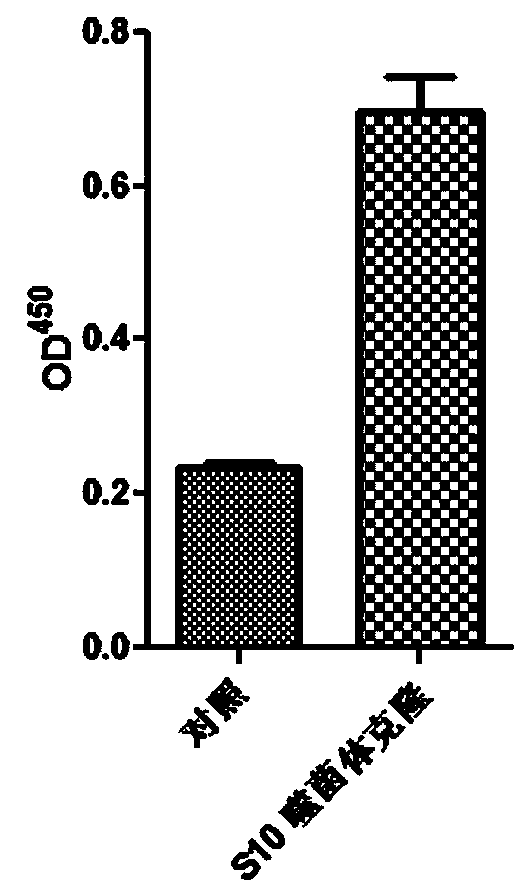
PD-L1 IgV affinity peptide S10 with antitumor activity
A technology of anti-tumor activity and affinity peptide, which is applied in the direction of anti-tumor drugs, medical preparations containing active ingredients, peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
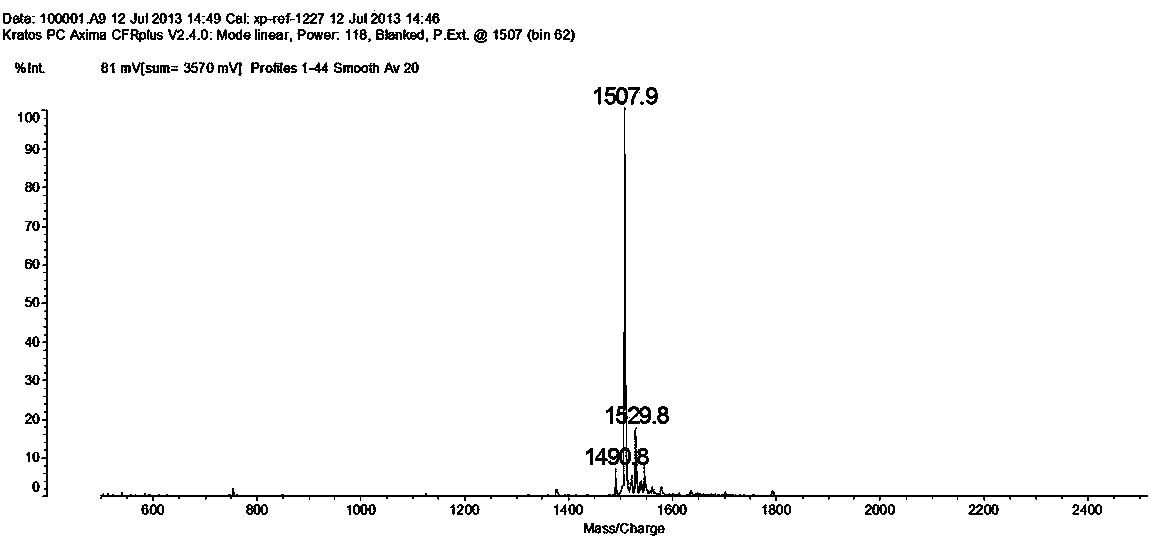
[0020] The anti-tumor activity targeting PD-L1 IgV affinity peptide S10 provided by the present invention specifically binds to the PD-L1 IgV region and is screened by using phage display peptide library technology. Its amino acid sequence is:
[0021] Trp-Ser-His-Gly-Gly-His-Gln-His-Phe-Ile-Arg-Phe, namely W-S-H-G-G-H-Q-H-FI-R-F, the molecular weight is 1507.7.
[0022] Due to the high cost of monoclonal antibody drugs and the inability to produce them on a large scale, we selected the PD-L1 IgV region protein expressed and purified from prokaryotic cells as the target, and screened the protein that can specifically bind to PD-L1 IgV through phage display technology. Peptides to block PD-1 / PD-L1 signaling pathway. For the convenience of those skilled in the art to implement the present invention, its screening process is briefly described as follows:
[0023] The preparation instructions for the media and master solutions used in the screening process are as follows:
[0...
Embodiment 2
[0076] The affinity peptide S10 of the PD-L1 IgV with anti-tumor activity is synthesized by the Fomc solid-phase peptide synthesis method, and the synthesis steps are briefly described as follows:
[0077] The main reagents used in the synthesis process are:
[0078] Heading liquid: acetic anhydride / pyridine solution (1:1, v / v);
[0079] Indene detection reagent: A. Ninhydrin / ethanol solution (5%, w / v)
[0080] B. Phenol / Ethanol (4:1, w / v)
[0081] C. Potassium cyanide / pyridine (2%, v / v)
[0082] Deprotection solution: piperidine / DMF solution (20%, v / v);
[0083] Cleavage reagent: by volume, TFA (82.5%), H 2 O (5%), phenol (5%), thioanisole (5%), ethanedithiol (2.5%).
[0084] The synthetic steps are briefly described as follows:
[0085] (1) Swell the resin, add the first amino acid
[0086] A. Swelling resin: Take 0.3~0.5 g Rink resin (the C-terminal amino acid of the peptide connected to the resin is an amide) and place it in a cleaned and dried peptide synthesize...
Embodiment 3
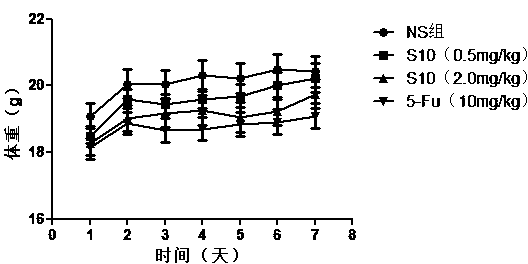
[0128] Taking the PD-L1 IgV affinity peptide S10 with anti-tumor activity prepared in Example 2 as an example, the inventors conducted further in vivo experiments on tumor-bearing mice. The specific experimental process is as follows:
[0129] (1) Affinity peptide S10 inhibits the growth of transplanted tumors in mice bearing CT26 colon cancer
[0130] Select 20 experimental Balb / c mice, adjust the cell concentration to 5×10 mouse colon cancer (CT26) cells with normal saline (NS) 6 cells / mL, 0.1 mL cell suspension (containing 5×10 5 cells) were inoculated subcutaneously in the armpit of the right forelimb of each Balb / c mouse, and the growth of the subcutaneous tumor was continuously observed.
[0131] The affinity peptide S10 prepared in Example 2 was dissolved in physiological saline to prepare a polypeptide drug, which was subpackaged and stored at -20°C for future use.
[0132] Nine days after inoculation with mouse-derived colon cancer (CT26) cells, the mice were divi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com