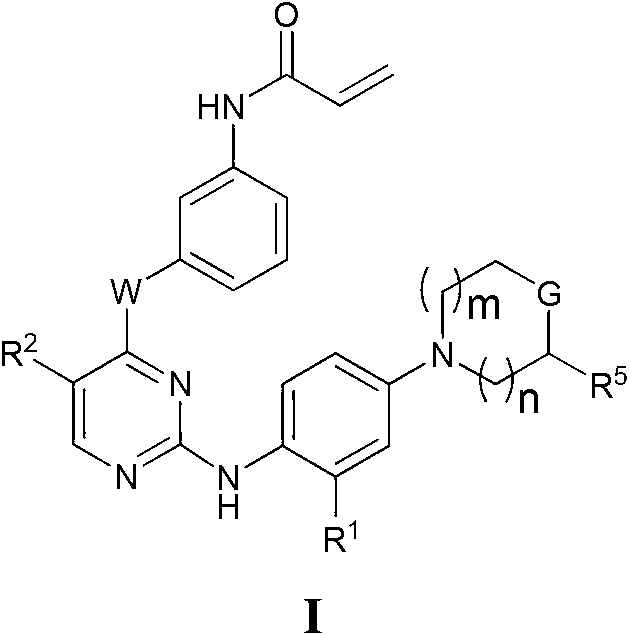
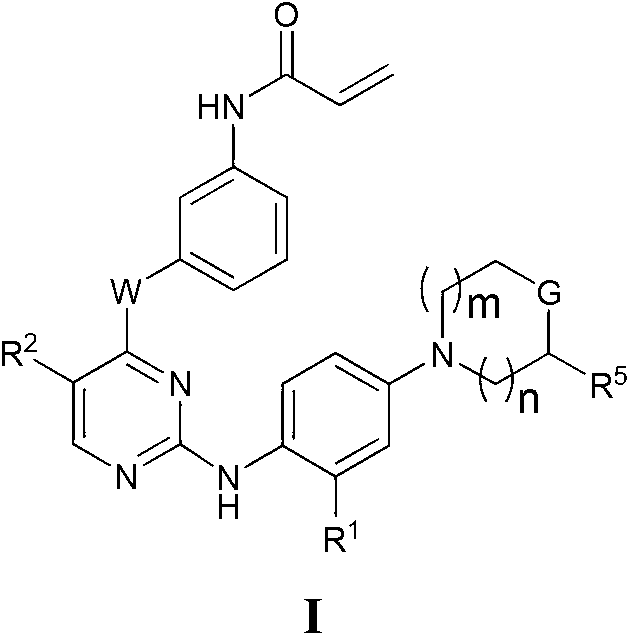
Heterocyclic compounds and uses thereof
A technology of compounds and compositions, applied in the field of treatment of various diseases, can solve problems such as dose-limiting toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0208] Intermediate 1
[0209] Process 1
[0210]
[0211] step 1:
[0212] Pre-equipped with magnetic stirrer, thermal bag and CaCl 2 N-Boc-1,3-diaminobenzene (0.96 g) and n-butanol (9.00 mL) were fed into the 25 mL three-neck RBF of the protective tube. The reaction mixture was cooled to 0°C. At 0°C, 2,4-dichloro-5-trifluoromethylpyrimidine (1.0 g) was added dropwise to the above reaction mixture. At 0°C, DIPEA (0.96 mL) was added dropwise to the above reaction mixture and the reaction mixture was stirred at 0°C to 5°C for 1 hour. Finally, the reaction mixture was allowed to warm to room temperature. The reaction mixture was stirred for another 4 hours at room temperature. Using hexane:ethyl acetate (7:3), the completion of the reaction was monitored by TLC. The precipitated solid was filtered off and washed with 1-butanol (2 mL). The solid was dried at 40°C under reduced pressure for 1 hour. 1 H-NMR (DMSO-d6, 400MHz) δ 1.48 (S, 9H), 7.02 (m, 1H), 7.26 (m, 2H), 7.58 (S, 1H)...
