Recombinant anti human IgE monoclonal antibody and preparation method and usage thereof
A monoclonal antibody and heavy chain technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
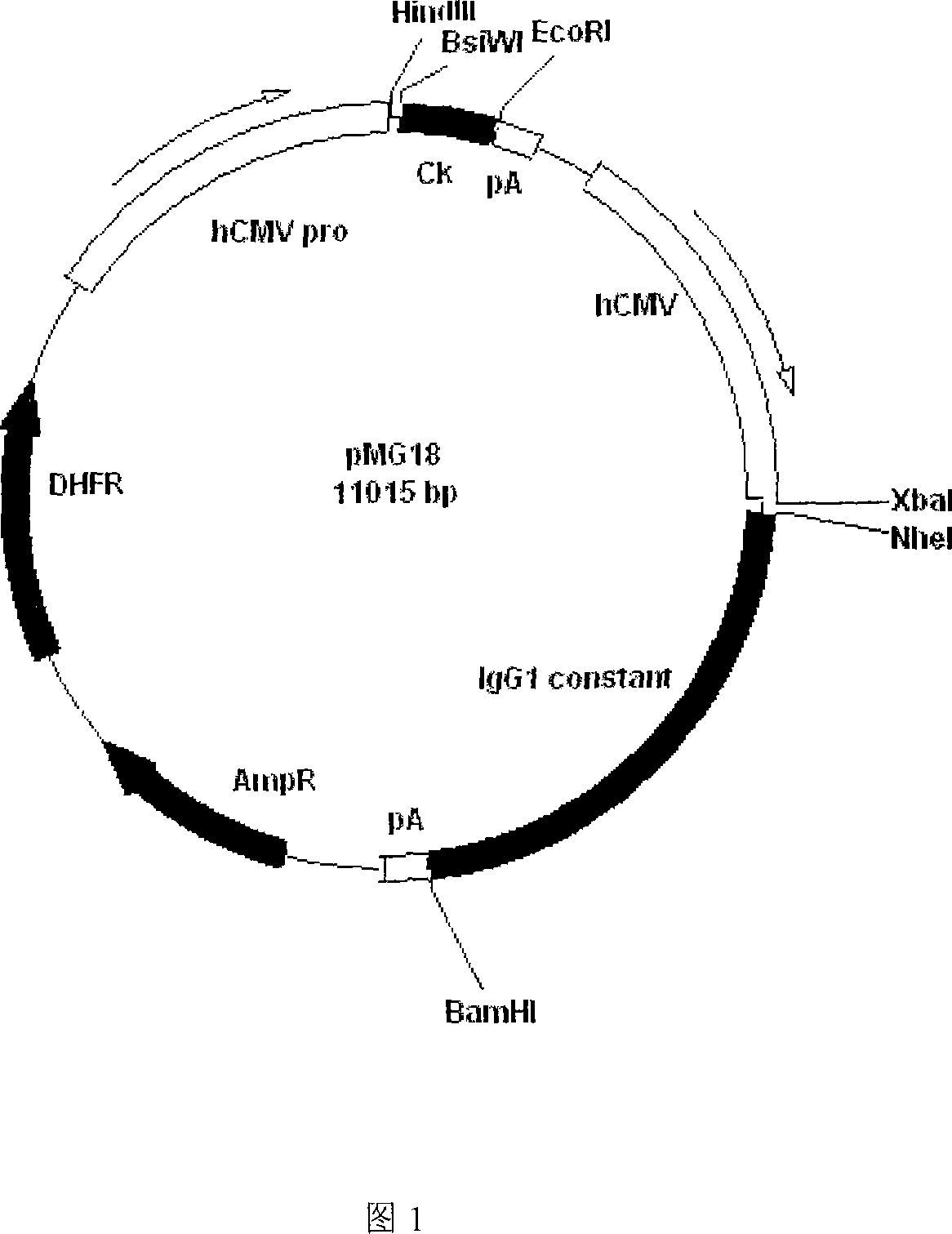
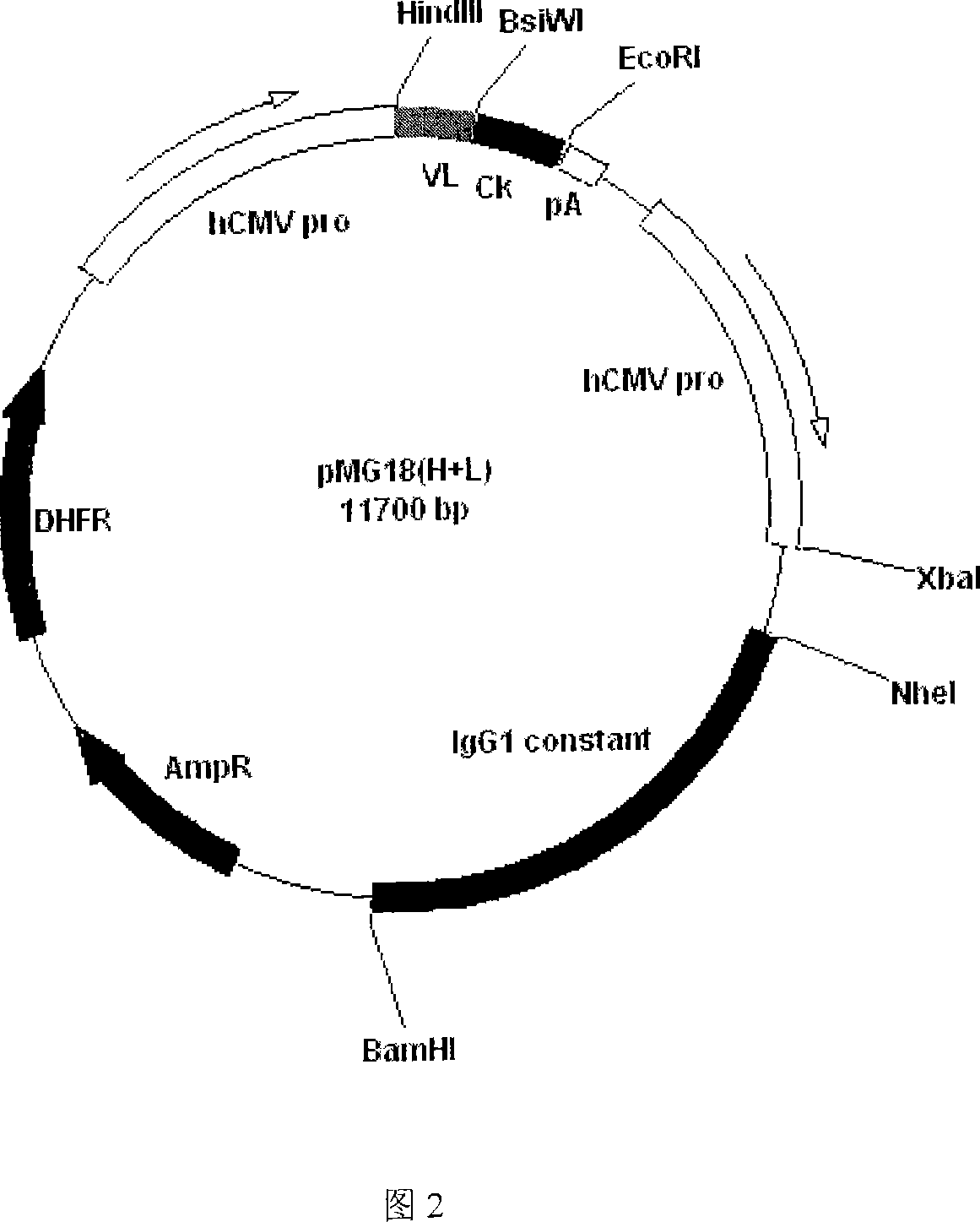
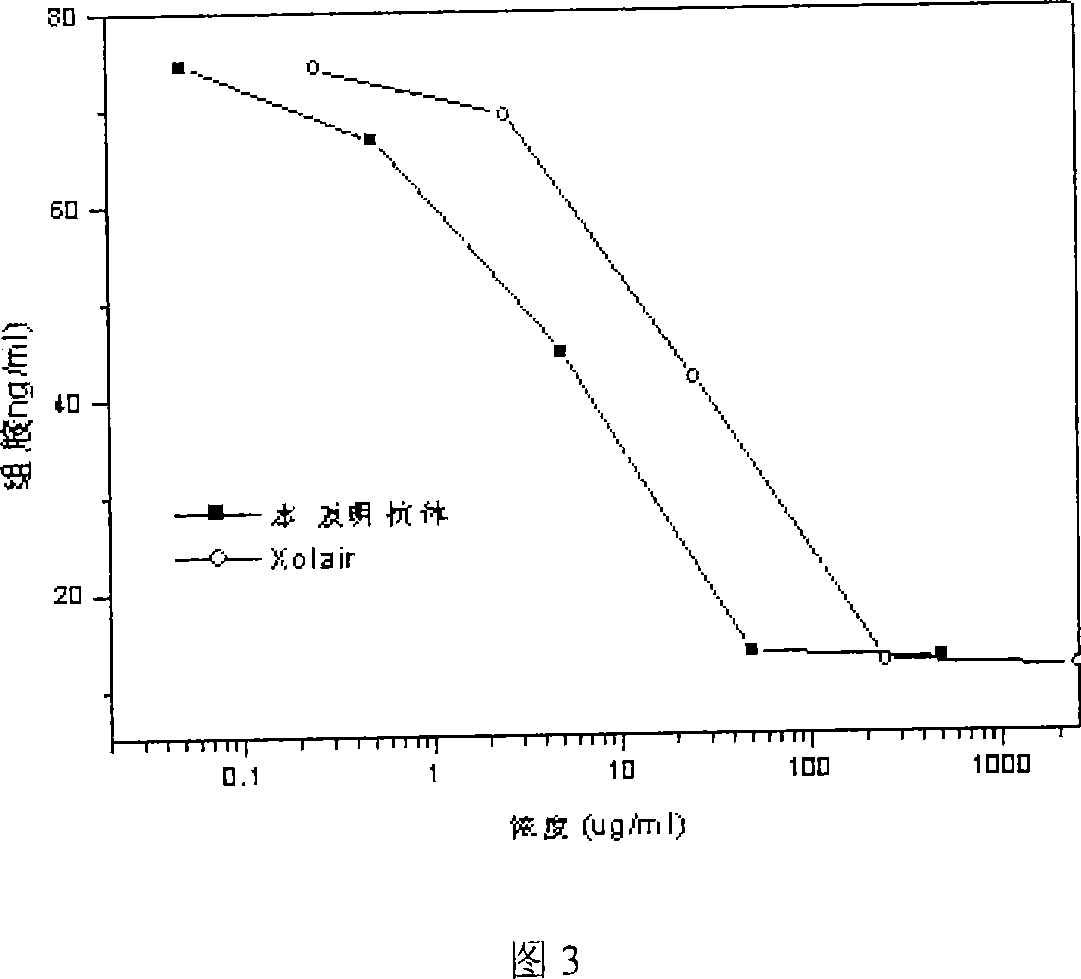
Image
Examples
Embodiment 1
[0040] Example 1 Screening the antibody gene variable region of IgE from the antibody library
[0041] 1) Construction of mouse antibody library
[0042] Human IgE (obtained by affinity chromatography of 8033 cell culture supernatant) and Freund's adjuvant were used to immunize Balb / C mice. After four times of immunization, the mouse serum was diluted 1:500 and showed a strong positive reaction with human IgE. Mouse spleen according to Marks et al. J. Mol. Biol., 222, 581-597. Hoogenboom and Winter, J. Mol. Biol., 227, 381-388. Haidaris CG et al. J Immunol Methods. 