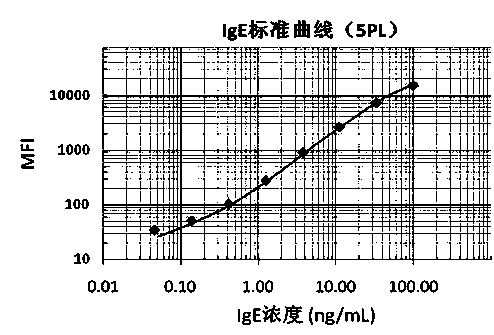
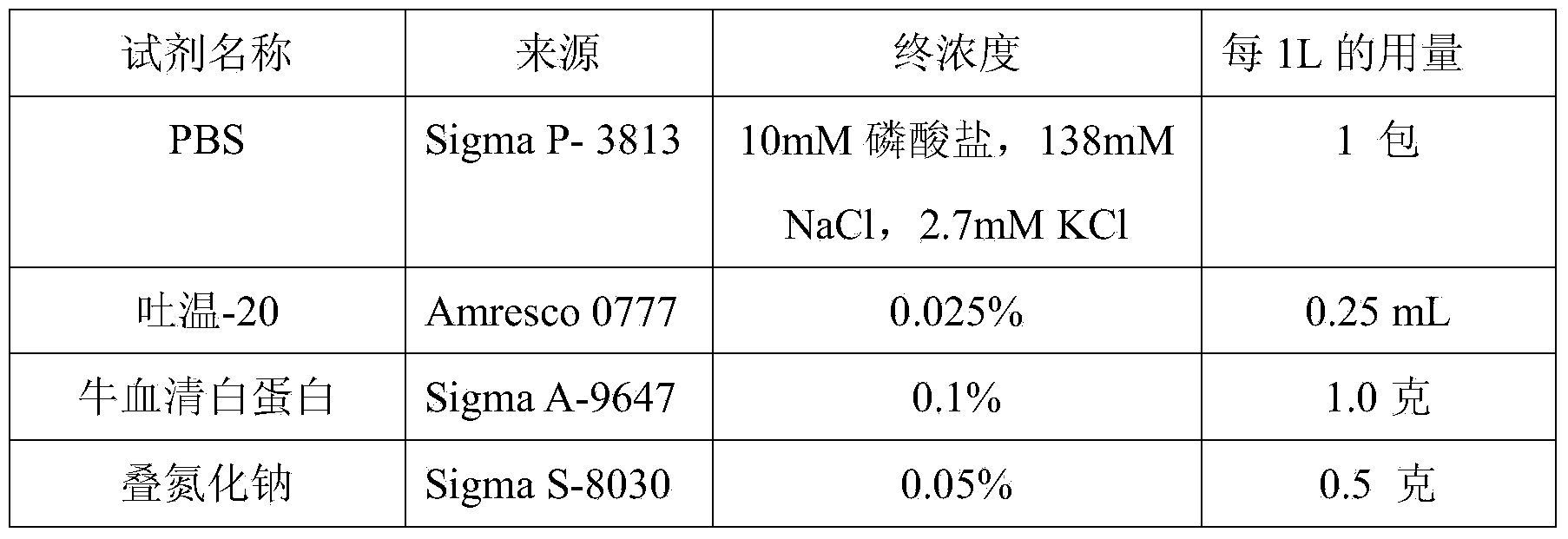
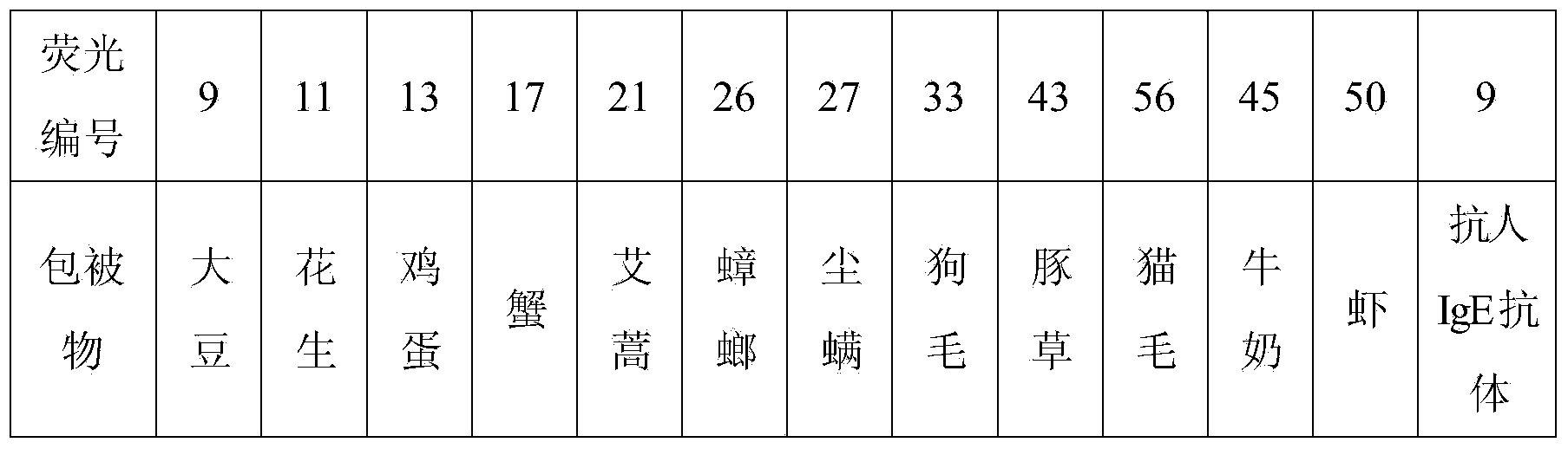
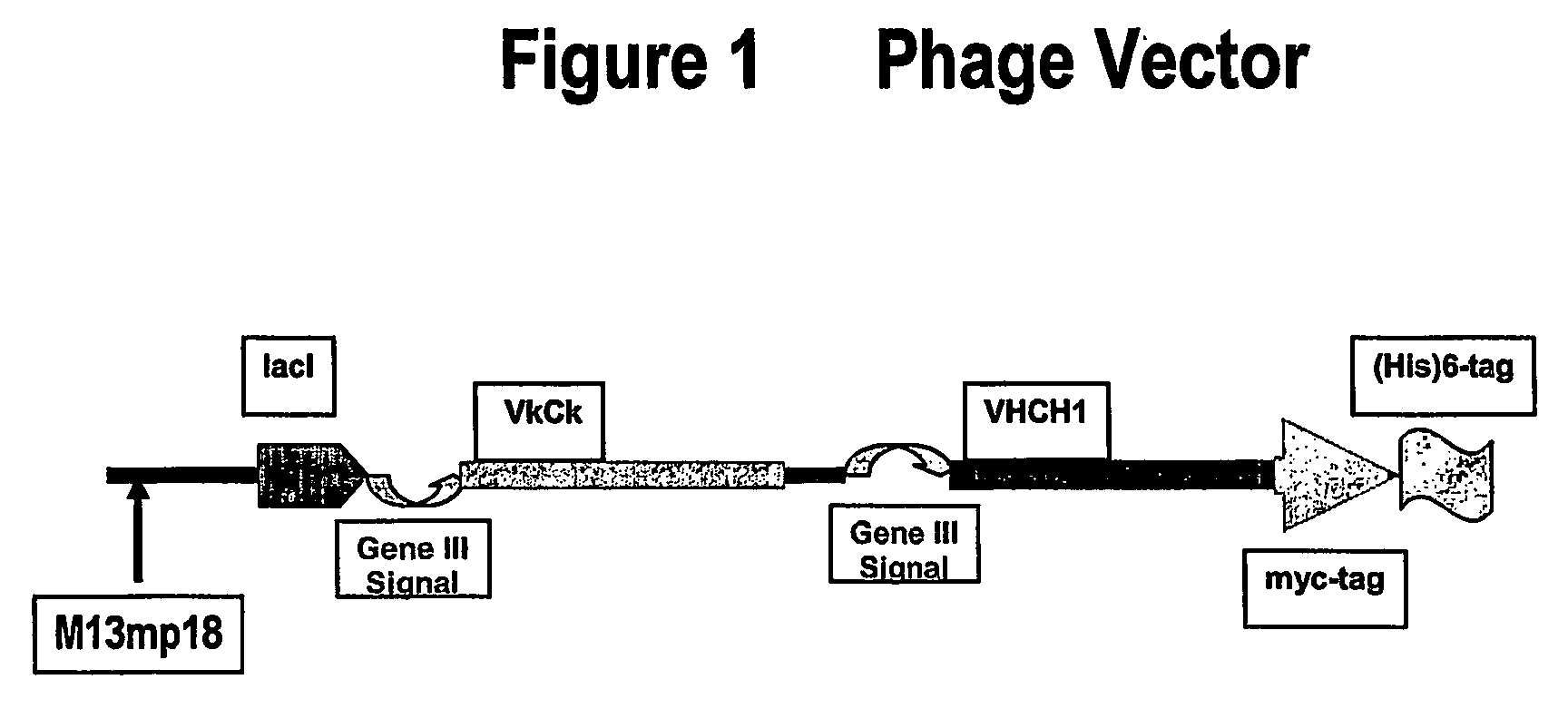
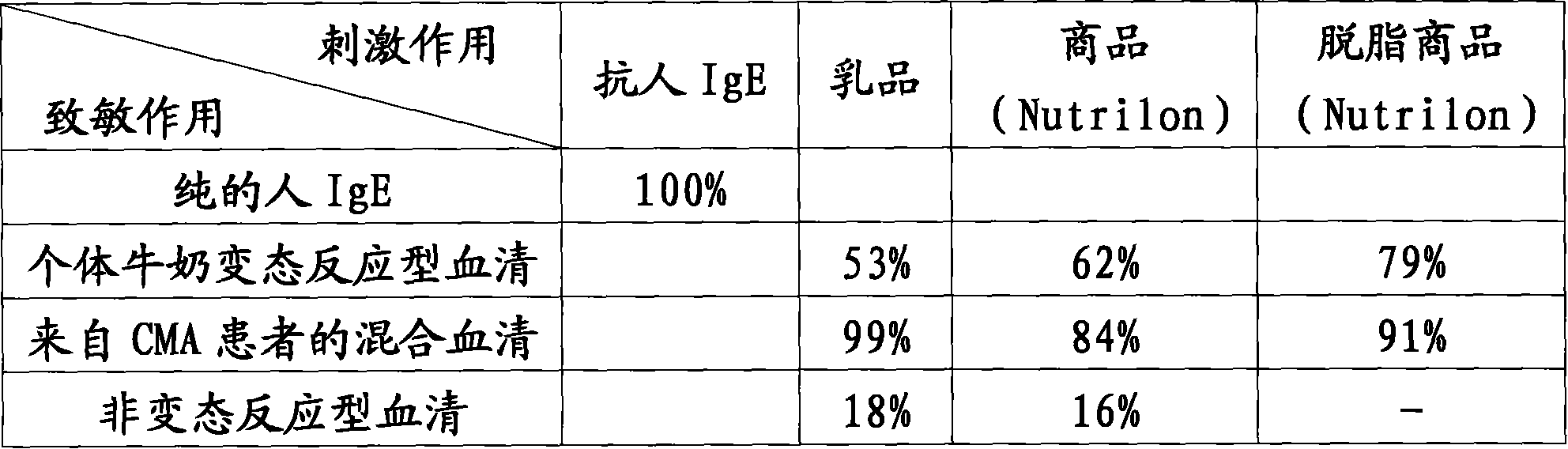
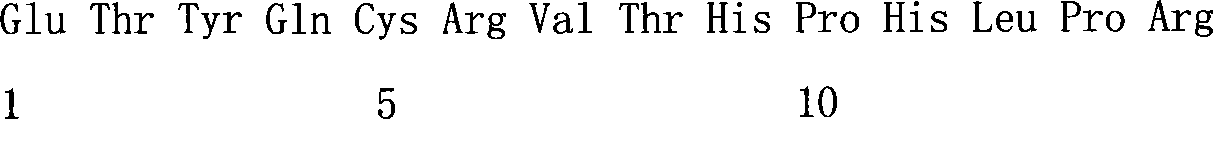Patents
Literature
79 results about "Human ige" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
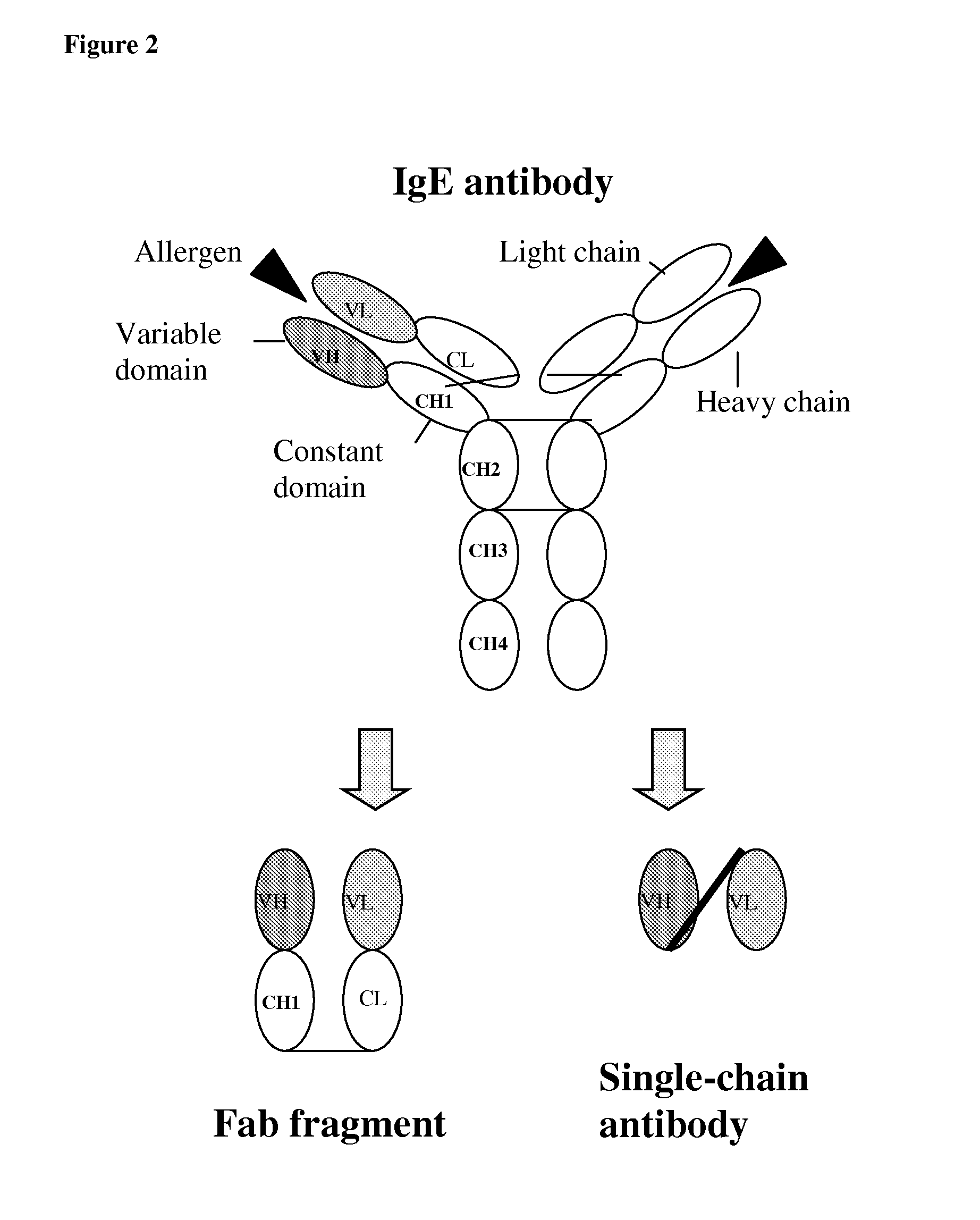
Antibody fragment-polymer conjugates and uses of same
Described are conjugates formed by an antibody fragment covalently attached to a non-proteinaceous polymer, wherein the apparent size of the conjugate is at least about 500 kD. The conjugates exhibit substantially improved half-life, mean residence time, and / or clearance rate in circulation as compared to the underivatized parental antibody fragment. Also described are conjugates directed against human vascular endothelial growth factor (VEGF), human p185 receptor-like tyrosine kinase (HER2), human CD20, human CD18, human CD11a, human IgE, human apoptosis receptor-2 (Apo-2), human tumor necrosis factor-α (TNF-α), human tissue factor (TF), human α4β7 integrin, human GPIIb-IIIa integrin, human epidermal growth factor receptor (EGFR), human CD3, and human interleukin-2 receptor α-chain (TAC) for diagnostic and therapeutic applications.
Owner:GENENTECH INC
High affinity anti-human IgE antibodies
The invention relates to high affinity human monoclonal antibodies, particularly those directed against isotypic determinants of immunoglobulin E (IgE), as well as direct equivalents and derivatives of these antibodies. These antibodies bind to their respective target with an affinity at least 100 fold greater than the original parent antibody. These antibodies are useful for diagnostics, prophylaxis and treatment of disease.
