Patents
Literature
124 results about "Immune reactivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cross-reactivity is the ability of an immune cell to attack a foreign cell that's different from the one that created it. Immune cells are made by the body to destroy disease-causing substances. Each immune cell attacks a certain type of invasive agent.
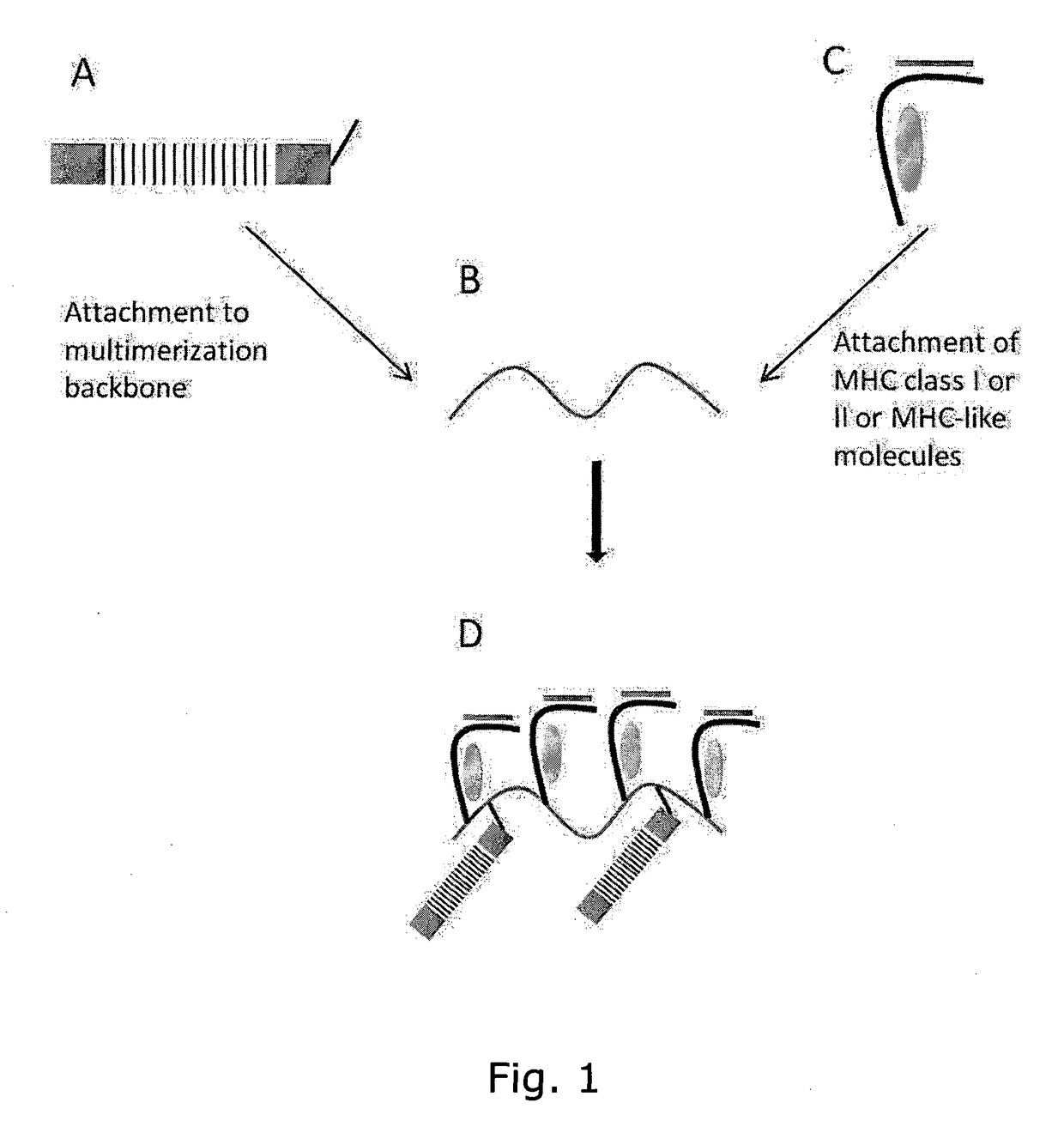
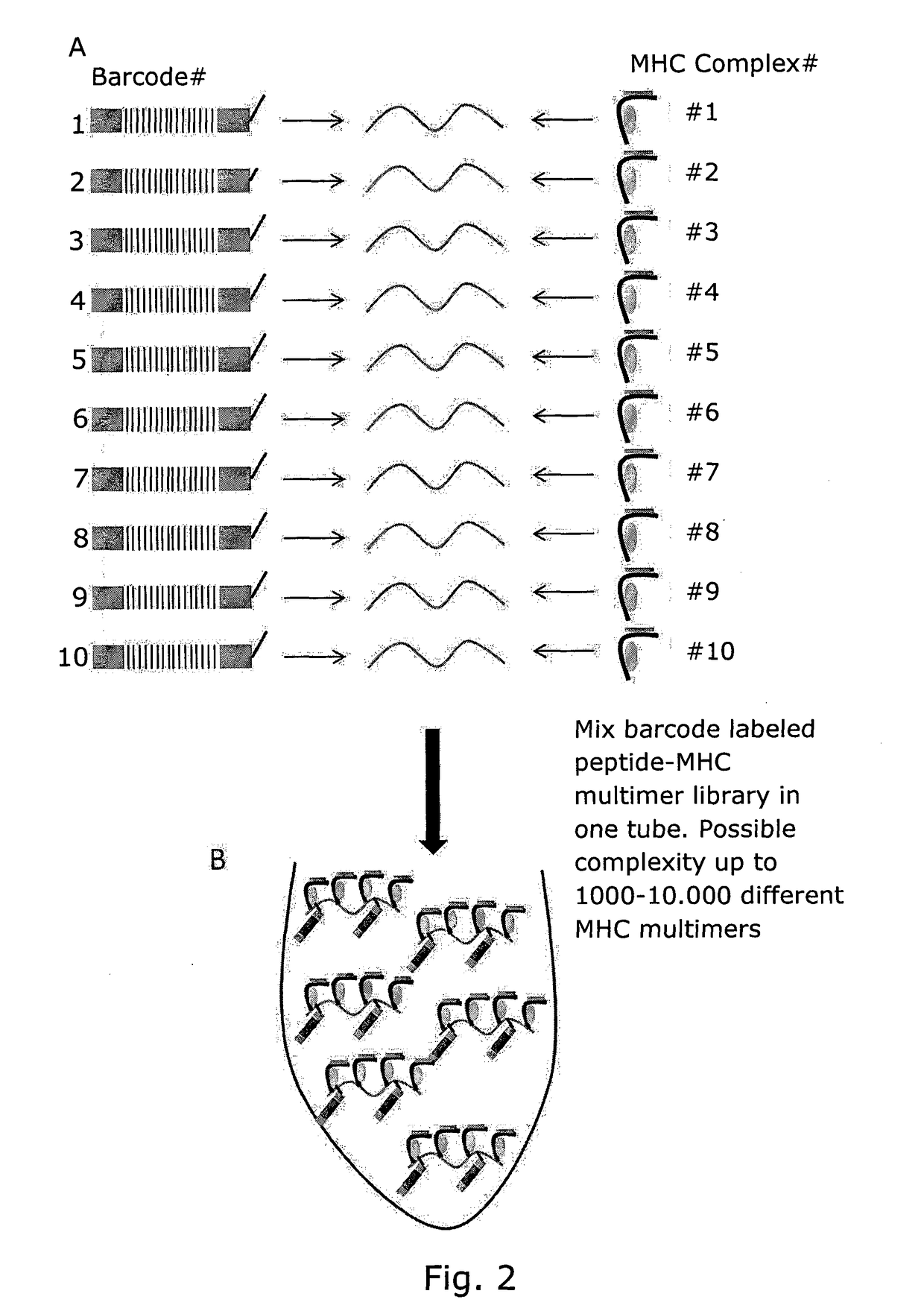