2001 Nov 1;257 (1-2): 185-202, method described in Griffiths, A.D. et al. EMBO J., 13, 3245-3260 (1994). Nissim, A. et al. EMBO J., 13, 692-698 (1994) , to construct a murine antibody library.
[0043] 2) screening
[0044] Add 1 ml of the revived antibody library strain to 14 ml of fresh LB medium, and culture in a 50 ml Erlenmeyer flask at 37°C for 16 hours.
[0045] Centrifuge at a high speed of 12000rpm for ...
Embodiment 2
[0063] Example 2 Expression Intensity of Antibody Genes in CHO Cells
[0064] The 12 high-expression candidate clones obtained from the above screening were cultured in a 10 cm tissue culture dish, and the expression level of the antibody was determined by ELISA as described below. Coat goat anti-mouse IgG (Fc) on an ELISA plate, overnight at 4°C, block with 2% BSA at 37°C for 2 hours, add the culture supernatant to be tested and a standard (mouse IgG1), incubate at 37°C for 2 hours, add HRP-goat anti-mouse IgG (κ) for binding reaction, incubated at 37°C for 1 hour, added TMB at 37°C for 10 minutes, and finally washed with H 2 SO 4 Termination reaction, test A 450 value. Table 1 below shows the expression levels of the 12 candidate clones obtained from the above screening.
[0065] Cell line number
[0066]As can be seen from Table 1 above, 8G1, 9B3 and 5H3 have very high expression levels.
Embodiment 3
[0067] Example 3 DNA sequencing of anti-IgE monoclonal antibody gene
[0068] According to the pedigree, DNA sequencing was performed on the anti-tumor necrosis factor receptor antibody gene of the 8G1 cell line obtained above. The results are as follows: SEQ ID NO: 3 shows its light chain variable region gene sequence (5' to 3', 387bp), and its deduced amino acid sequence is shown in SEQ ID NO: 1; SEQ ID NO: 3 shows the 8G1 heavy Chain variable region gene sequence ((5', to 3', 426 bp), its deduced amino acid sequence is shown in SEQ ID NO:2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com