Owner:TANOX
Allergenic specific IgE antibody immunoassay detection kit and preparation method thereof
InactiveCN101696973AHigh sensitivityImprove stabilityChemiluminescene/bioluminescenceImmune profilingMicrosphere
The invention relates to an immunoassay detection kit, and provides an allergenic specific IgE antibody immunoassay detection kit and a preparation method thereof. The detection kit comprises magnetic microspheres coupled with allergenic protein, magnetic microspheres coupled with anti-human IgE antibody, a human IgE antibody standard substance, alkali phosphatase labeled anti-human IgE antibody and an alkali phosphatase chemical luminous substrate AMPPD. The preparation method for the detection kit comprises the following steps: coupling the allergenic protein to the magnetic microspheres, coupling the anti-human IgE antibody to the magnetic microspheres, preparing the human IgE antibody standard substance, preparing the alkali phosphatase labeled anti-human IgE antibody, preparing the alkali phosphatase chemical luminous substrate AMPPD and packing the combination. The detection kit has the advantages of high sensitivity, high accuracy, quick response, convenient usage, low dosage and low cost, can assist in precisely and quantitatively measuring sensitive allergene, and guides clinical anaphylactic reaction treatment.
Owner:深圳市博卡生物技术有限公司
Diagnostic kit for determination of serum total IgE, preparation method and application method
InactiveCN102798725AShorten detection timeReduce biasBiological testingFluorescence/phosphorescenceBiotin-streptavidin complexMicrosphere
The invention provides a diagnostic kit for determination of serum total IgE, a preparation method and an application method. The kit comprises an IgE standard substance, a biotin labeled rate anti-human IgE monoclonal antibody solution, a rabbit anti-human IgE polyclonal antibody coated acceptor microspheres solution and a streptavidin donor microspheres solution. The kit provided by the present invention solves a tedious washing step of current heterogeneous immunoassay kits, overcomes the defects of environmental pollution caused by radioimmunoassay and short shelf life, and overcomes the defects of poor repeatability of enzyme immunoassay analysis and easy hook effect generation, and the detection precision is higher than that of immuno-turbidimetric analysis.
Owner:天津中企华科生物科技发展有限公司
Antibody fragment-polymer conjugates and uses of same
Described are conjugates formed by an antibody fragment covalently attached to a non-proteinaceous polymer, wherein the apparent size of the conjugate is at least about 500 kD. The conjugates exhibit substantially improved half-life, mean residence time, and / or clearance rate in circulation as compared to the underivatized parental antibody fragment. Also described are conjugates directed against human vascular endothelial growth factor (VEGF), human p185 receptor-like tyrosine kinase (HER2), human CD20, human CD18, human CD11a, human IgE, human apoptosis receptor-2 (Apo-2), human tumor necrosis factor-α (TNF-α), human tissue factor (TF), human α4β7 integrin, human GPIIb-IIIa integrin, human epidermal growth factor receptor (EGFR), human CD3, and human interleukin-2 receptor α-chain (TAC) for diagnostic and therapeutic applications.
Owner:GENENTECH INC
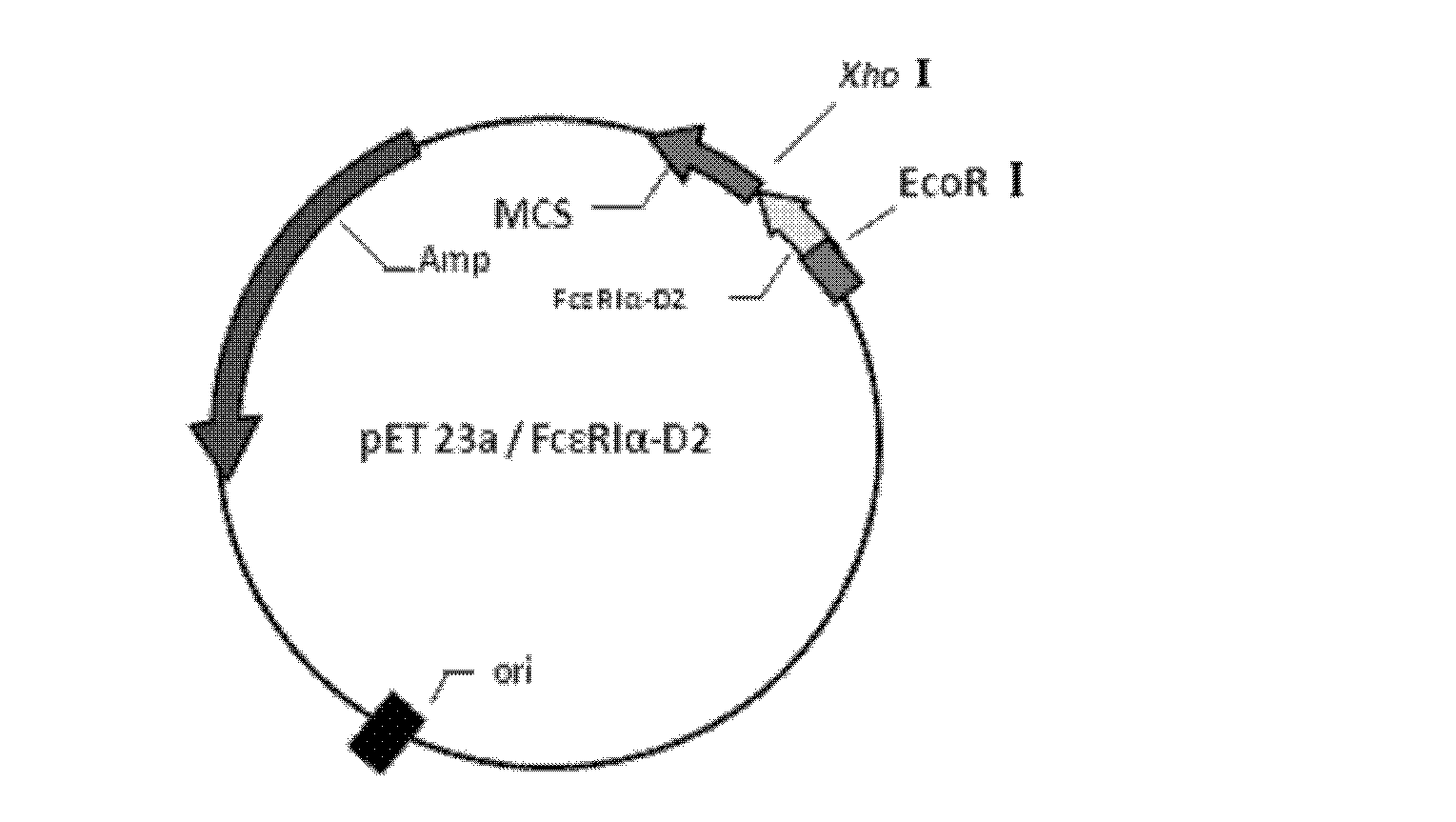
Method for preparing recombinant human IgE receptor protein and application of recombinant human IgE receptor protein
ActiveCN102660569AImprove bindingGuaranteed removal effectBacteriaPeptide/protein ingredientsSorbentHypersensitive response
The invention discloses a method for preparing recombinant human IgE receptor protein and application of the recombinant human IgE receptor protein, and belongs to the field of biological engineering and blood purification technology. Fc epsilon RI alpha-D2 with high binding force with IgE is highly expressed by means of gene recombination technology, recombinant protein is obtained by means of purification and used as a genin of an adsorbent, and the prepared adsorbent fixed onto a solid phase can be used for binding the IgE in a high-affinity manner. The method solves the problems of low safety and high cost of the prior art in the field of IgE removal by means of the blood purification technology. The method has an excellent application prospect in terms of removing excessive IgE in blood of an allergic reaction patient by means of the blood purification technology, treating allergic reaction mediated by the IgE, purifying the IgE and the like.
Owner:DALIAN UNIV OF TECH
Light-activating chemiluminescence immunoassay kit for serum specific IgE (immunoglobulin E)
InactiveCN105785030AEasy to operateImprove automationChemiluminescene/bioluminescenceBiological testingAntigenBiotin-streptavidin complex
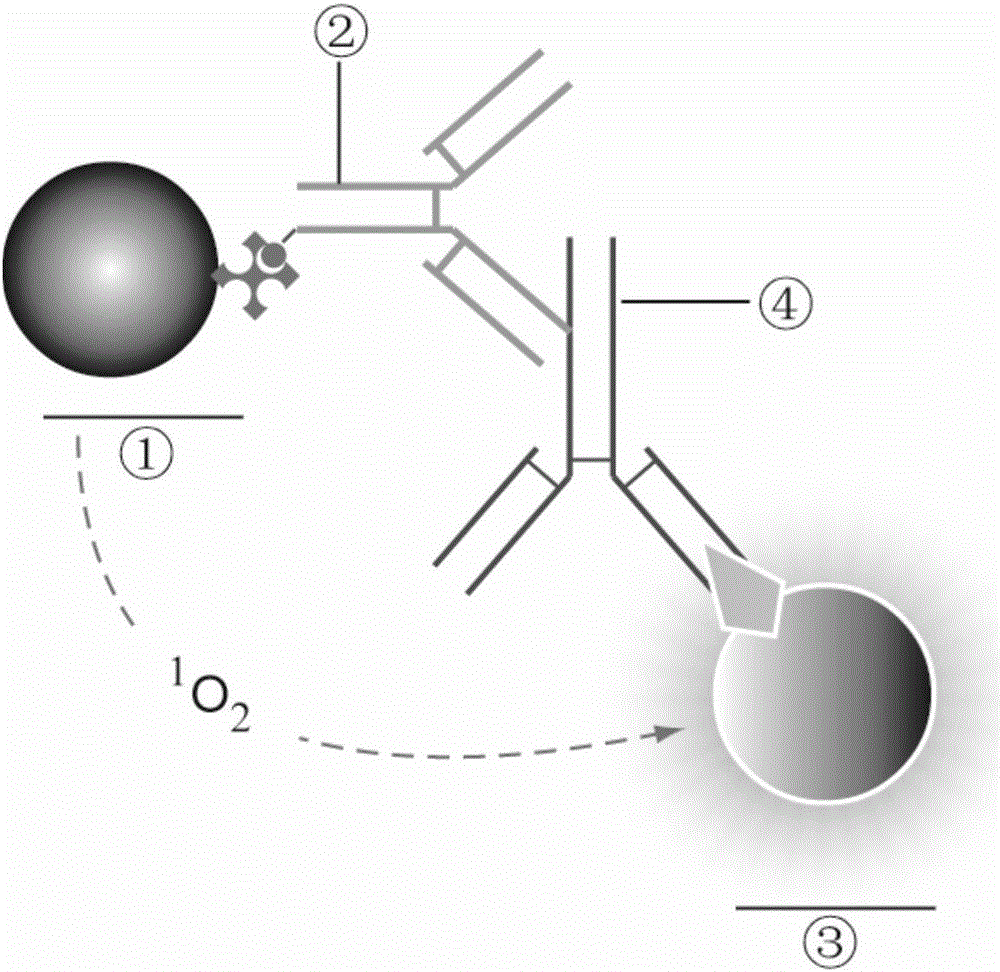
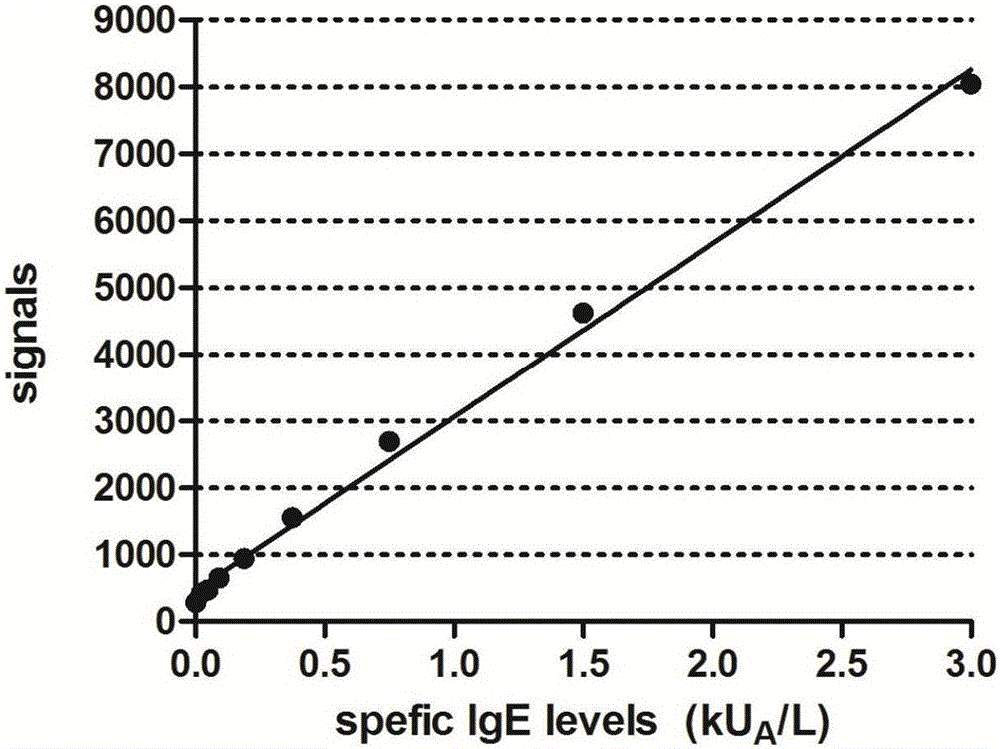
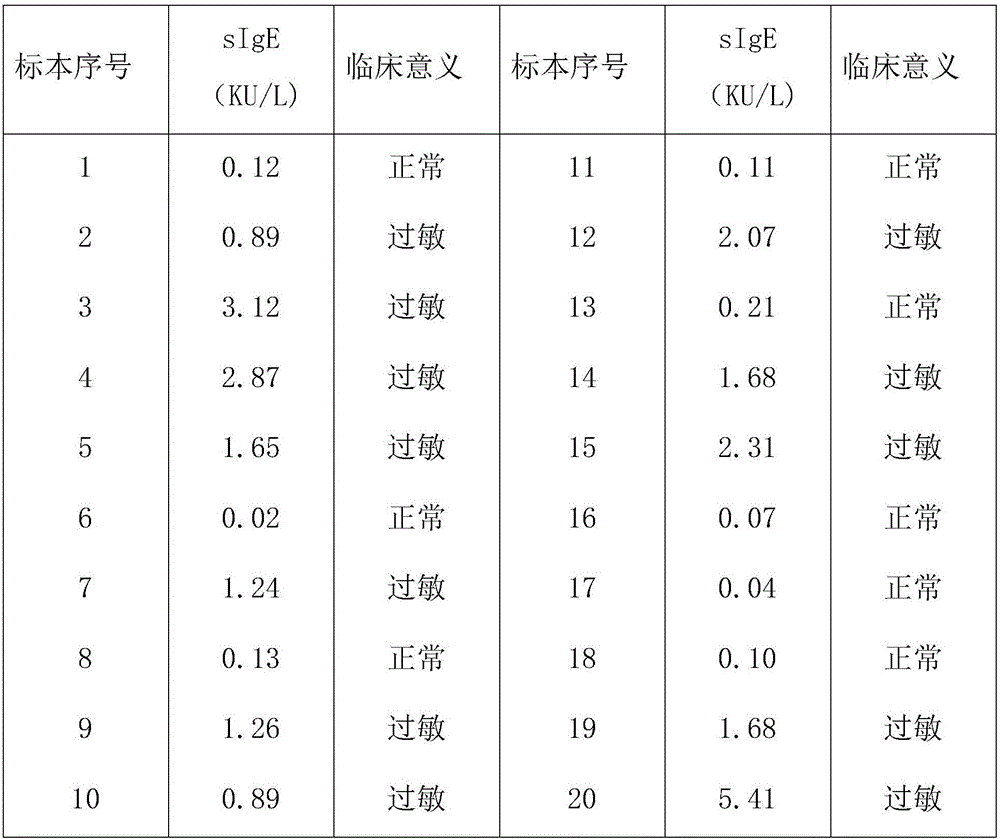
The invention provides a light-activating chemiluminescence immunity analysis kit for serum specific IgE (immunoglobulin E). The kit adopts a luminous microsphere coated with a specific single-component allergen (prepared by naturally purification or recombination), a biotin-labeled anti-human-IgE antibody and a light-sensitive microsphere precoated with streptavidin to form detecting reagents. Firstly, to-be-detected serum, the luminous microsphere coated the allergen and the a biotin-labeled anti-human-IgE antibody are incubated together, and the specific IgE antibody in the to-be-detected serum is bound with an antigen molecule on the surface of the luminous microsphere and the biotin-labeled anti-human-IgE antibody respectively; secondly, the light-sensitive microsphere precoated with streptavidin is added for continuous incubation, and is bridged with the luminous microsphere through the binding of biotin and streptavidin; thirdly, a light-activating chemiluminescence analyzing instrument is used for detecting the strength of light signals. The kit has various advantages of being easy and quick to operate, facilitating automation and quantitative analysis, and the like.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Liquid phase chip for detecting allergen specific antibody and preparation method of liquid phase chip
ActiveCN103454412AComprehensive clinical testingAccurate clinical detection meansFluorescence/phosphorescenceBiotin-streptavidin complexFluorescence
The invention discloses a liquid phase chip for detecting an allergen specific antibody. The liquid phase chip mainly comprises (1) a plurality of common allergen probes, comprising 12 fluorescence coded microspheres respectively coated with mite, cockroach, ragweed, felon herb, dog hairs, cat hairs, soybean, peanut, milk, egg, shrimp and crab allergen extracts; (2) a biotin labeling detection antibody; (3) a streptavidin phycoerythrin; (4) a total IgE (immunoglobulin E) probe used when semiquantitative or quantitative detection is carried out on the allergen specific IgE antibody, namely, a fluorescence coded microsphere coated with a rat anti-human IgE monoclonal antibody. The invention also discloses a preparation method of the liquid phase chip probe. The liquid phase chip disclosed by the invention is mainly used for detecting the allergen specific IgE antibody in a serum sample, also for detecting an allergen specific IgG4 (Intravenous Gamma Globulin 4) antibody and a total IgE antibody, and has the advantages of few used samples, parallel detection of a plurality of indexes, high flux, high sensitivity and the like.