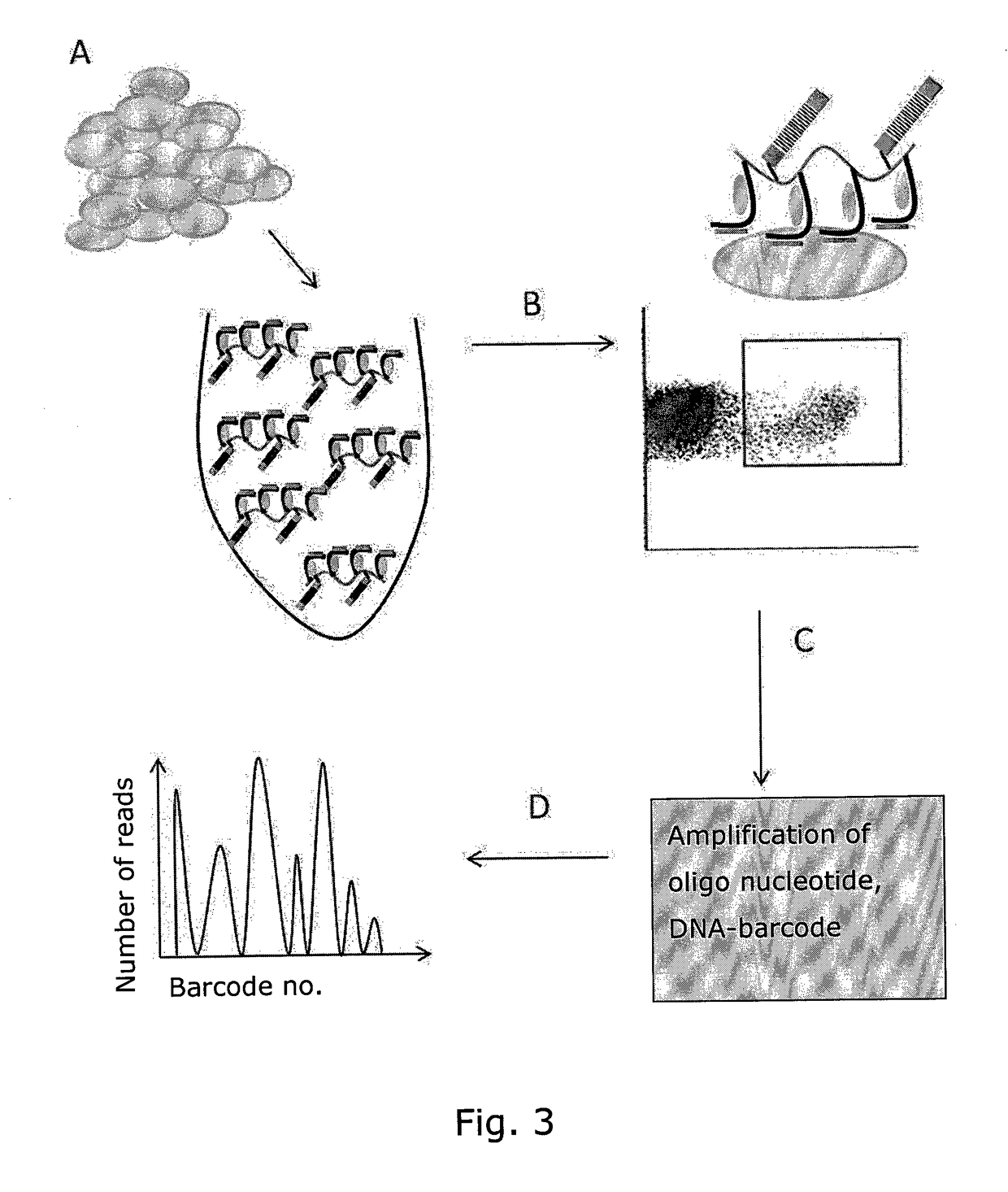
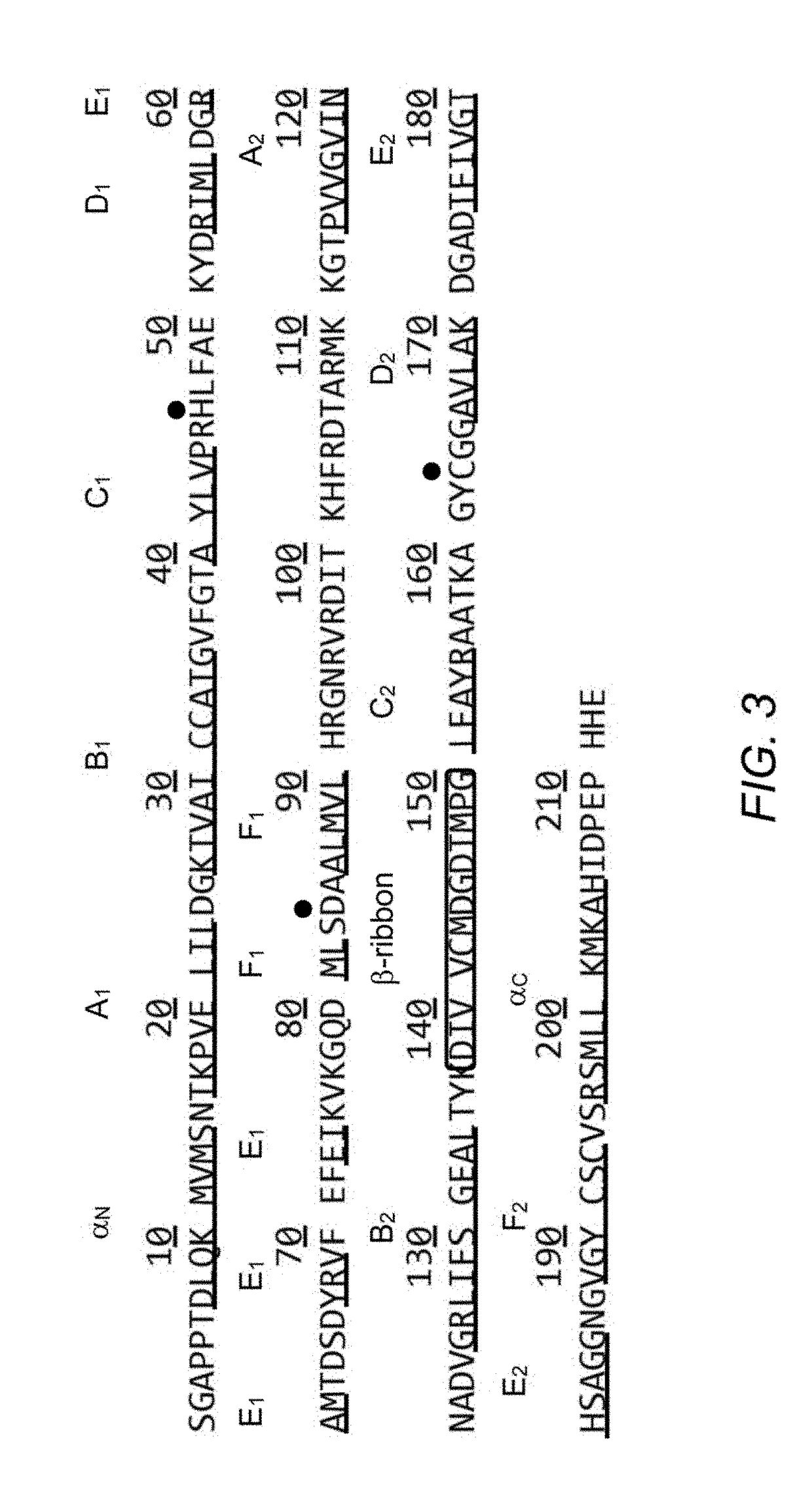
Determining Antigen Recognition through Barcoding of MHC Multimers
PendingUS20170343545A1Improve understandingMicrobiological testing/measurementBiological material analysisSingle sampleVaccination
The present invention describes the use of nucleic acid barcodes as specific labels for MHC multimers to determine the antigen responsiveness in biological samples. After cellular selection the barcode sequence will be revealed by sequencing. This technology allows for detection of multiple (potentially >1000) different antigen-specific cells in a single sample. The technology can be used for T-cell epitope mapping, immune-recognition discovery, diagnostics tests and measuring immune reactivity after vaccination or immune-related therapies.