example 2
[0218] Compound I-2N-(3-(2-(2-methoxy-4-morpholinylphenylamino)-5-(trifluoromethyl)pyrimidin-4-ylamino)phenyl)acrylamide)
[0219]
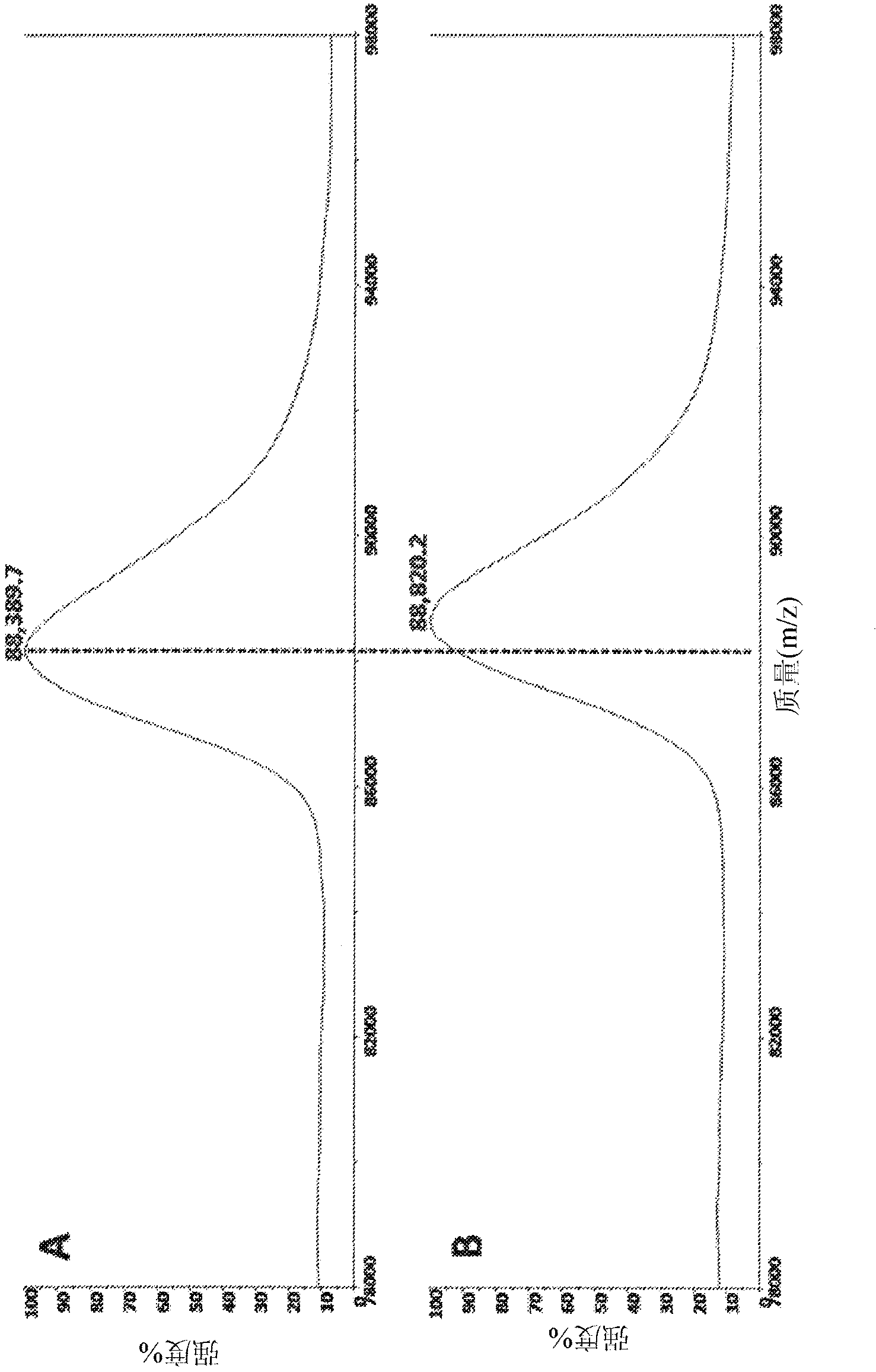
[0220] In order to obtain the title compound I-2, the intermediate 1 (16 mg) and 2-methoxy-4-morpholinoaniline in Example 1 were mixed in dioxane (1.0) with catalytic trifluoroacetic acid at 50°C. mL) was stirred overnight. The crude material was concentrated under reduced pressure and purified using HPLC (TFA modifier) to give the title compound as a TFA salt. 1 H-NMR (DMSO-d6, 400MHz) δ 10.4 (S, 1H), 9.72 (br, 1H), 9.18 (br, 1H), 8.49 (br, 1H), 7.83 (S, 1H), 7.59 (d , J=8.4Hz, 1H), 7.31-7.48(m, 2H), 7.41(t, J=15.2Hz, 1H), 7.12(br, 1H), 6.67(S, 1H), 6.49(dd, J= 10.0, 16.8 Hz, 1H), 6.25 (dd, J = 2.0, 16.8 Hz, 1H), 5.77 (dd, J = 2.0, 10.0 Hz, 1H), 3.7-3.9 (m, 7H), 3.1 (br, 4H) ); C 25 H 25 F 3 N 6 O 3 Calculated value of the mass: 514.2, experimental value: 515.5 (M+H + ).
example 3
[0222] Compound I-4N-(3-(2-(4-(4-acetylpiperazin-1-yl)-2-methoxyphenylamino)-5-(trifluoromethyl)pyrimidin-4-yl Amino) phenyl) acrylamide)
[0223]
[0224] The title compound 1-4 was prepared as described in Example 2 using 2-methoxy-4-(4-acetylpiperazinyl)aniline and Intermediate 1 in Example 1. 1 H-NMR (DMSO-d6, 400MHz) δ 10.2 (S, 1H), 8.2 (br, 1H), 8.30 (S, 1H), 7.73 (br, 1H), 7.52 (d, J = 7.8 Hz, 1H ), 7.45 (d, J = 7.8 Hz, 1H), 7.26 (J = 8.2 Hz, 1H), 7.14 (be, 1H), 6.60 (S, 1H), 6.42 (dd, J = 11.4, 16.9 Hz, 1H) ), 6.24 (d, J = 16.9 Hz, 1H), 5.75 (d, J = 11.4 Hz, 1H), 3.76 (S, 3H), 3.04 (br, 4H), 2.04 (S, 3H); C 27 H 28 F 3 N 7 O 3 Calculated value of the mass: 555.2, experimental value: 556.2 (M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com