Owner:NANJING BOMINDA BIO TECH
High affinity anti-human ige antibodies
The invention relates to high affinity human monoclonal antibodies, particularly those directed against isotypic determinants of immunoglobulin E (IgE), as well as direct equivalents and derivatives of these antibodies. These antibodies bind to their respective target with an affinity at least 100 fold greater than the original parent antibody. These antibodies are useful for diagnostics, prophylaxis and treatment of disease.
Owner:TANOX
IgE antigenicity sequence and preparation of branched multiple antigenic peptides
InactiveCN101503460AStrong specificityOvercome the disadvantage of weak immunogenicityDepsipeptidesPeptide preparation methodsHplc methodImmunogenicity
The invention relates to IgE antigenicity sequence in the immunological technical field and a preparation method for branch multiple antigenic peptides; wherein, the IgE antigenicity sequence has amino acid sequence shown in the SEQ ID NO.I; the preparation method for the branch multiple antigenic peptides comprises the following steps: the peptide sequence shown in the SEQ ID NO.I is used, a Fmoc solid phase peptide synthesis method is used for synthesizing eight branch multiple antigen peptide taking lysine as a bracket; after being collected and freezing out, the eight branch multiple antigen peptide is reserved; a HPLC method purifies the branch multiple antigenic peptides obtained in the step one. The branch multiple antigenic peptides prepared by the method can solve weak immunogenicity when the antigen epitope linear peptide is separately used and effectively gives rises to antibody response aiming at human IgE heavy chain C epsilon3 structure in immune animals.
Owner:SHANGHAI JIAO TONG UNIV
Chemiluminescence quantitative detection kit for food allergens, and preparation method and detection method thereof
ActiveCN103901215ANo pre-dilution requiredImprove accuracyDisease diagnosisBiological testingImmunglobulin eMicroparticle
Owner:SUZHOU HAOOUBO BIOPHARML
Detecting kit for specific IgE antibody of food allergen as well as preparation and detecting methods of detecting kit
InactiveCN104820090AEasy to operateHigh sensitivityChemiluminescene/bioluminescenceBiological testingMicroparticleBiotin
The invention belongs to the technical field of biology and particularly relates to a detecting kit for a specific IgE antibody (quantitative) of a food allergen as well as preparation and detecting methods of the detecting kit. The detecting kit is used for quantitatively detecting the content of the specific IgE antibody (quantitative) of the food allergen in human serum in vitro. The detecting kit for the specific IgE antibody of the food allergen contains the following components: a magnetic separation reagent, an allergen reagent, an enzyme reactant, a calibrated product, a quality control product, a concentrated cleaning solution and a substrate solution, wherein the magnetic separation reagent contains magnetic particles on which human IgE antibodies are coated, and the allergen reagent contains a biotin-labeled specific allergen. The detecting method of the detecting kit for the specific IgE antibody of the food allergen is realized by combining a chemiluminescence immune assay technology and a magnetic particle separation technology, and meanwhile, an enzyme-free capturing method is adopted, so that the detecting method has the advantages of convenience in operation, high sensitivity, good accuracy, high speed and no pollution.
Owner:HANGZHOU AOMIN BIOLOGICAL TECH CO LTD
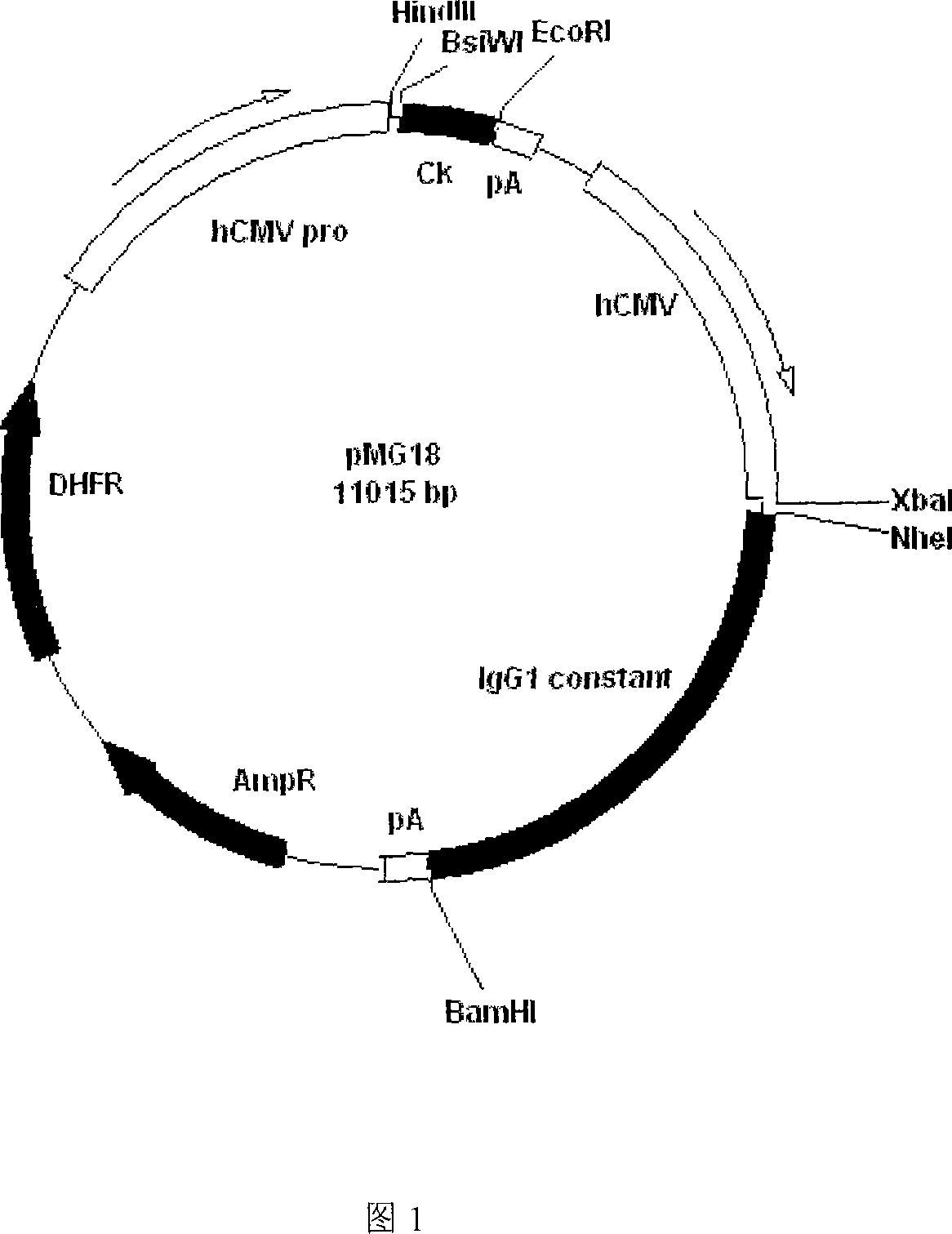
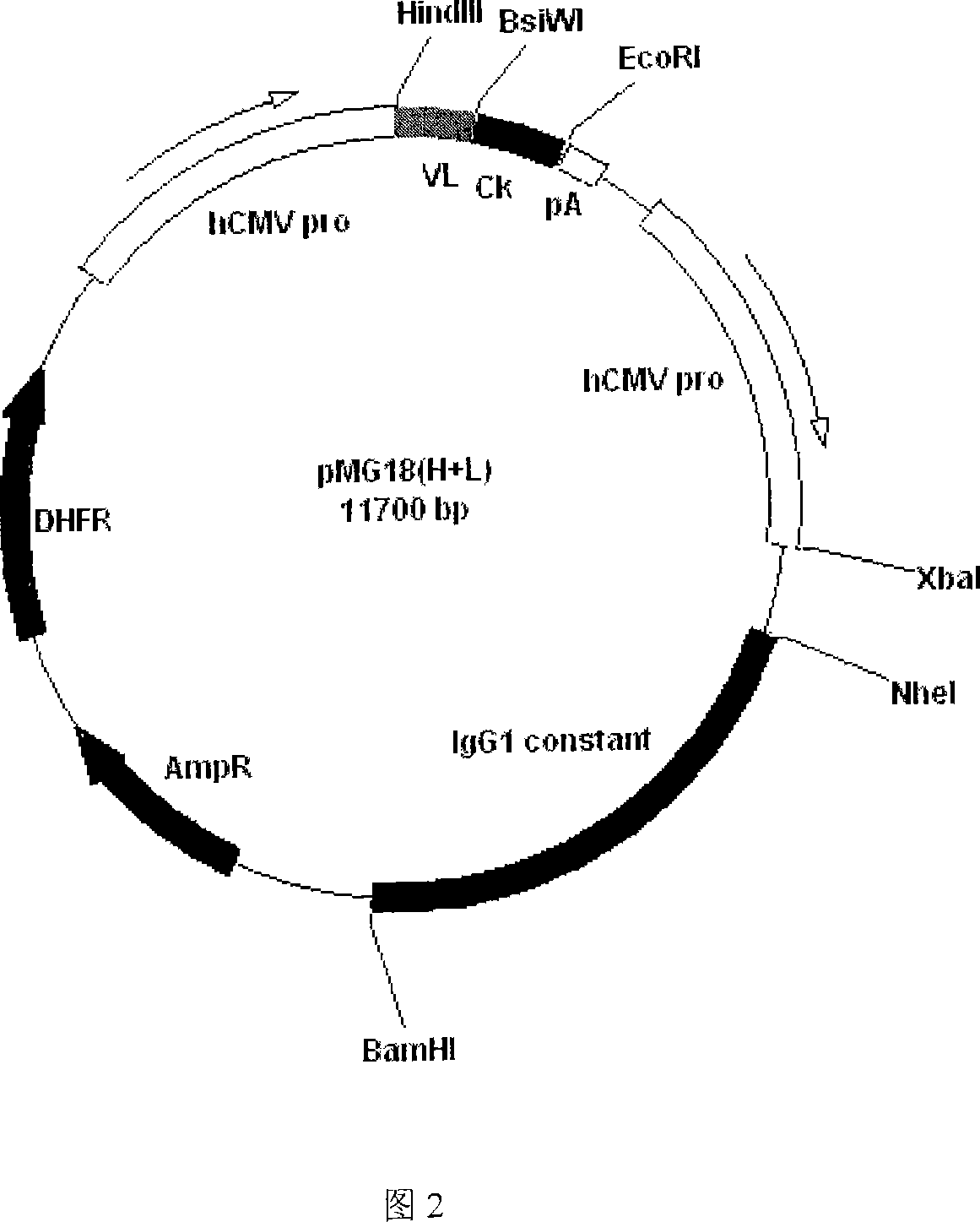
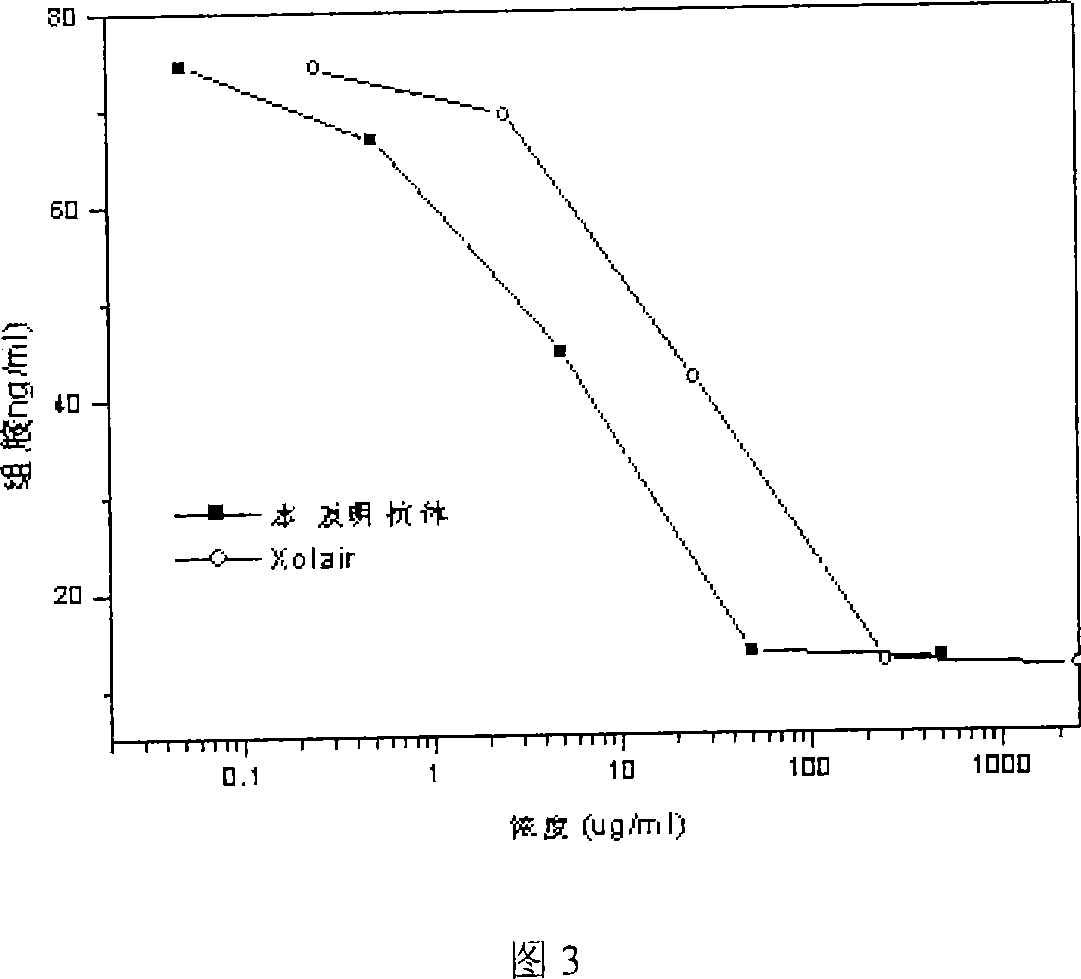
Recombinant anti human IgE monoclonal antibody and preparation method and usage thereof
InactiveCN101062949AImmunoglobulins against animals/humansAntibody ingredientsDiseaseMonoclonal antibody
The invention discloses a restructuring antihuman IgE monoclonal antibody, which is characterized by the following: possessing amino acid sequence of SEQ ID NO: 1 in light chain variable zone; possessing amino acid sequence of weight chain variable zone of SEQ ID NO: 2 in weight chain variable zone. This invention also discloses a DNA molecule to encode monoclonal antibody, a carrier to express the monoclonal antibody and eukaryotic host cell transformed by the expression carrier. This invention also discloses an usage to prepare I type hypersensitivity disease medicine of restructuring antihuman IgE monoclonal antibody.