Owner:IMMUDEX APS +1
RNA preparations comprising purified modified RNA for reprogramming cells
ActiveUS9012219B2Promote growthMicrobiological testing/measurementArtificial cell constructsGeneticsSingle strand
The present invention provides compositions and methods for reprogramming somatic cells using purified RNA preparations comprising single-strand mRNA encoding an iPS cell induction factor. The purified RNA preparations are preferably substantially free of RNA contaminant molecules that: i) would activate an immune response in the somatic cells, ii) would decrease expression of the single-stranded mRNA in the somatic cells, and / or iii) active RNA sensors in the somatic cells. In certain embodiments, the purified RNA preparations are substantially free of partial mRNAs, double-stranded RNAs, un-capped RNA molecules, and / or single-stranded run-on mRNAs.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Dual-signal independent chimeric antigen receptors (dsCAR) and uses thereof
ActiveCN103483452APromote proliferationHigh activityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorsViral infectious disease
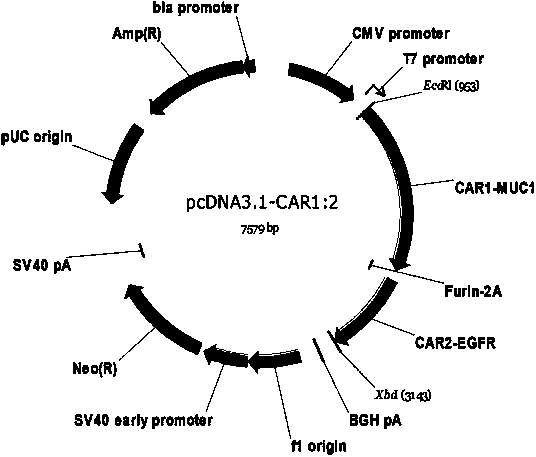
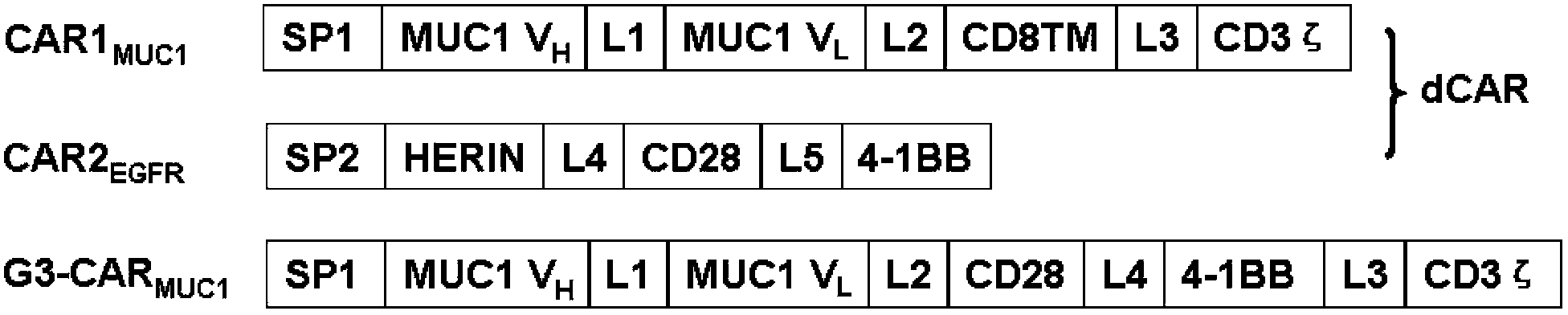
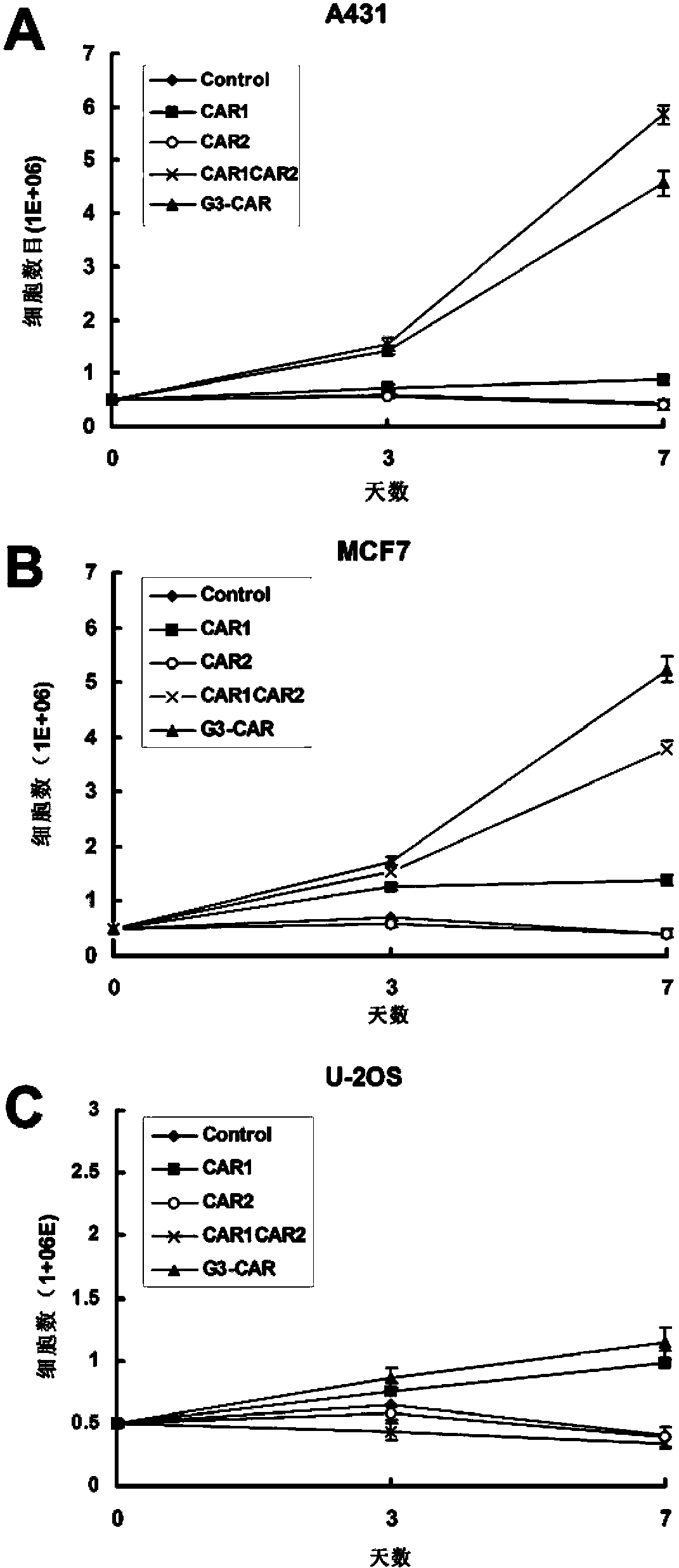
The invention relates to chimeric antigen receptors (CAR), particularly relates to dual-signal independent chimeric antigen receptors (dsCAR), and also relates to immune response cells of the dual-signal independent chimeric antigen receptors (dsCAR) and uses of the immune response cells in preparation of drugs for treatment of malignant tumor and virus infected diseases. In detail, the dual-signal independent chimeric antigen receptors (dsCAR) can respectively identify two different family antigens of tumor cells and can respectively transmit two T-cell-activation related signals. One of the CAR can transmit a first T-cell-activation related signal by combing