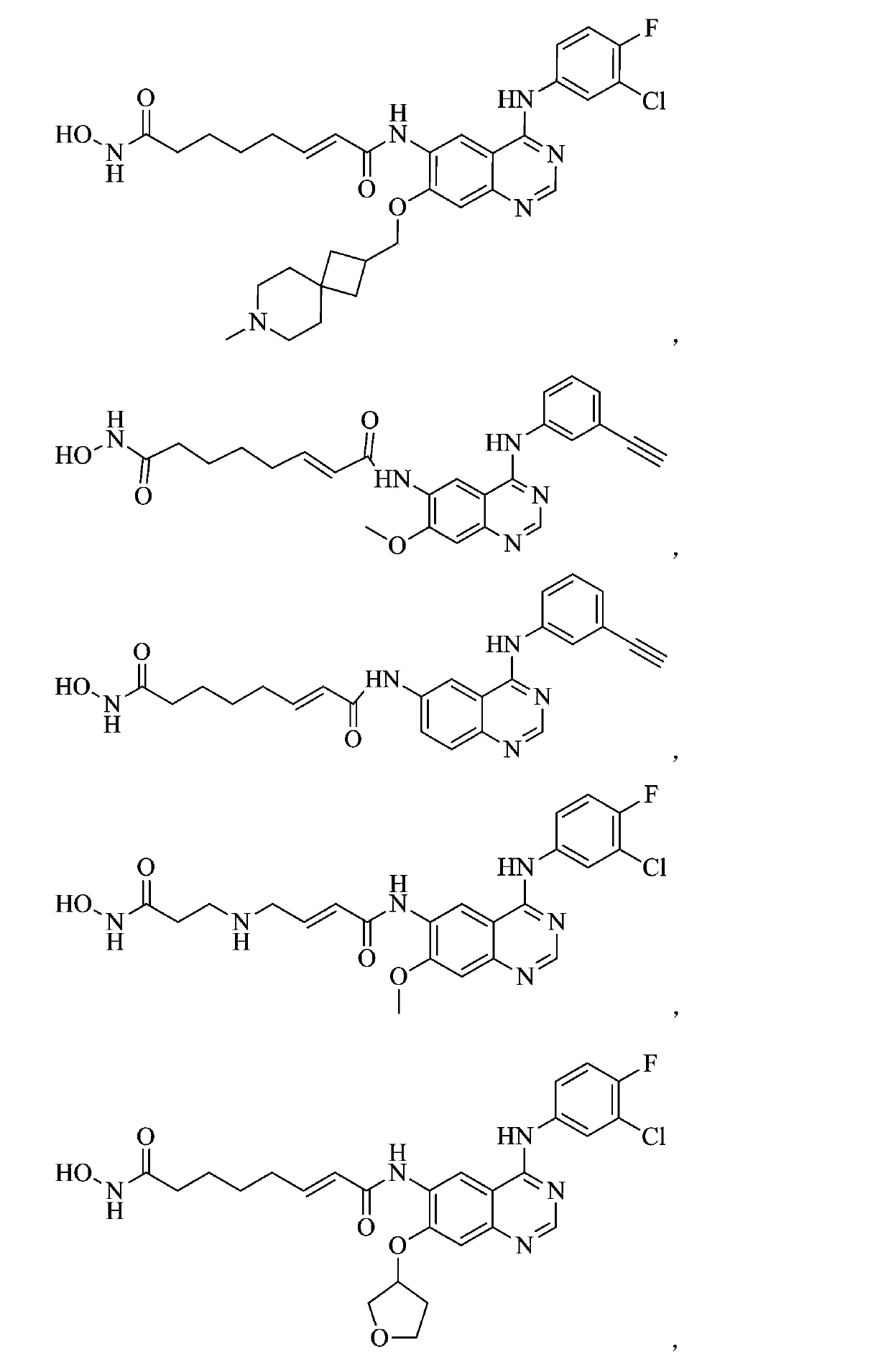
Zinc binding group-containing irreversible EGFR tyrosine kinase inhibitor
An alkyl and alkenyl technology, applied in the field of medicine, can solve problems such as the difficulty of EGFR tyrosine kinase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0129] (1) Preparation of intermediate 1
[0130] Suspend raw material 1 potassium carbonate in the middle chamber of raw material 2, add 50% aqueous sodium hydroxide solution, and heat the mixed solution to 70 °C for reaction. After the reaction is completed, pour the mixed solution into water and stir thoroughly, filter, wash with water, and dry under reduced pressure. Intermediate 1 was obtained.
[0131] (2) Preparation of Intermediate 2
[0132] Dissolve the intermediate 1 in an appropriate amount of tetrahydrofuran, add the catalyst Raney nickel for reduction, filter after the reaction is completed, and remove the solvent by rotary evaporation to obtain the intermediate 2.
[0133] (3) Preparation of Intermediate 3
[0134] Dissolve the tetrahydrofuran solution of intermediate 2 in an appropriate amount of dichloromethane, add triethylamine, add raw material 3 at 0 °C, and stir for reaction. After the reaction is completed, separate and purify by chromatography to obta...
experiment example
[0156] Experimental example The in vitro enzymatic activity experiment of the compound of the present invention
[0157] Test product: the compound of the present invention, its chemical name, structural formula and preparation refer to the examples of each compound.
[0158] Control drug: CUDC-101, compound 12 in patent US7547781, prepared with reference to patent example 8, its structural formula is: .
[0159] Experimental method Determination of inhibitory activity of HDAC enzyme
[0160] 1. Preparation of test reagents
[0161] 1x buffer (50 mM hydroxyethylpiperazineethanesulfonic acid, pH=7.4, 100 mM potassium chloride, 0.001% Tween-20, 0.05% bovine serum albumin, 20 μM trichloroethyl phosphate)
[0162] 2. Compound Serial Dilution
[0163] ① 0.15 mM DMSO (dimethyl sulfoxide) compound solution: 15 μL of 10 mM compound solution was added to 985 μL of 100% DMSO.
[0164] ② Serially dilute the compound on a 96-well plate: Dilute the compound 4 times (20 μL of 0.15 mM...
Embodiment 1
[0194] Example 1 (E)-N 1 -(4-((3-Chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-N 8 Preparation of -Hydroxyoct-2-enedioamide (Compound 1)
[0195]
[0196] (1) Preparation of tert-butyl diethylphosphonoacetate
[0197]
[0198] A mixed solution of triethyl phosphite (1.66 g, 10 mmol) and tert-butyl bromoacetate (1.93 g, 10 mmol) was stirred at 100 °C for 3 hours. After the reaction was completed, the product was concentrated under reduced pressure to obtain the product (2.48 g, Yield 98%).
[0199] (2) Preparation of ethyl 6-oxohexanoate
[0200]
[0201] Pyridinium chlorochromate (21.5 g, 0.1 mol) was added to a solution of ethyl 6-hydroxyhexanoate (3.20 g, 20 mmol) in dichloromethane (40 mL), stirred at room temperature for about 6 hours, and the reaction mixture was subjected to preparative chromatography The product was isolated and purified (2.6 g, yield 82%).
[0202] (3) Preparation of (E)-1-tert-butyl 8-ethyl octa-2-enedioate
[0203] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com