Owner:SHANGHAI NAT ENG RES CENT OF ANTIBODY MEDICINE
Method of anaphylactogen screening
InactiveCN1869699ANo painSimple and fast operationMaterial analysis by observing effect on chemical indicatorBiotin-streptavidin complexSerum ige
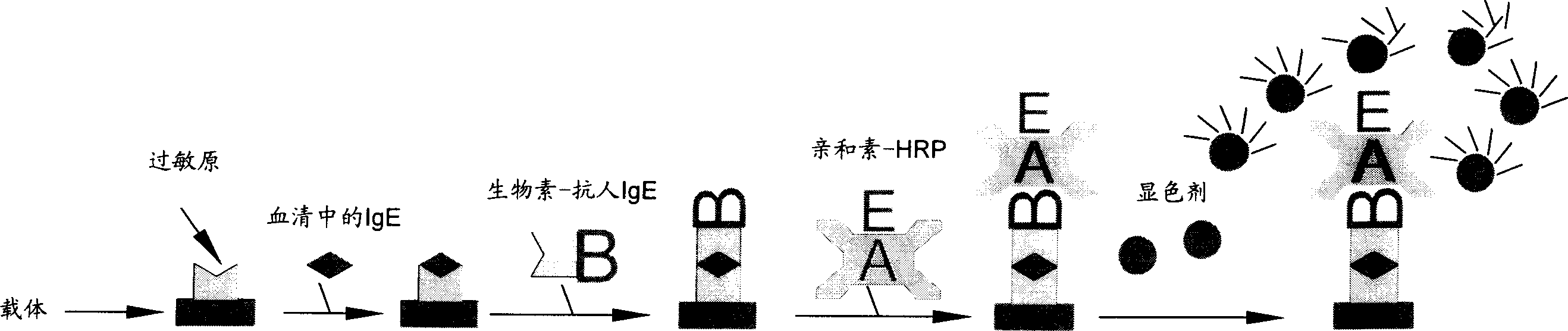
The invention is an allergen screening method, comprising the steps of: a. taking removable ELISA plate stripe as allergen screening carrier, and coating common allergens in ELISA plate holes; b. adding human serum into the ELISA plate stripe to make IgE antibodies in the serum specifically bind with the allergens, and then adding in biotin-labeled anti-human IgE to specifically form combination; c. successively adding in streptavidin-Horse Radish Peroxidase (streptavidin-HRP) and using biotin-avidin specific binding to form compound; d. finally adding in allergen color developing agent, and under the action of HRP, making the color developing agent change color, and determining the concentration of IgE antibodies in the sample according to the shade of color. And it is simple to operate and has accurate result and low cost.
Owner:深圳市博卡生物技术有限公司
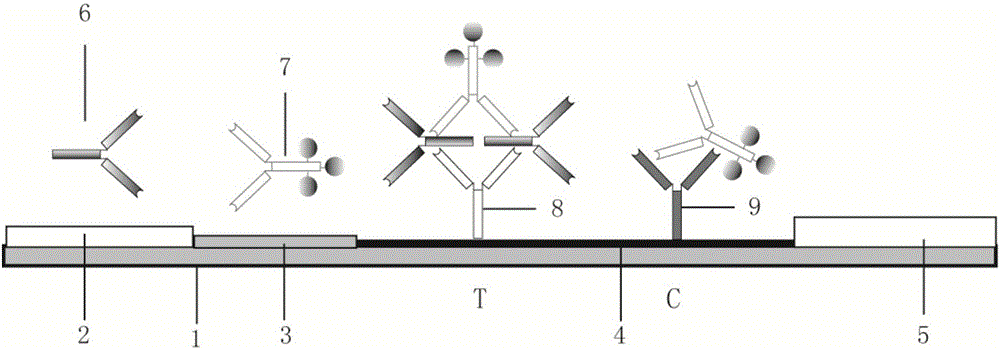
Rapid specific antibody IgE detection kit and preparation method thereof
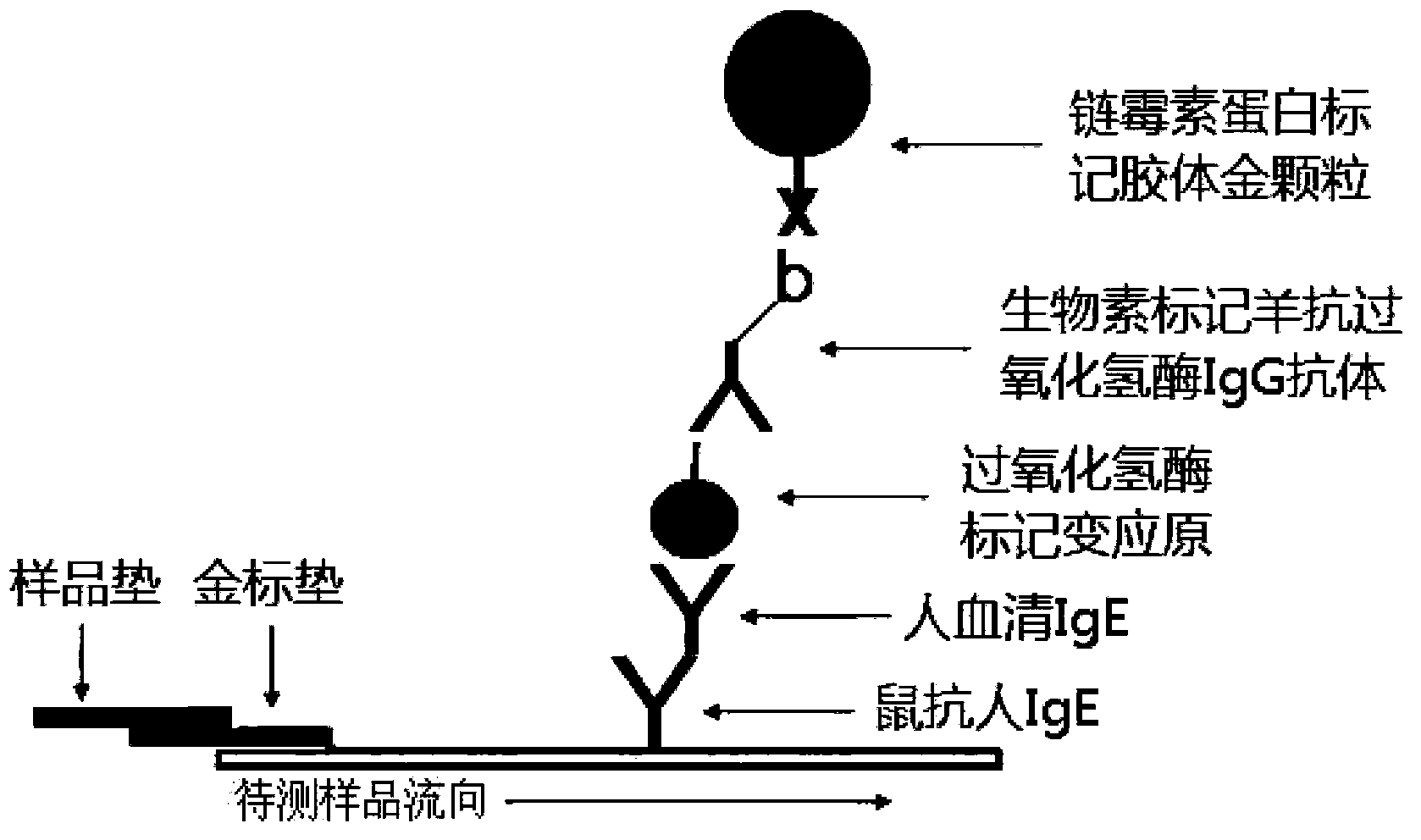
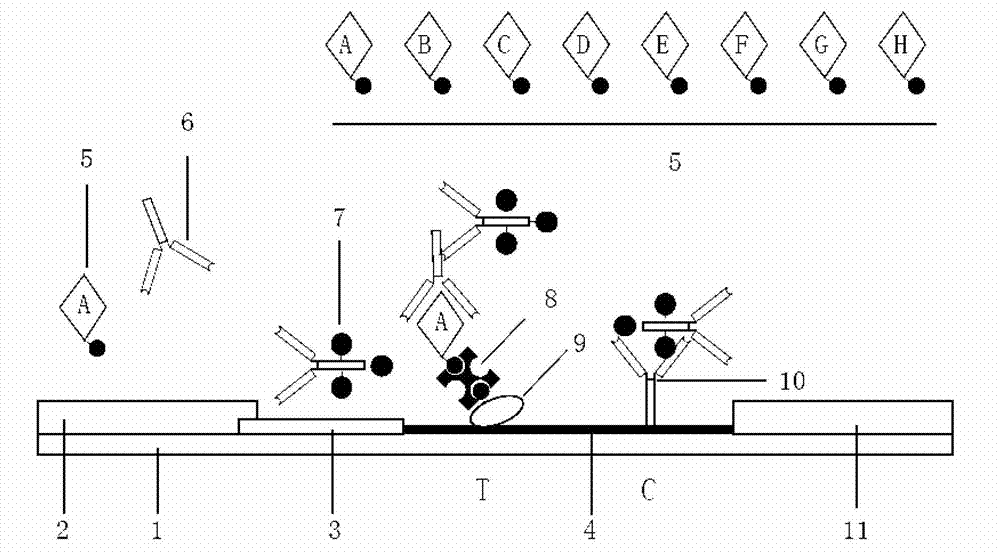
The present invention relates to a rapid allergic disease allergen diagnosis kit through a two-step method, a preparation method and a use method thereof. During use of the kit, a serum sample requiring detection and catalase-labeled allergen are subjected to equal volume mixing, room temperature incubation is performed for 5-15 min, the mixed sample is added to a sample hole of colloidal gold test paper strip, and the result is read after 5-15 min, wherein the test strip comprises the sample hole, a colloidal gold pad, a film strip and a water suction pad, colloidal gold is conjugated with biotin labeled goat anti-catalase antibody through streptomycin protein and mouse anti-human IgE monoclonal antibody is coated on the film strip so as to form the colloidal gold-streptomycin protein-biotin-goat anti-catalase antibody-catalase-allergen-specific human IgE antibody-mouse anti-human IgE antibody complex, and the quality control zone is coated with mouse anti-goat IgG antibody. The total detection time of the kit is 10-30 min. The kit has characteristics of strong specificity, high sensitivity, simpleness, rapidness, on-site inspection and no requirement of professional training on operators, wherein operations on allergen specific IgE detection reagents can be completed according to the instruction.
Owner:北京新华联协和药业有限责任公司
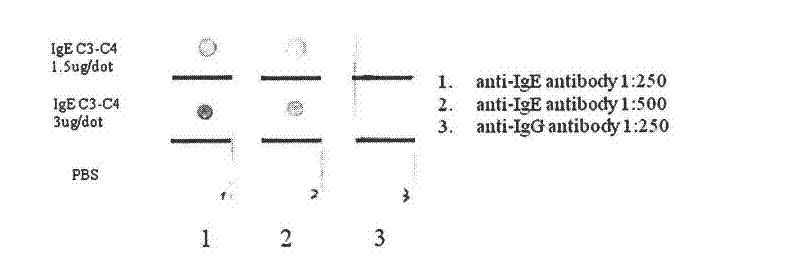
Human IgE (immunoglobulin E) Fab (fragment ab) against human and coding gene and application thereof
The invention belongs to the technical field of biotechnology and relates to a human IgE (immunoglobulin E) Fab (fragment ab) against human and a coding gene and application thereof. In the invention, a recombined IgE C3-C4 protein is used for screening a natural human immunoglobulin gene library, and a human antibody Fab of IgE against human is screened from the library through ELISA (enzyme-linked immunosorbent assay) sequencing analysis and the like; extensive expression purification and further identification prove that the antibody Fab of human IgE against human is fully human and without Fc (fragment c), has an effect of inhibiting IgE mediated anaphylaxis without activating complements or causing human immune response or other pathological lesions and can be used for preparing antibody drugs for treating allergic diseases caused by IgE.
Owner:FUDAN UNIV
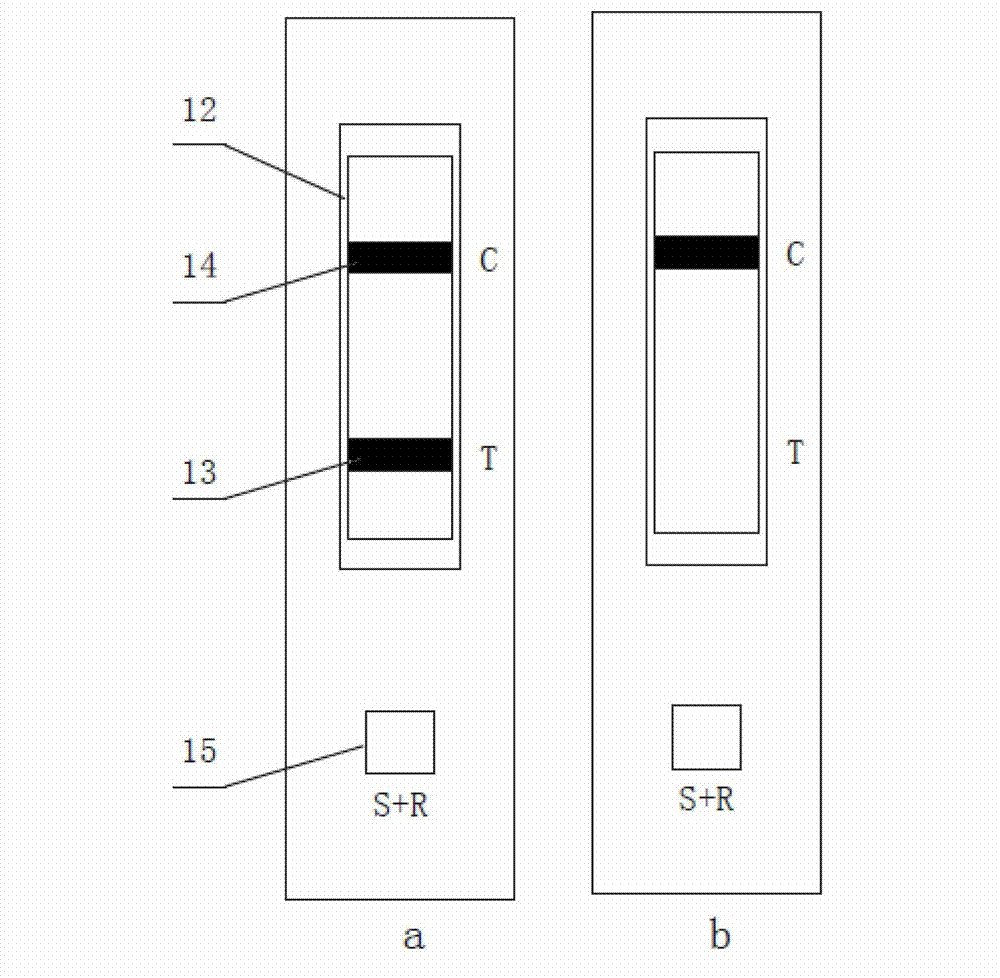
Kit and test paper strip for serum immunoglobulin E colloidal gold chromatography quantitative detection
InactiveCN106370860AEasy to operateMaster fastBiological testingMonoclonal antibodyPolyclonal antibodies
The invention provides a kit and a test paper strip for serum immunoglobulin E colloidal gold chromatography quantitative detection. The kit includes: a colloidal gold marked mouse-anti-human IgE monoclonal antibody, a rabbit-anti-human IgE polyclonal antibody, and a goat-anti-mouse IgG antibody. In the invention, the colloidal gold immunochromatography technology is employed, and the kit and the test paper strip need to be combined with an immunochromatography result interpretoscope. The kit and the test paper strip are easy to use, are free of training and can be rapidly learnt, are free of large-size devices and are suitable for primary laboratories or community laboratories.
Owner:天津中新科炬生物制药股份有限公司
Antibody fragment-polymer conjugates and uses of same
Owner:GENENTECH INC
Kit and method for achieving highly-sensitive multiterm joint inspection of allergen-specific IgE antibodies
InactiveCN105044326AReduce dosageSuitable for high-throughput detectionMaterial analysisSerum igePolymerase L
The invention discloses a kit and method for achieving highly-sensitive multiterm joint inspection of allergen-specific IgE antibodies. The kit comprises a membrane strip, the polymerase coupling anti-human IgE antibodies and a substrate color development agent matched with the polymerase coupling anti-human IgE antibodies; str-bio-allergens are fixed on the membrane strip, and the allergen-specific IgE antibodies are detected by adopting the kit. In this way, According to the kit and method for achieving highly-sensitive multiterm joint inspection of the allergen-specific IgE antibodies, the concentration of the allergen-specific antibodies IgE in the human serum or plasma can be detected highly sensitively, qualitatively or semi-quantitatively and quickly, the allergen usage amount can be reduced, dozens of allergens can be screened for one time, the advantages of being quick, accurate and little in sample usage amount are achieved, and high throughput detection is suitably performed.
Owner:SUZHOU HAOOUBO BIOPHARML
Kit and method for detecting specific allergen antibody IgE in high-sensitivity manner
ActiveCN104237527AReduce dosageSuitable for high-throughput detectionBiological testingBiotin-streptavidin complexSerum ige
The invention provides a kit and a method for detecting specific allergen antibody IgE in a high-sensitivity manner. The kit mainly comprises a fiber material membrane provided with allergen protein to be detected, double biotin-streptavidin optimized liquid, a biotin coupled anti-human IgE antibody, biotin or streptavidin marked polymerase, and a substrate color developing agent corresponding to polymerase in a curing manner; the kit can qualitatively or semi-quantitatively detect the concentration of the specific allergen antibody IgE in human serum or plasma rapidly in the high-sensitivity manner, can screen dozens of allergens, is rapid and accurate, and is suitable for high-throughput testing, and a relatively small quantity of samples are used.