a ligand of a tumor specific antigen or a tumor-associated antigen to decide T-cell killing specificity, and the other CAR can transmit a second T-cell-activation related signal by combing a ligand of a membrane receptor (such as EGFR (epidermal growth factor receptor) family protein) widely expressed by the tumor cells to promote T cell activation, proliferation and survival. The dual-signal independent chimeric antigen receptors (dsCAR) can avoid the potential safety problems on the basis of maintaining curative effects of second generation and third generation CAR.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
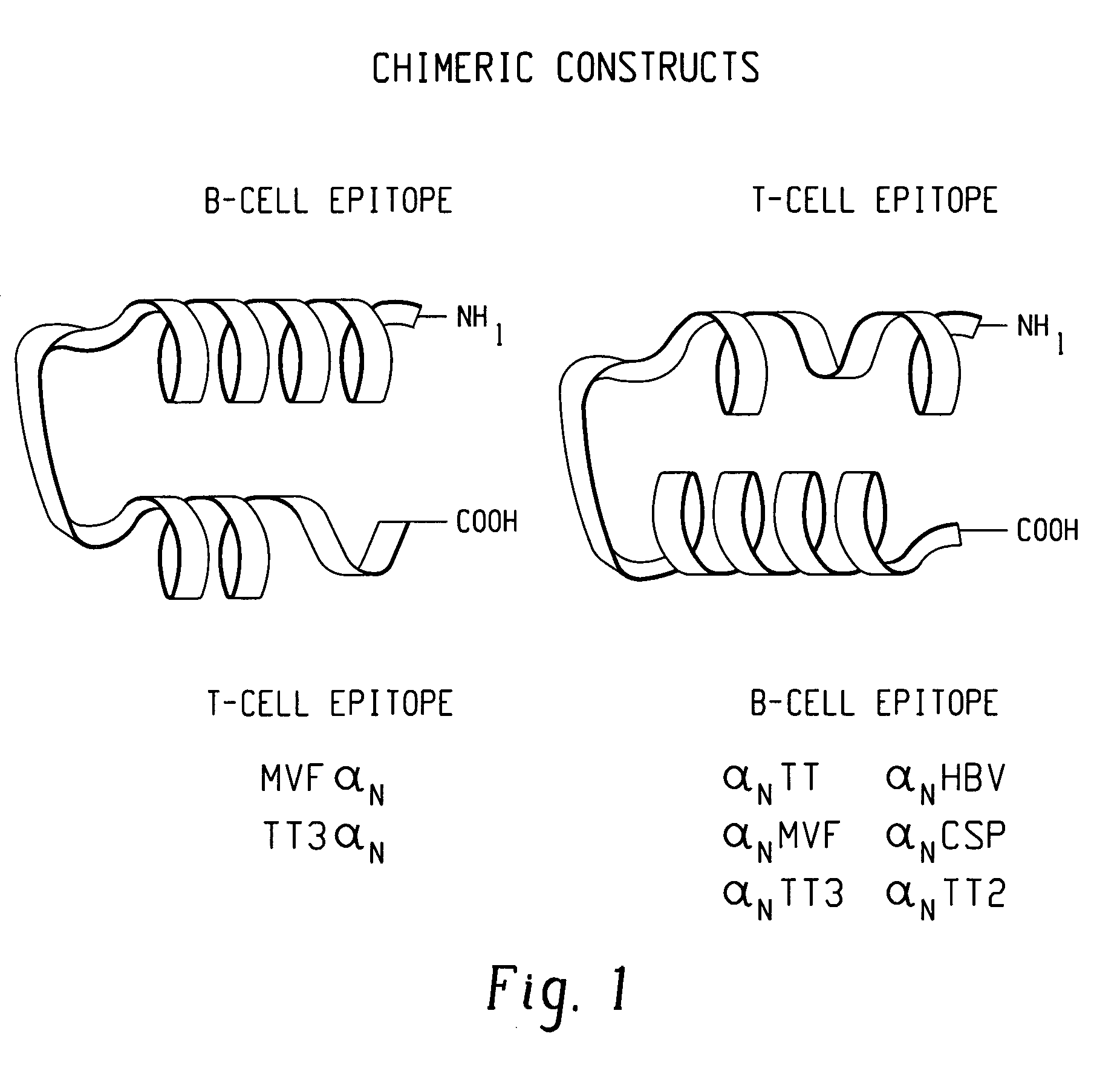
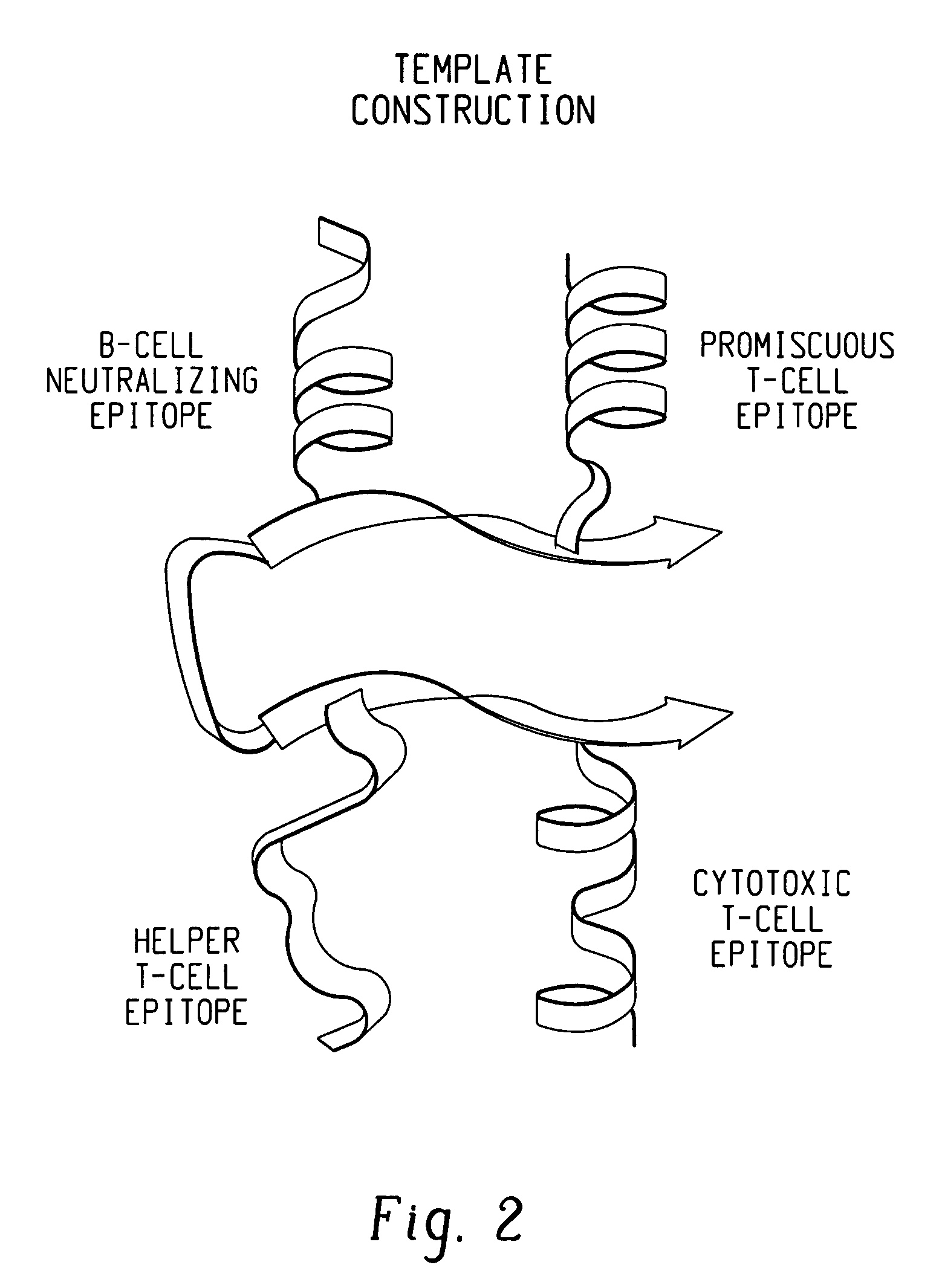
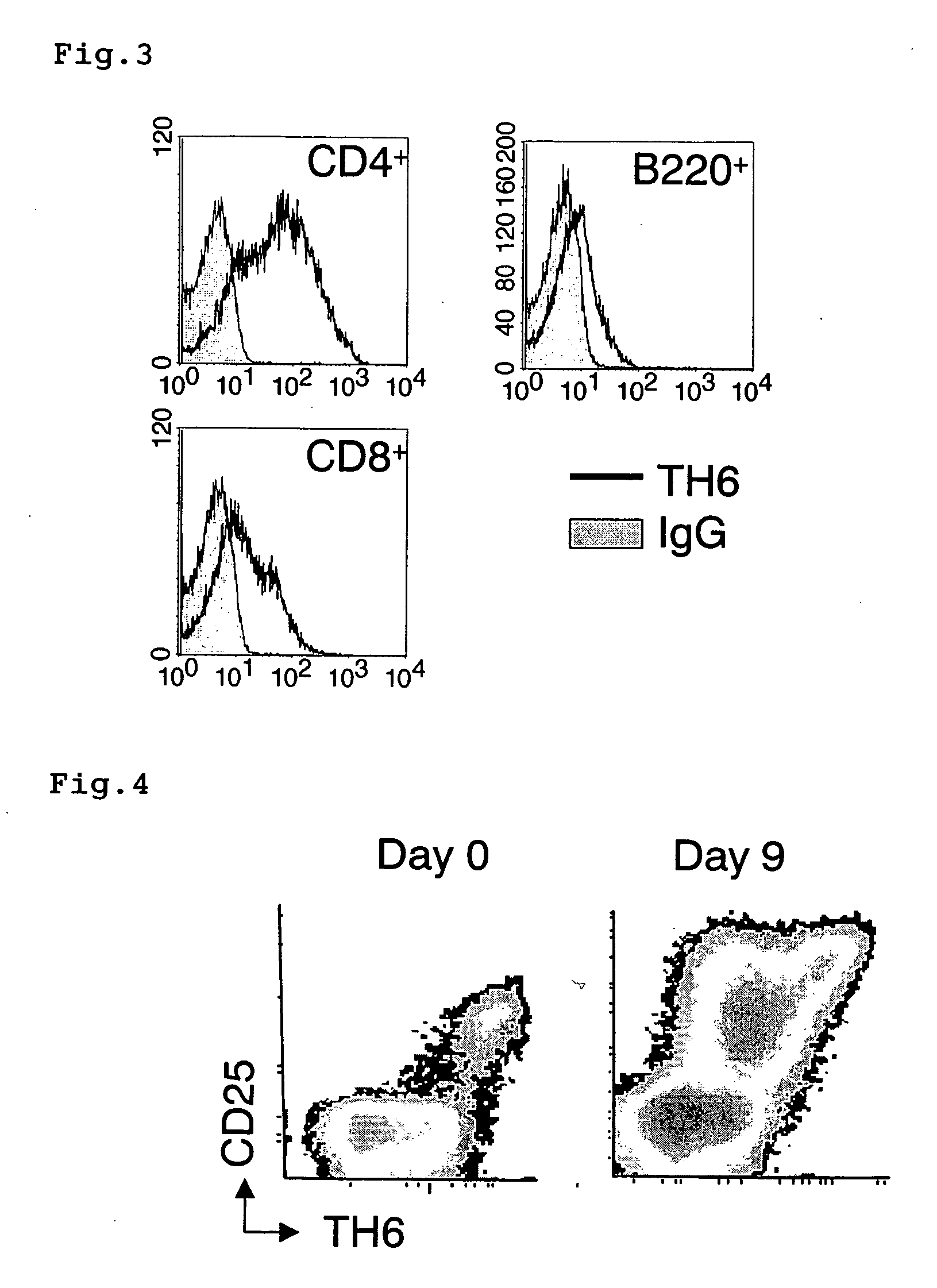
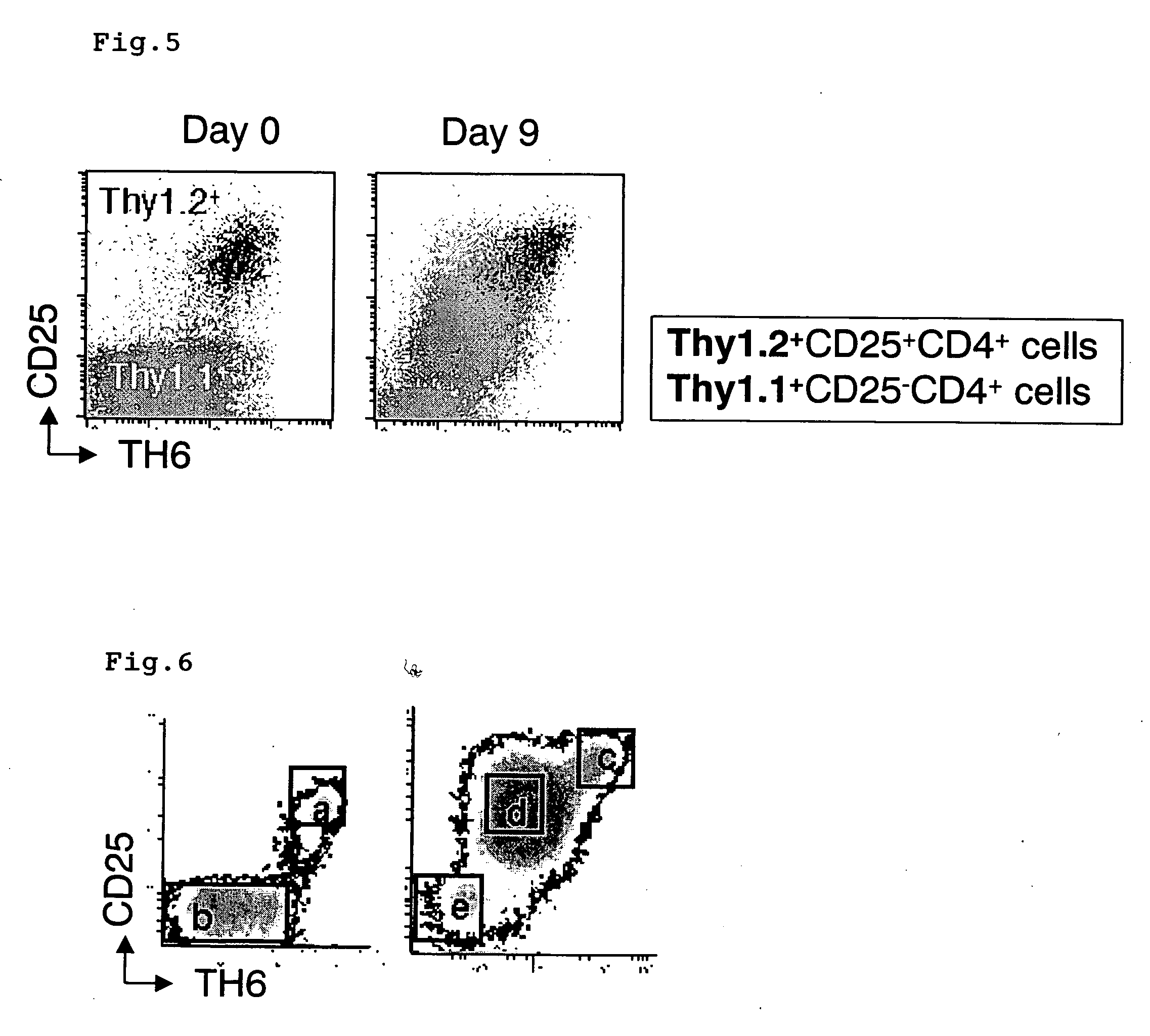
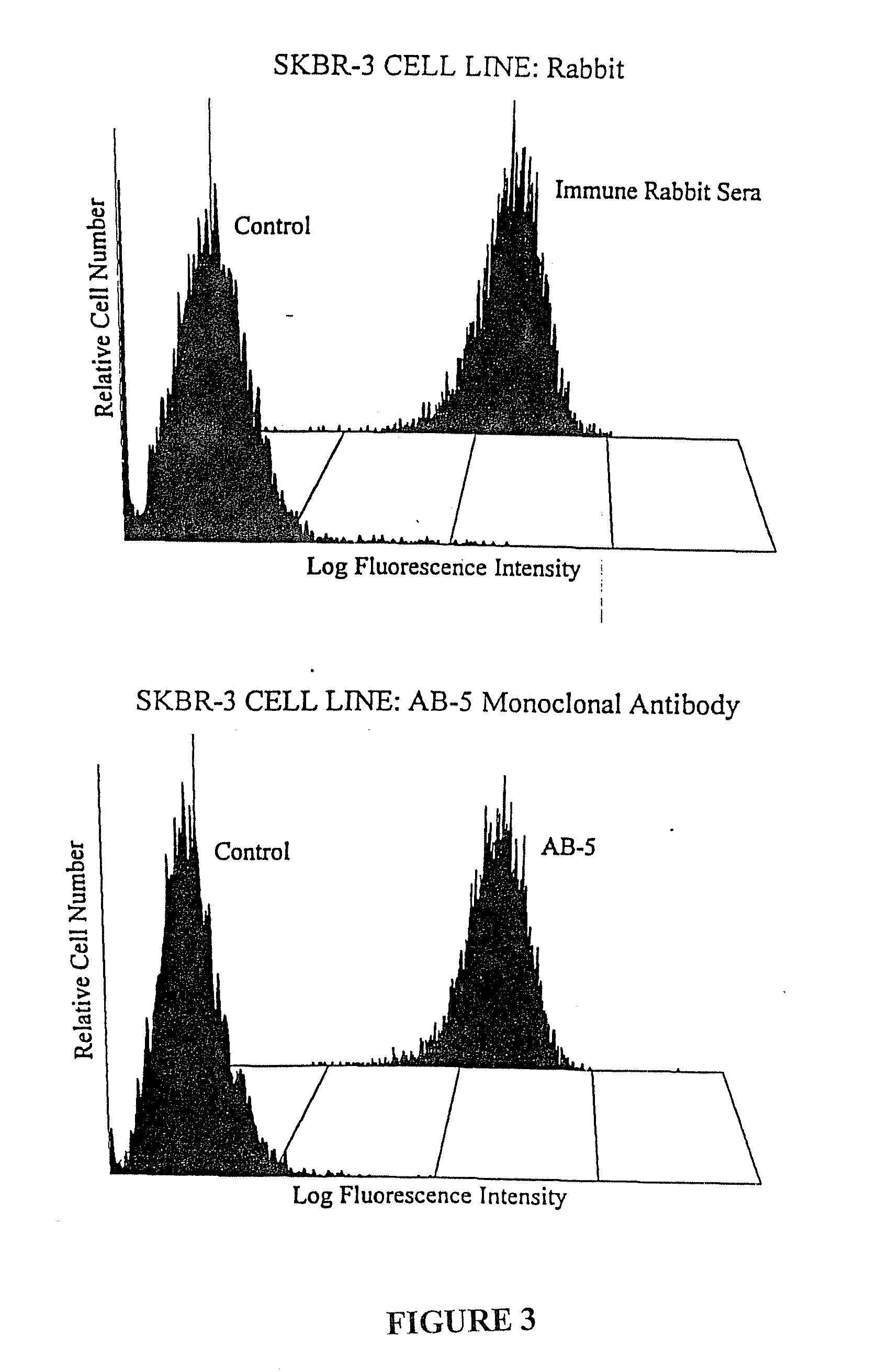
Polypeptides and polynucleotides for enhancing immune reactivity to HER-2 protein