Owner:ZHEDA DIXUN BIO GENE ENG
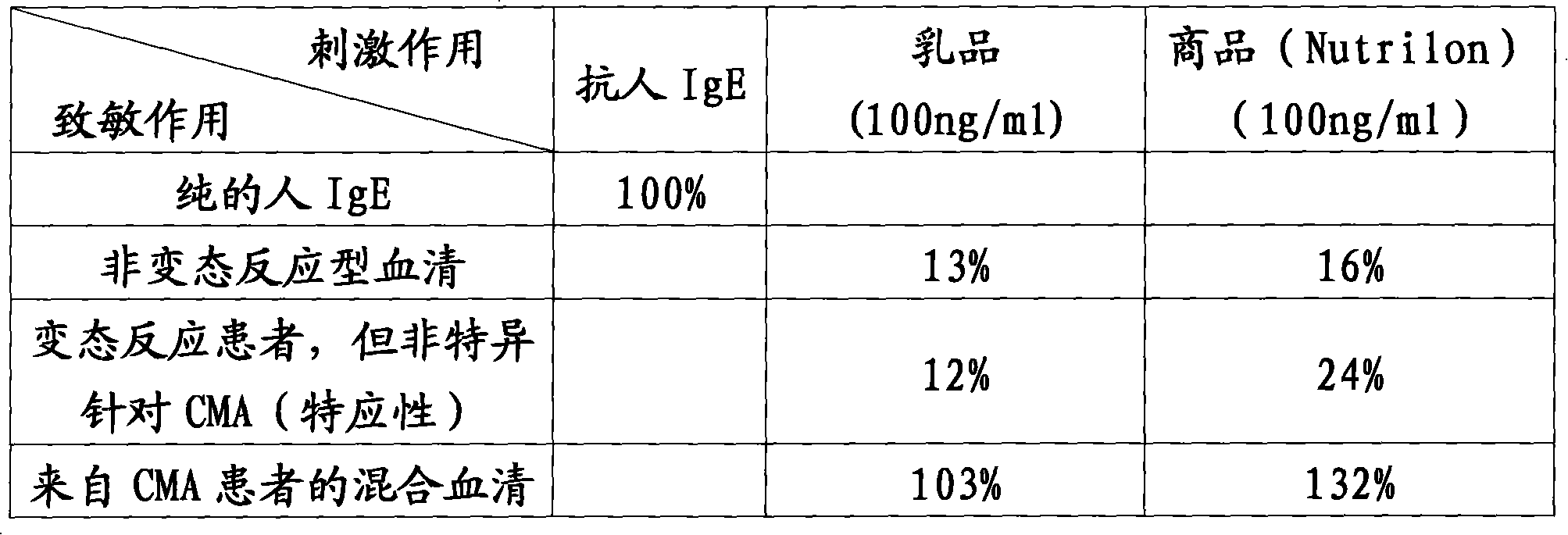
Method for measuring allergic response
InactiveCN101142480AGood allergiesMicrobiological testing/measurementBiological testingAllergic reactionHuman ige
The present invention discloses a method for measuring allergic reaction with measuring the degranulation degree when the human IgE sensitized cell is activated by the allergen in the food.
Owner:NV NUTRICIA
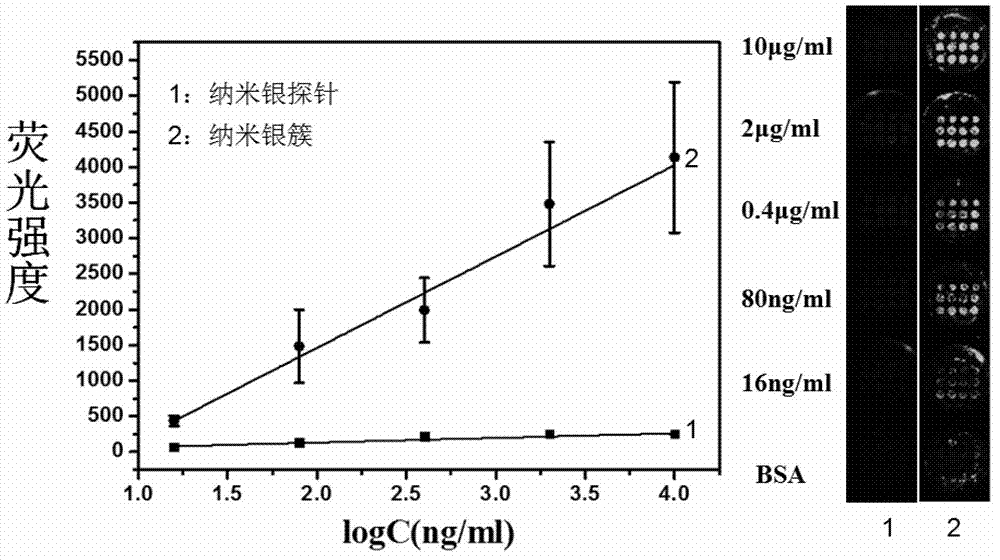
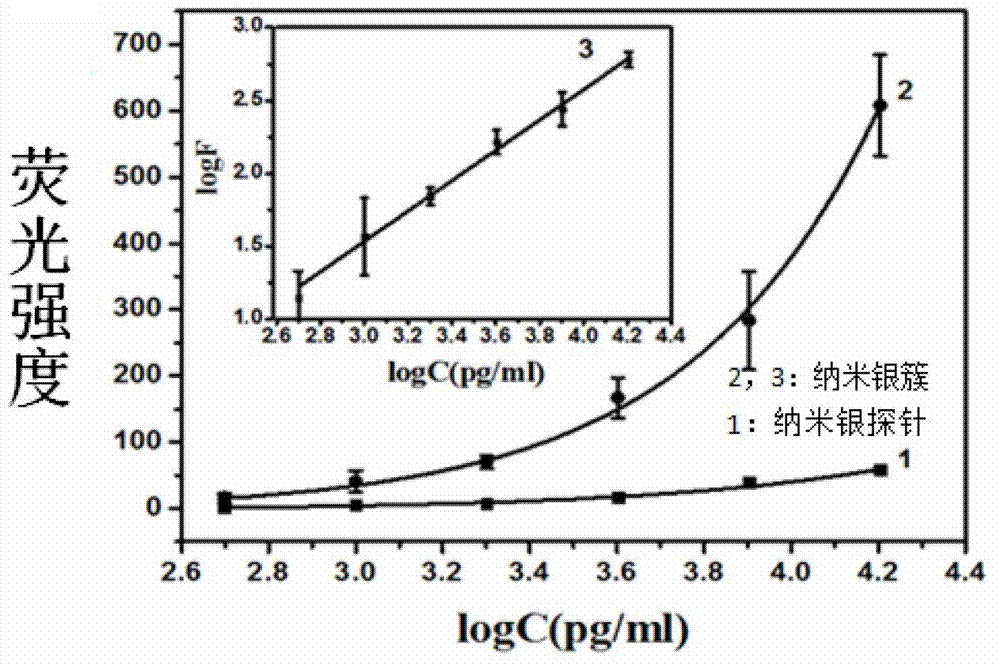
Nanometer silver probe modified by fluorescent molecule, kit thereof, and application thereof in detecting human IgE
ActiveCN102830103AHigh sensitivityEasy to detectFluorescence/phosphorescenceLuminescent compositionsProtein markersFluorescence
The invention discloses a nanometer silver probe modified by fluorescent molecule. The nanometer silver probe uses a nanometer silver ball having a diameter of 10 to 30 nm as a core, and the external surface of the silver ball is bonded with a fluorescent molecule chain and an aptamer chain. The invention further discloses a kit containing the probe and an application in detecting human IgE. The nanometer silver probe provided by the invention can greatly improve sensitivity of fluorescence detection by coupling a fluorescence enhancing liquid, and a linear range of human IgE detection is from 0.5 ng / ml to 10 [mu]g / ml, thereby being low in detection limit and good in specificity. Such nanometer silver probe is mature in synthesis and modification method of, the fluorescence enhancing liquid is cheap and easily available, and sensitivity of human IgE detection can be greatly enhanced by combining the aforementioned two factors. Such signal amplified method provides an excellent method for fluorescence analysis and detection of protein markers.
Owner:NANJING UNIV
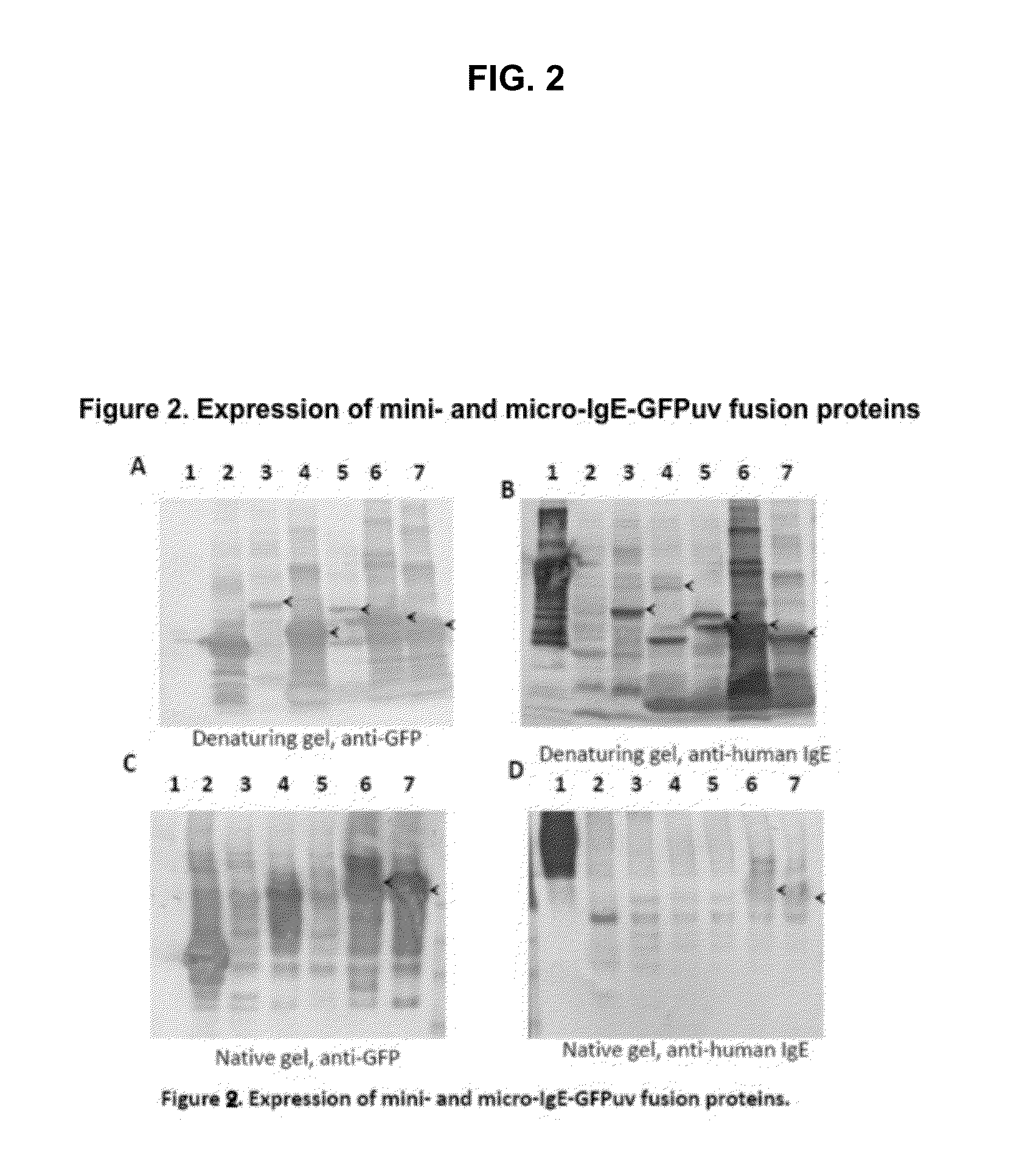
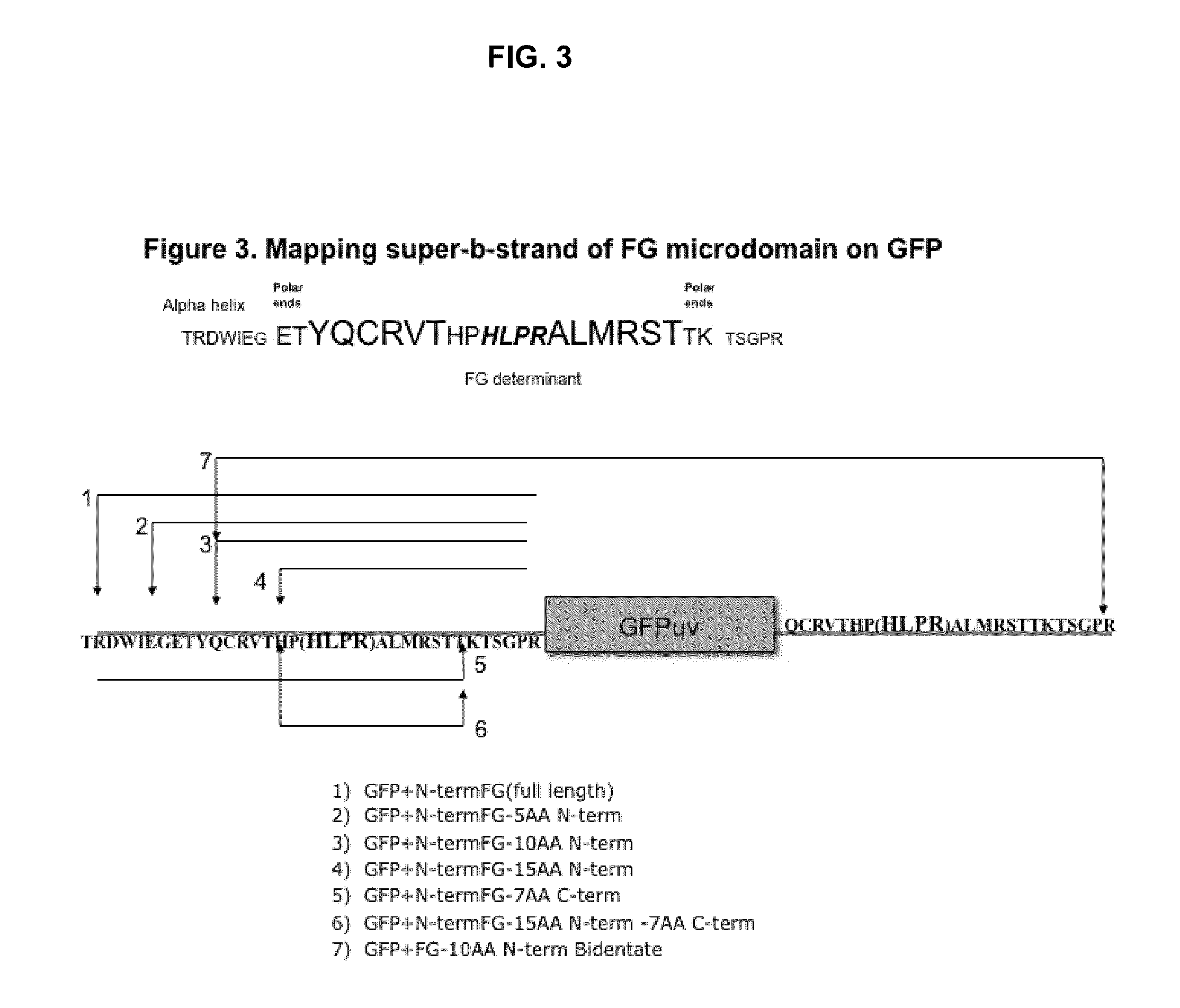
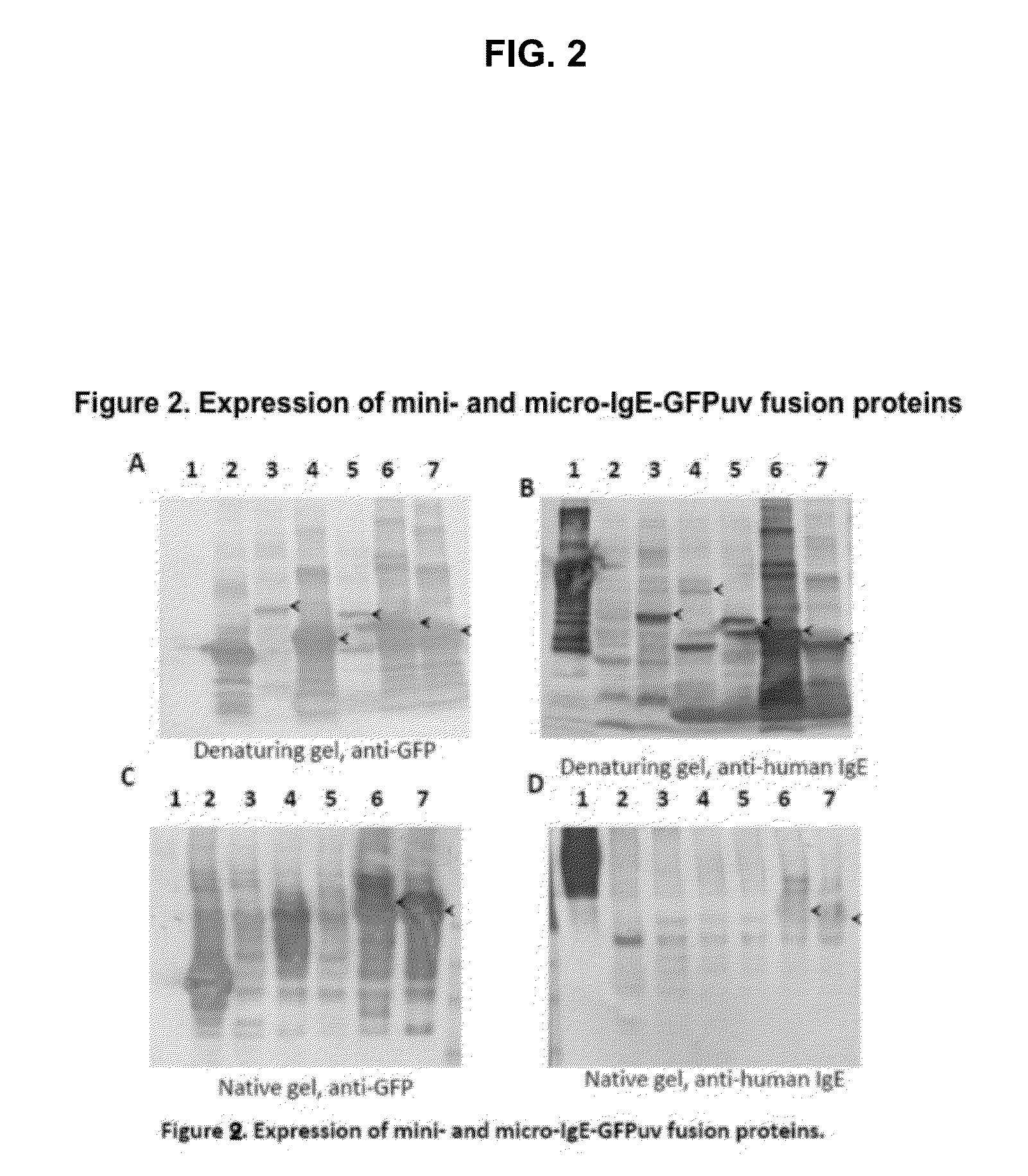
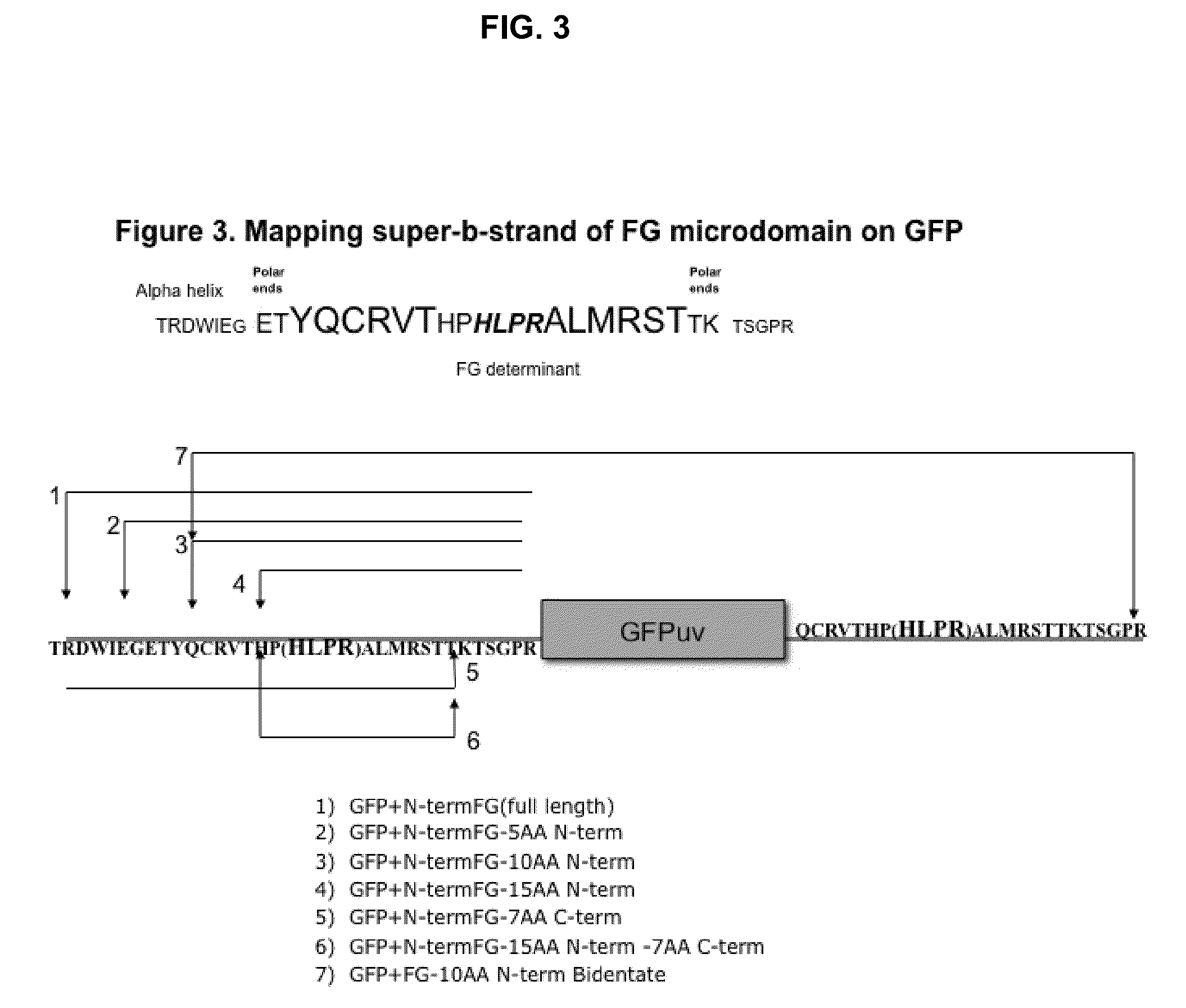
Displaying native human IgE neutralizing FceRla-contacting IgE B-cell epitopes by constraining super beta(b)-strands and cystine knots on thermostable protein scaffold
Vaccine displaying native antigenic loops of immunoglobulin E is critical for eliciting neutralizing anti-IgE antibodies. The embodiment of the invention enables the display of native antigenic IgE receptor-contacting loops as IgE B-cell vaccines via three steps of constraining methods. The loops of multiple antigenic B-cell epitopes can be molecularly grafted in, and conformationally constrained by the energy favorable flanking beta (b)-stands, i.e., the super b-strands identified in this invention. The constrained loops can be further stabilized in replacing a selective loop within the cystine knot peptide. These dual constrained antigenic loops are then integrated onto thermostable protein scaffolds, folded in the oxidative milieu that provides further conformational constraint and high yield.
Owner:CHEN SWEY SHEN
Chemiluminescence quantitative detection kit for inhaled allergens, and preparation method and detection method thereof
ActiveCN103901188ANo pre-dilution requiredImprove accuracyChemiluminescene/bioluminescenceBiological testingIntravenous gammaglobulinGlobulin
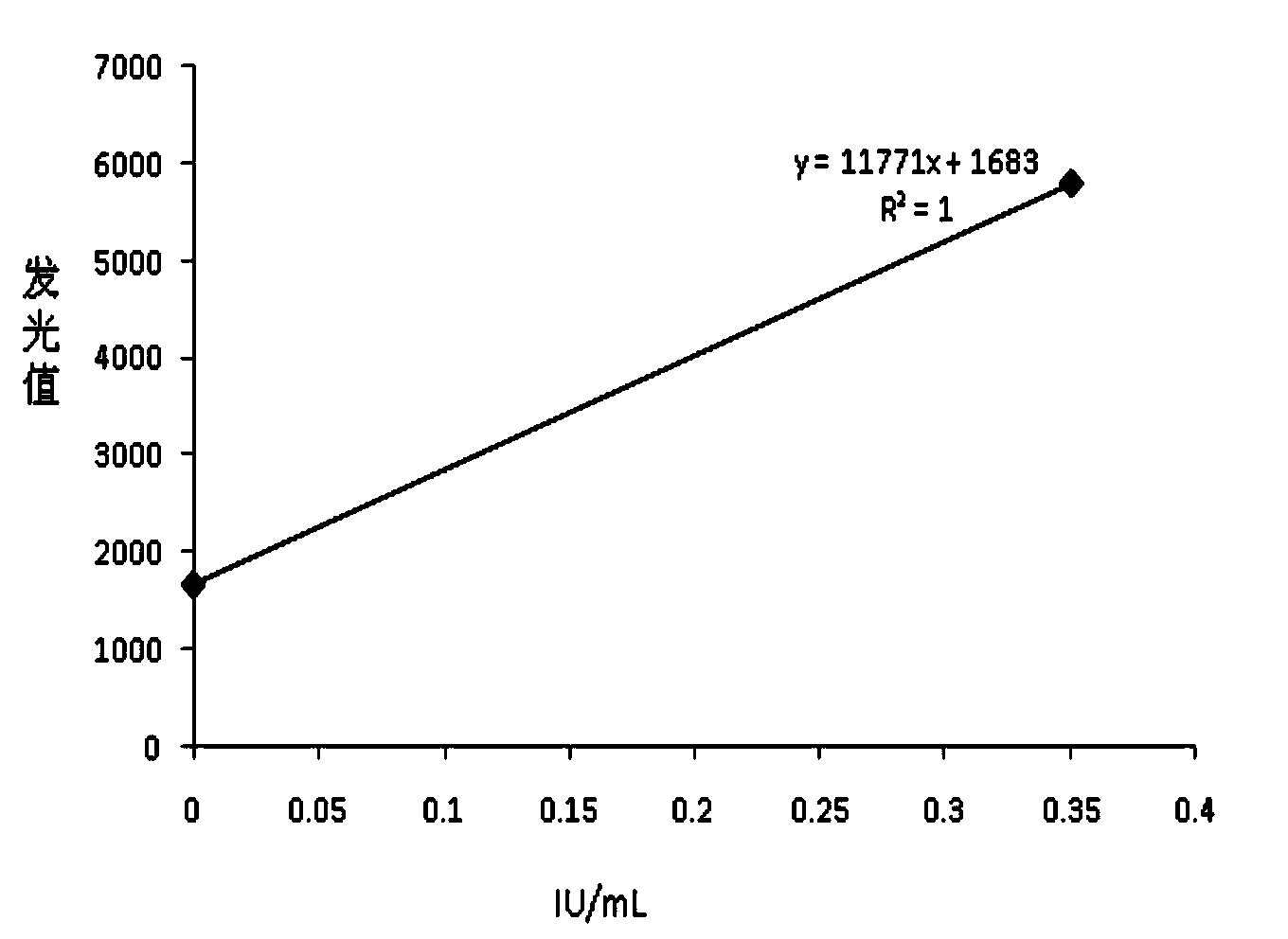
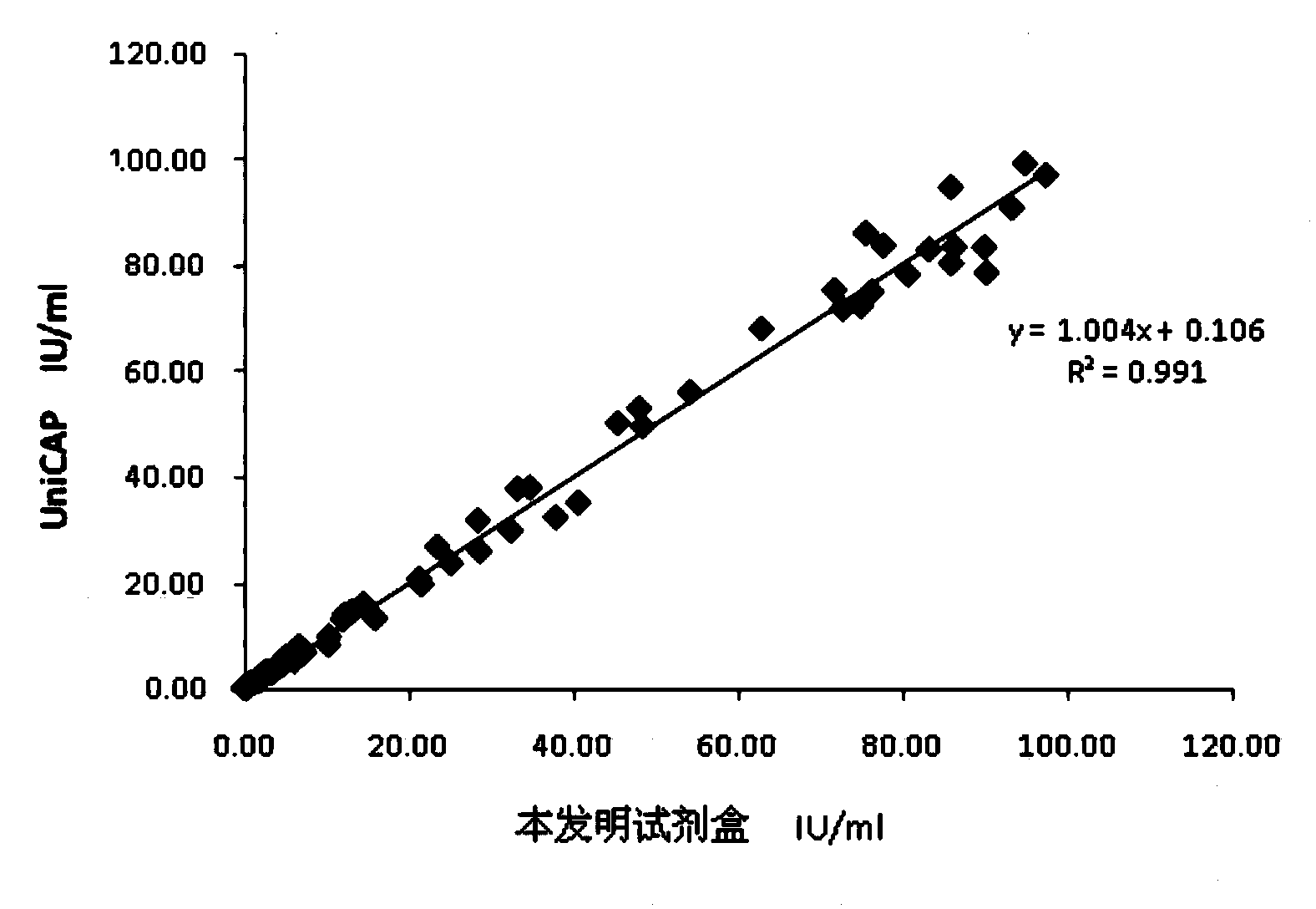
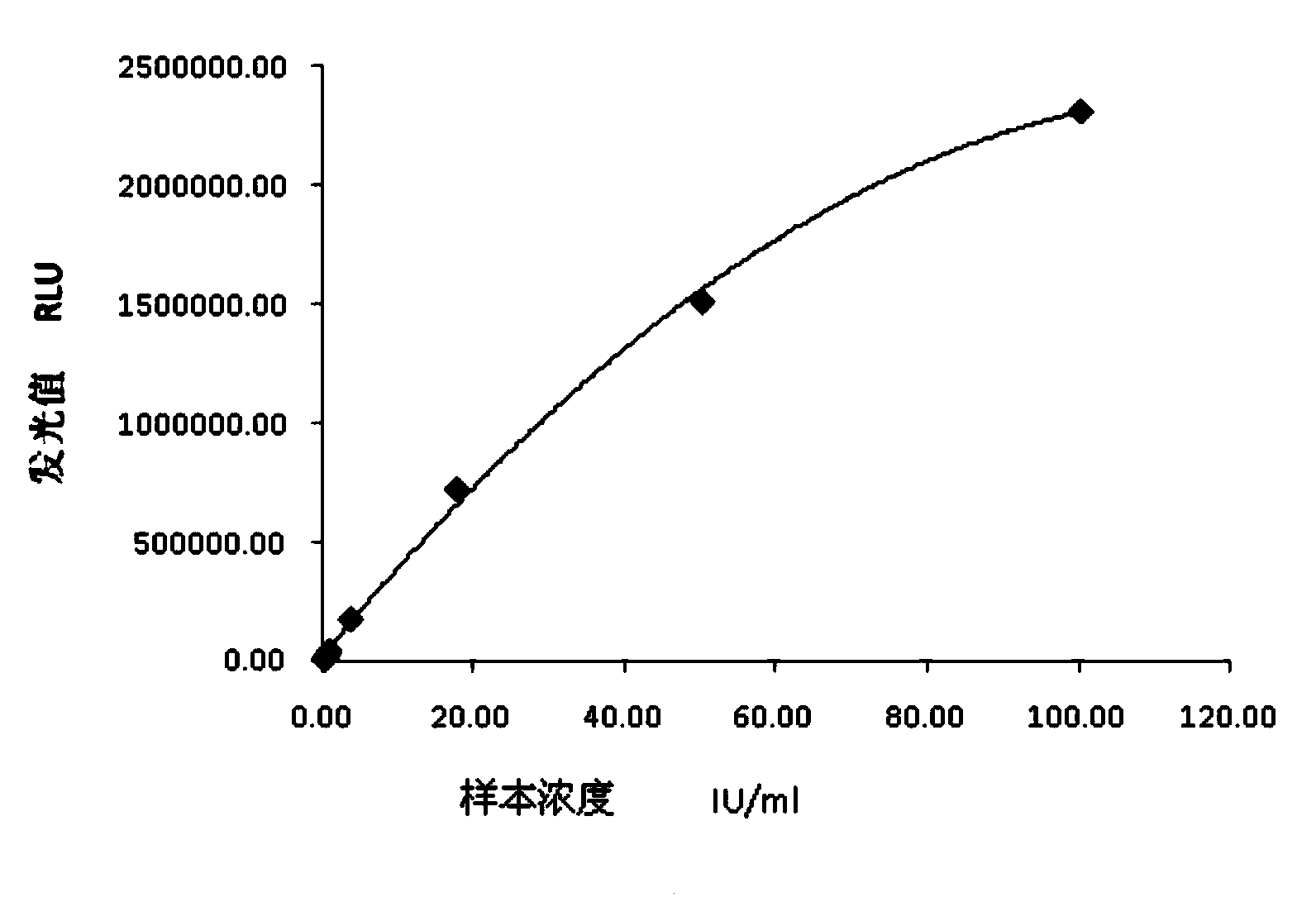
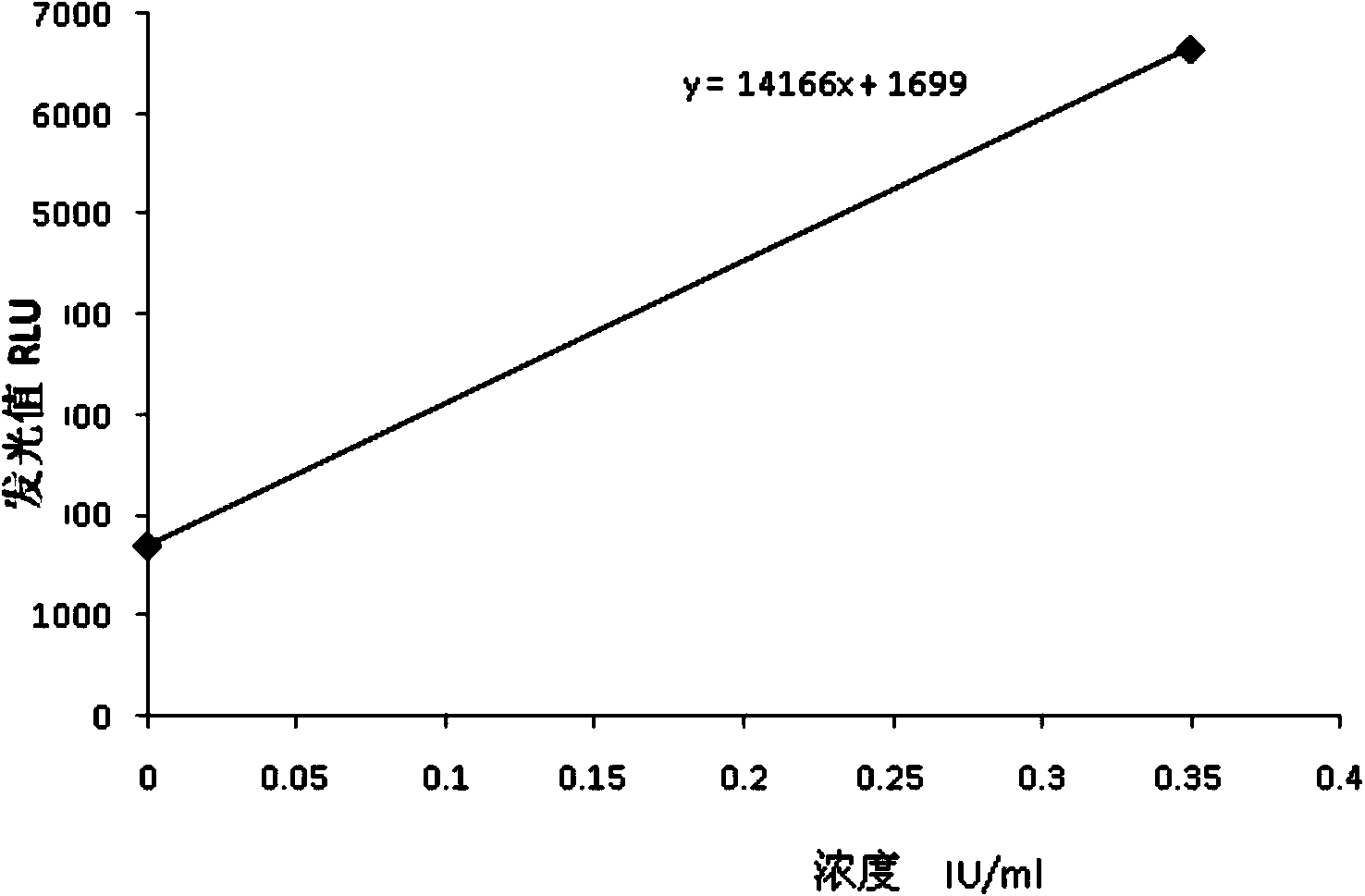
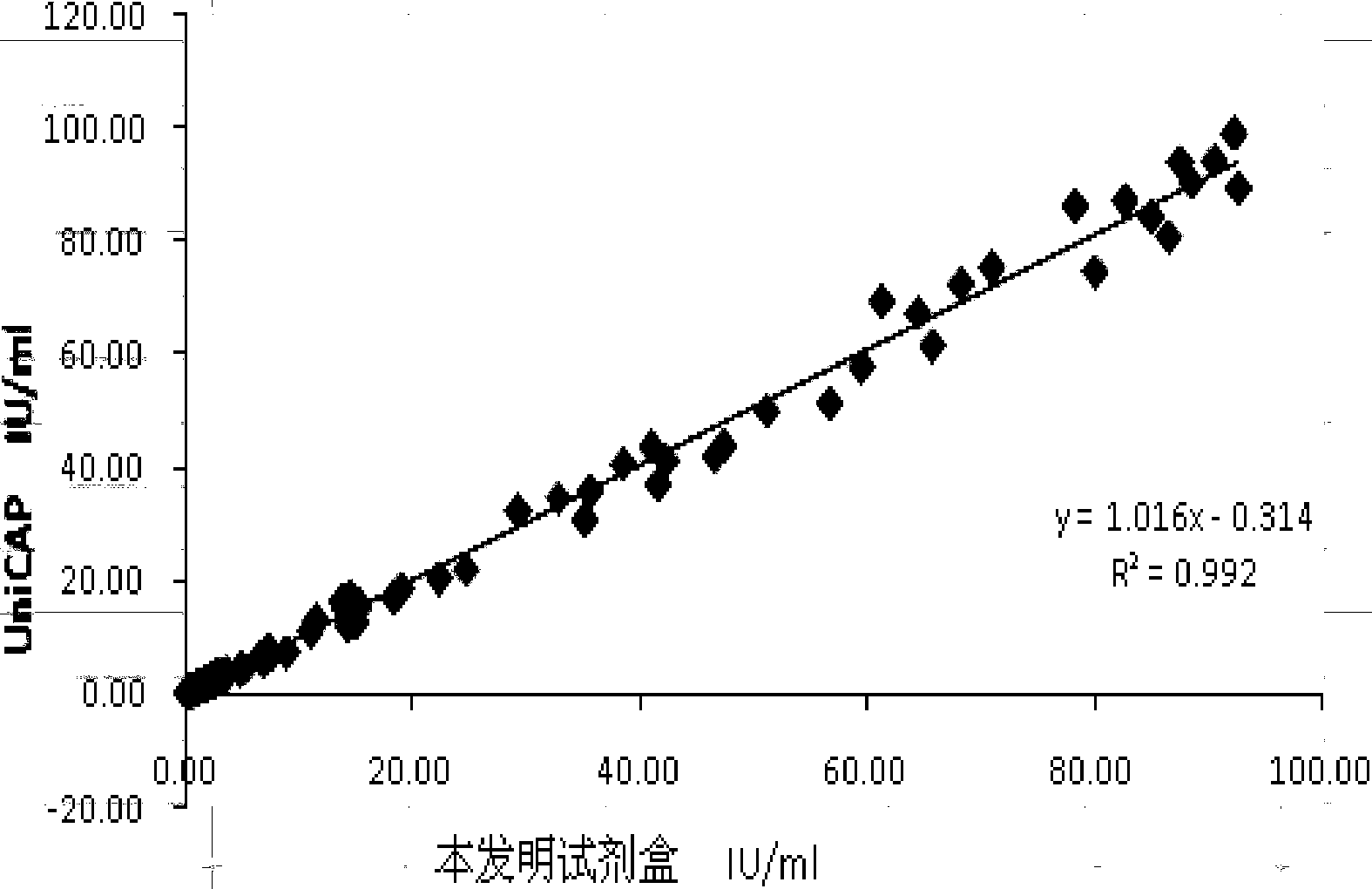
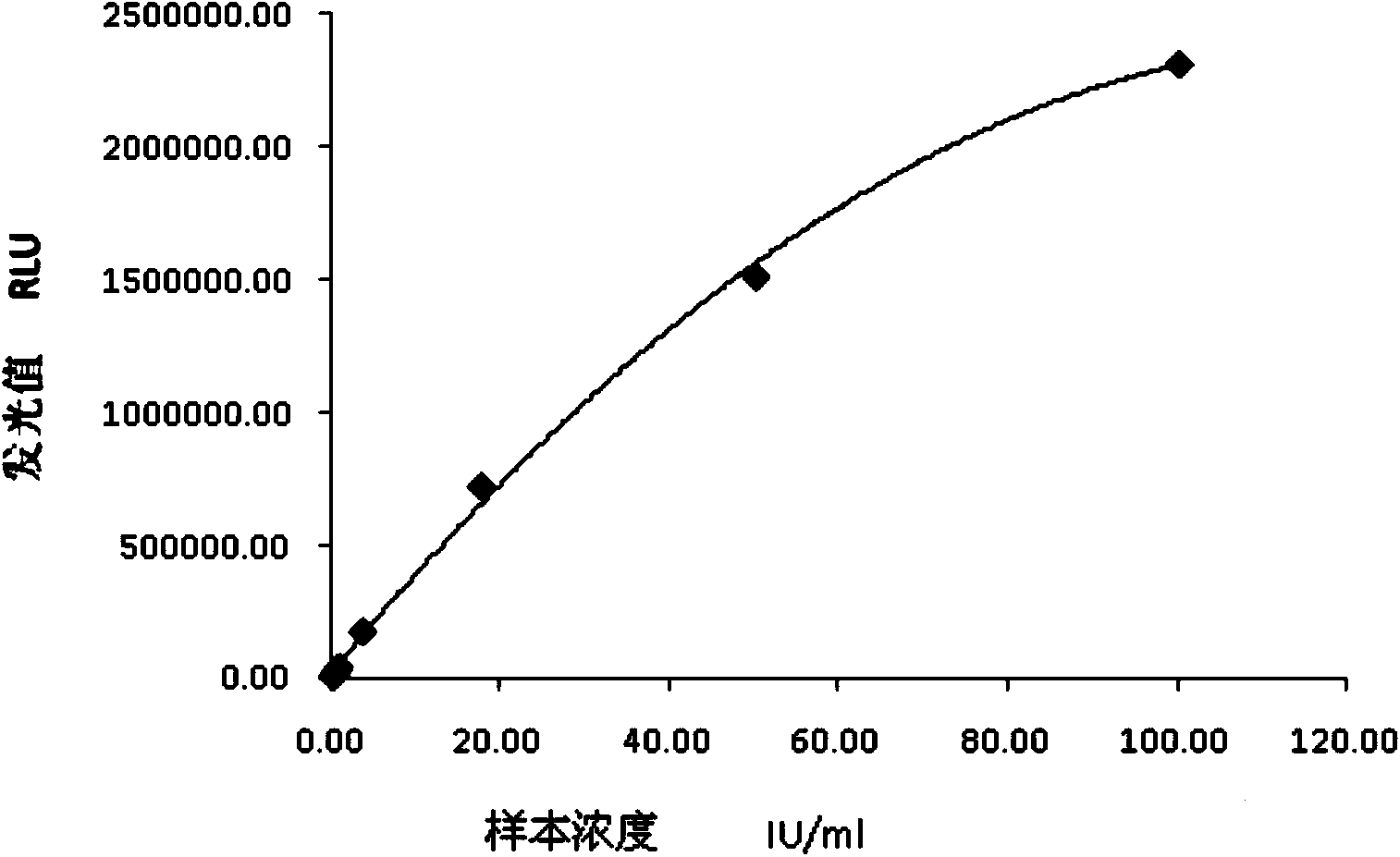
The invention relates to a chemiluminescence quantitative detection kit for inhaled allergens, which comprises a magnetic separation reagent (a 0.1-1.0 mg / ml inhaled-allergen-labeled magnetic particle suspension) and an ELIASA reagent (a 0.5-1 mu g / ml anti-human IgE antibody solution containing alkaline phosphatase label). The sensitivity of the kit prepared from the inhaled-allergen-labeled magnetic particle suspension and anti-human IgE antibody solution containing alkaline phosphatase label is up to 0.008 IU / ml, and the cross reaction rates of the kit with other immunoglobulins are respectively less than 0.04%; and the kit has the advantages of high accuracy, high precision, no need of prediluting the sample, and wide detection range, and is simple and time-saving to operate.
Owner:SUZHOU HAOOUBO BIOPHARML
Elisa screening method for inhibitors of human IgE binding to the high affinity receptor, FcepsiRIalpha
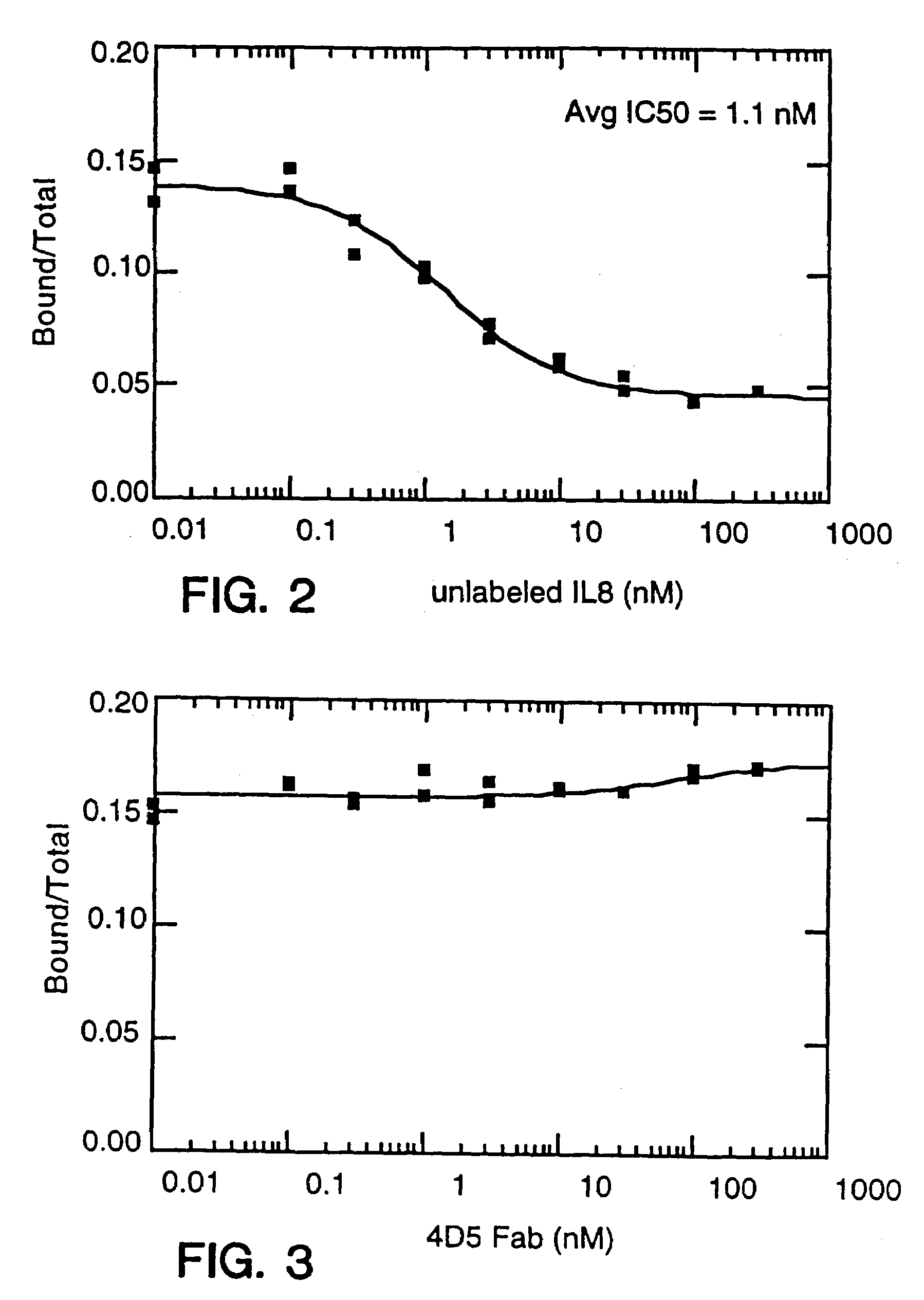
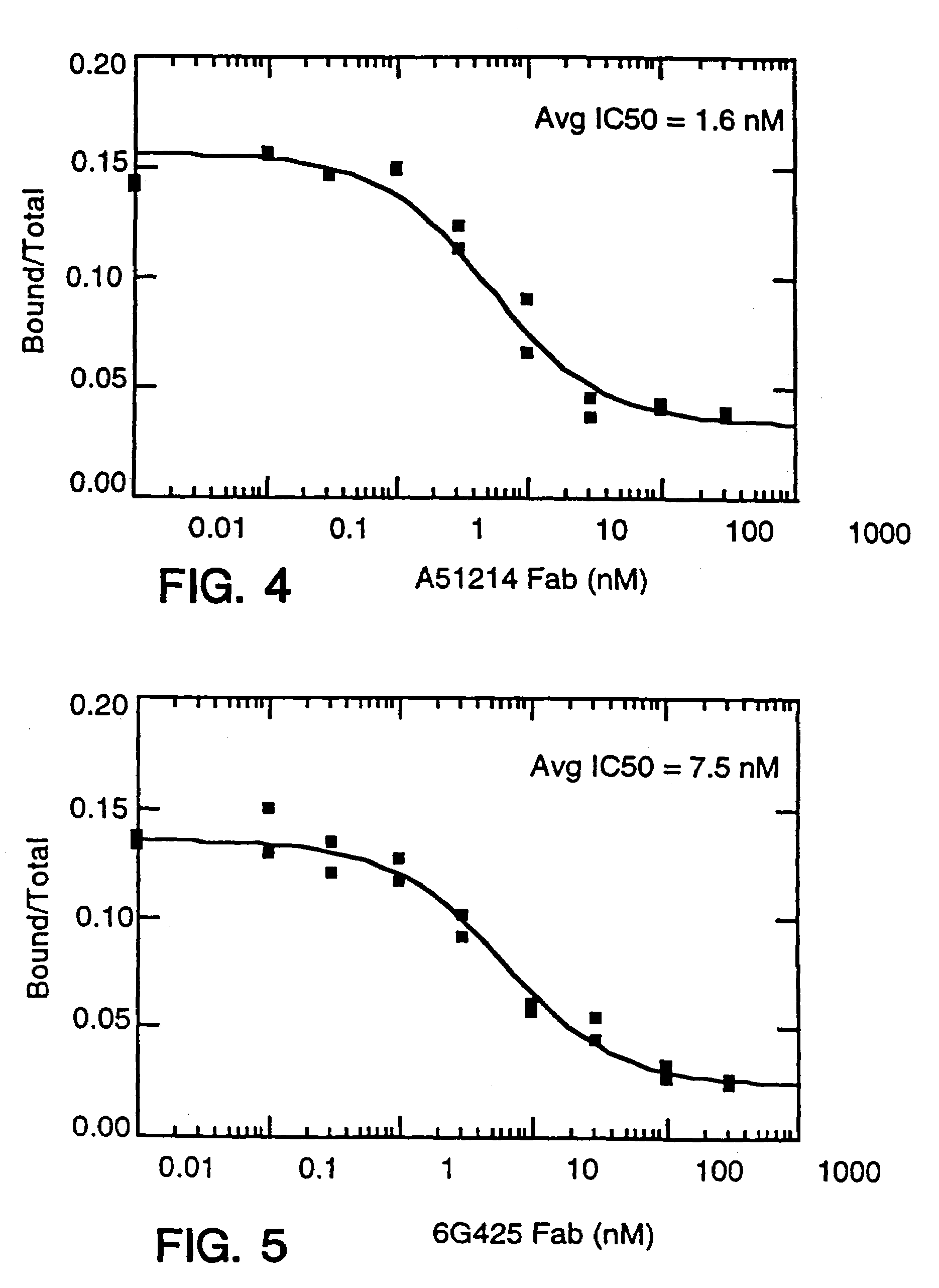
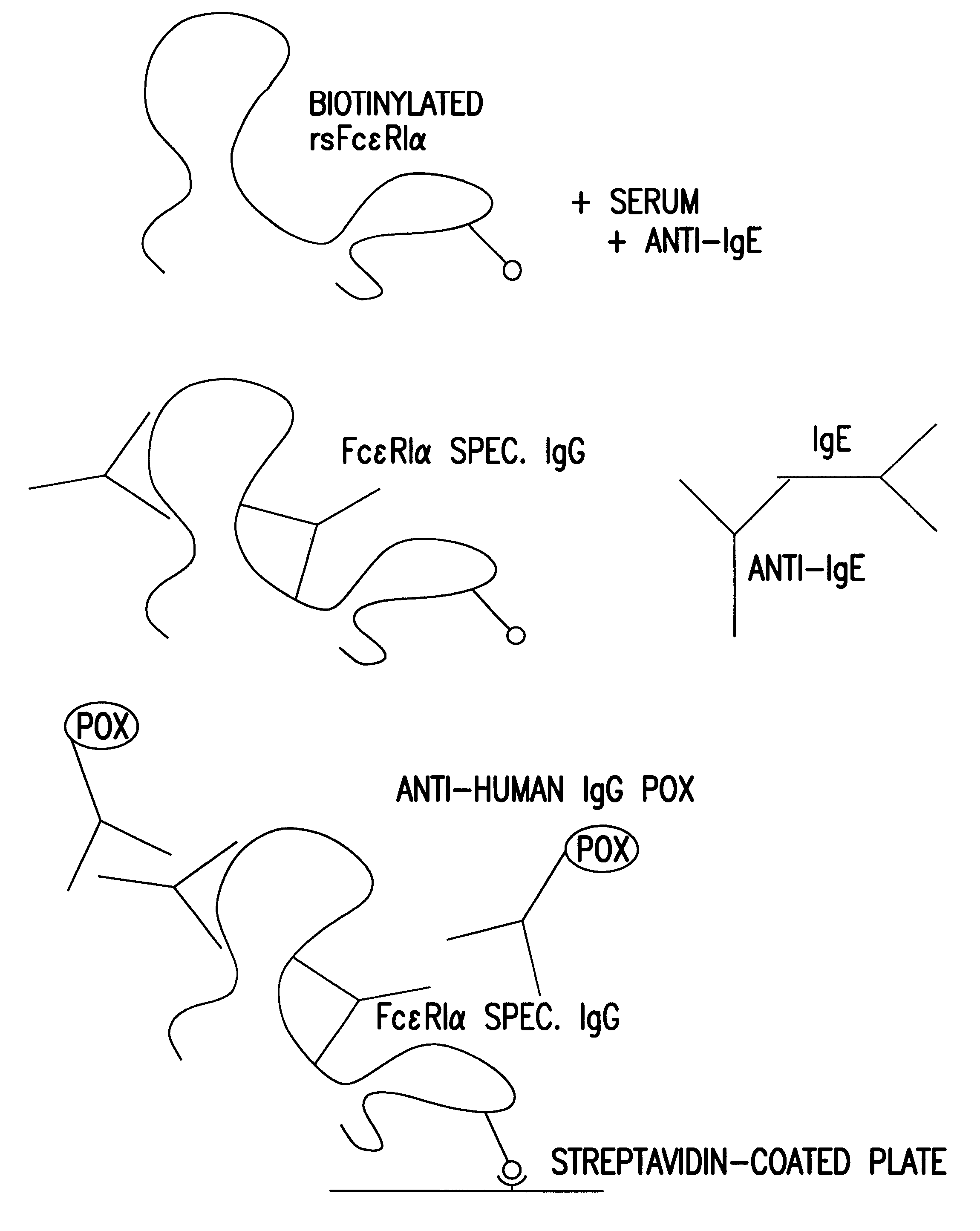
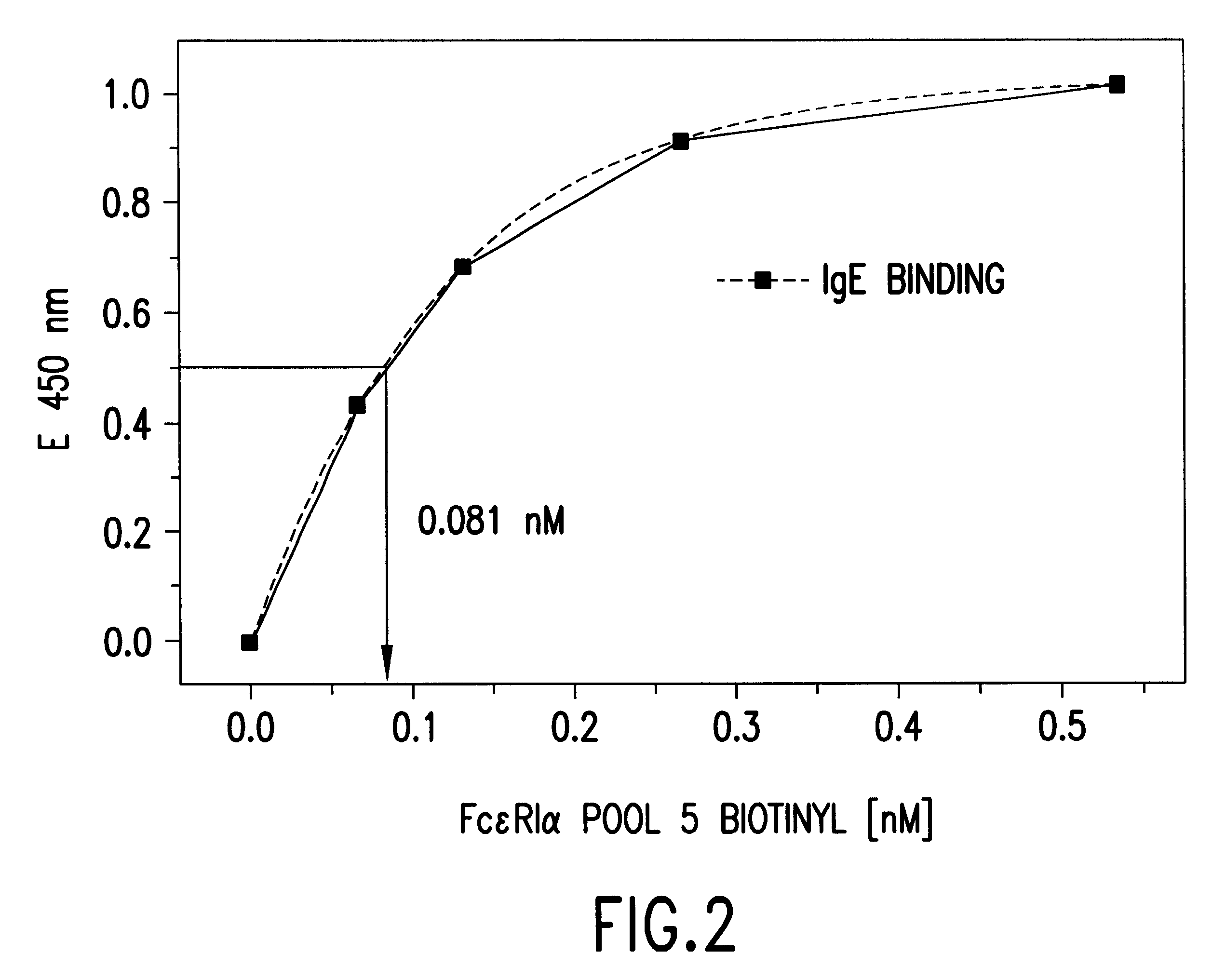
The present invention is directed to an Assay for high capacity screening of substances interfering with the attachment of human IgE to its high affinity receptor and / or of substances capable of detaching already bound IgE from this receptor and for the differential analysis between autoimmune disorders and classical allergies.
Owner:NOVARTIS AG
Food specificity IgE detection kit and preparation method thereof
InactiveCN102778572AMeet the need for rapid screeningShorten time to see a doctorBiological testingNumerical controlBiotin-streptavidin complex
The invention provides a food specificity IgE detection kit and a preparation method thereof. The kit comprises a test reaction plate, and a gold mark cushion and a NC (Numerical Control) film used as a solid phase carrier are arranged on the reaction plate. The kit further comprises various biotin labeled allergens independent from the test reaction plate; the gold mark cushion contains a colloidal gold marked mouse anti-human IgE monoclonal antibody; the NC film is provided with a detection region coated by a biotin labeled bovine serum albumin and a quality control region coated by a goat anti-mouse IgG polyclonal antibody; and the biotin labeled bovine serum albumin is combined with the streptavidin. The kit provided by the invention can be used for 'individualized' combined detection according to specific situation of a patient, is simple to operate and can obtain the detection result within short time.
Owner:WATSON & CRICK TIANJIN BIOTECH
Anti-human FcepsilonRIalpha subunit monoclonal antibody and application thereof
InactiveCN104212770APromote growthStrong divisionMicroorganism based processesImmunoglobulins against cell receptors/antigens/surface-determinantsAllergic dermatitisDisease
The invention discloses an anti-human FcepsilonRIalpha subunit monoclonal antibody and application thereof. A hybridoma cell strain producing the monoclonal antibody is collected in China Center for Type Culture Collection, and the collection number thereof is CCTCC NO:201211. The anti-human FcepsilonRIalpha subunit monoclonal antibody prepared by the invention can be combined with a human IgE high-affinity receptor FcepsilonRIalpha, and can be used for detecting FcepsilonRIalpha expression on the surface of a cell; and the monoclonal antibody is of great significance for prevention and treatment of mast cell and basophilic granulocyte related diseases such as allergic dermatitis, dermatomyositis, psoriasis and the like, and has great clinical application value. The hybridoma cell strain provided by the invention is favorable in cell strain growth vigor, uniform in cell body size, muddy or transparent and vigorous in division, and has a proliferative potential of infinite division.
Owner:李莉 +1
Displaying native human IgE neutralizing FcepsilonRIa-contacting IgE B-cell epitopes by constraining super beta(b)-strands and cystine knots on thermostable protein scaffold
Vaccine displaying native antigenic loops of immunoglobulin E is critical for eliciting neutralizing anti-IgE antibodies. The embodiment of the invention enables the display of native antigenic IgE receptor-contacting loops as IgE B-cell vaccines via three steps of constraining methods. The loops of multiple antigenic B-cell epitopes can be molecularly grafted in, and conformationally constrained by the energy favorable flanking beta (b)-stands, i.e., the super b-strands identified in this invention. The constrained loops can be further stabilized in replacing a selective loop within the cystine knot peptide. These dual constrained antigenic loops are then integrated onto thermostable protein scaffolds, folded in the oxidative milieu that provides further conformational constraint and high yield.
Owner:CHEN SWEY SHEN
Method for producing novel ige based reagents
InactiveUS20100311604A1High affinityStrong specificityPeptide librariesLibrary screeningEpitopeProtein engineering
This invention relates to protein engineering technology. More particularly, the present invention relates to a method for preparing human IgE antibodies and derivatives thereof, which bind epitope structures that are weakly IgG or IgM immunoreactive.
Owner:TEKNOLOGIAN TUTKIMUSKESKUS VTT
Kit detecting dog-hair allergen specific IgE antibody and method
InactiveCN105353134AImprove stabilityStrong specificityChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexClinical research
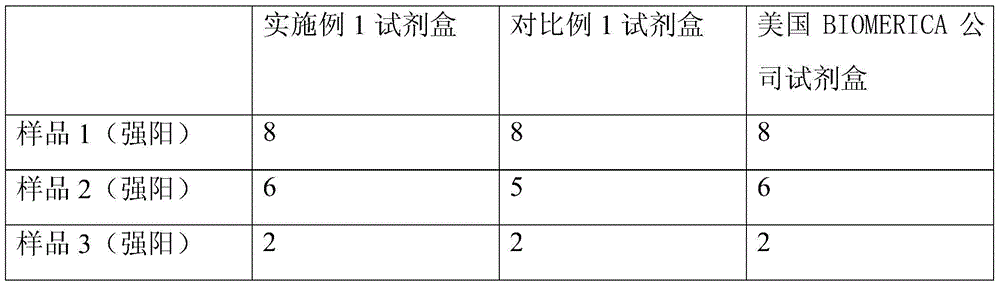
The invention discloses a kit detecting dog-hair allergen specific IgE antibody and a method. The kit comprises a calibration substance, a quality-control substance, a biotin-labeled dog-hair allergen solution, an alkaline-phosphatase-labeled mouse anti-human IgE secondary-antibody solution and a streptavidin-labeled nanometer-magnetic-particle suspension. The kit is utilized to detect the dog-hair allergen specific IgE antibody. Through the above manner, the reagent compositions in the kit are good in stability, the expiration data is one year or more, the detection sensitivity is high, the specificity is good, and variation is small. An improved unified technology is obtained after a large amount of experiments are performed for technology optimization, and production is performed strictly according to standard production operation rules and quality control rules. A user can perform standard operation and obtain a reliable result only according to the operation instruction. During clinic research, the coincidence correlation with a foreign imported reagent is up to 90% or more, and the cost only is 1 / 2 of the foreign imported reagent.
Owner:SUZHOU HAOOUBO BIOPHARML
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com