Compositions for stimulating the immune system and for treating malignancies associated with overexpression of the HER-2 protein are provided. Such compositions include immunogenic epitopes of the HER-2 proteins and chimeric and multivalent peptides which comprise such epitopes. The present invention also relates to polynucleotides which encode the chimeric peptides. Also provided are pharmaceutical compositions comprising such immunogenic compositions. Methods for stimulating an immune response to HER-2 protein are provided. Methods for treating breast cancer, ovarian cancer, prostate cancer, colon cancer and lung cancer are provided.
Owner:OHIO STATE INNOVATION FOUND
Microfluidic devices and methods of preparing and using the same
InactiveUS20100261286A1Constant flowAccurate samplingMaterial nanotechnologyComponent separationFluorescenceCapillary action
Microfluidic devices include a photoresist layer in which an inlet chamber, an optional reaction chamber and at least one detection chamber are in fluid contact, a support arranged under the photoresist layer and a cover arranged above the photoresist layer. The devices further include a set of absorbent channels downstream of the last detection chamber. Biogenic or immunoreactive substances are placed in the reaction chamber and detection chamber(s). When a liquid sample is dropped into the inlet chamber, the sample liquid is drawn through the devices by capillary action. Detection methods include electrochemical detection, colorimetric detection and fluorescence detection.
Owner:NANO DITECH CORP
Modified mrnas encoding cell-penetrating polypeptides
InactiveUS20140371302A1Polypeptide with localisation/targeting motifSugar derivativesCell biologyNucleic acid
This invention relates to modified nucleic acid compositions encoding cell-penetrating polypeptides to provoke an innate immune response in a cell and methods of delivering protein-binding partners to target cells.
Owner:MODERNATX INC
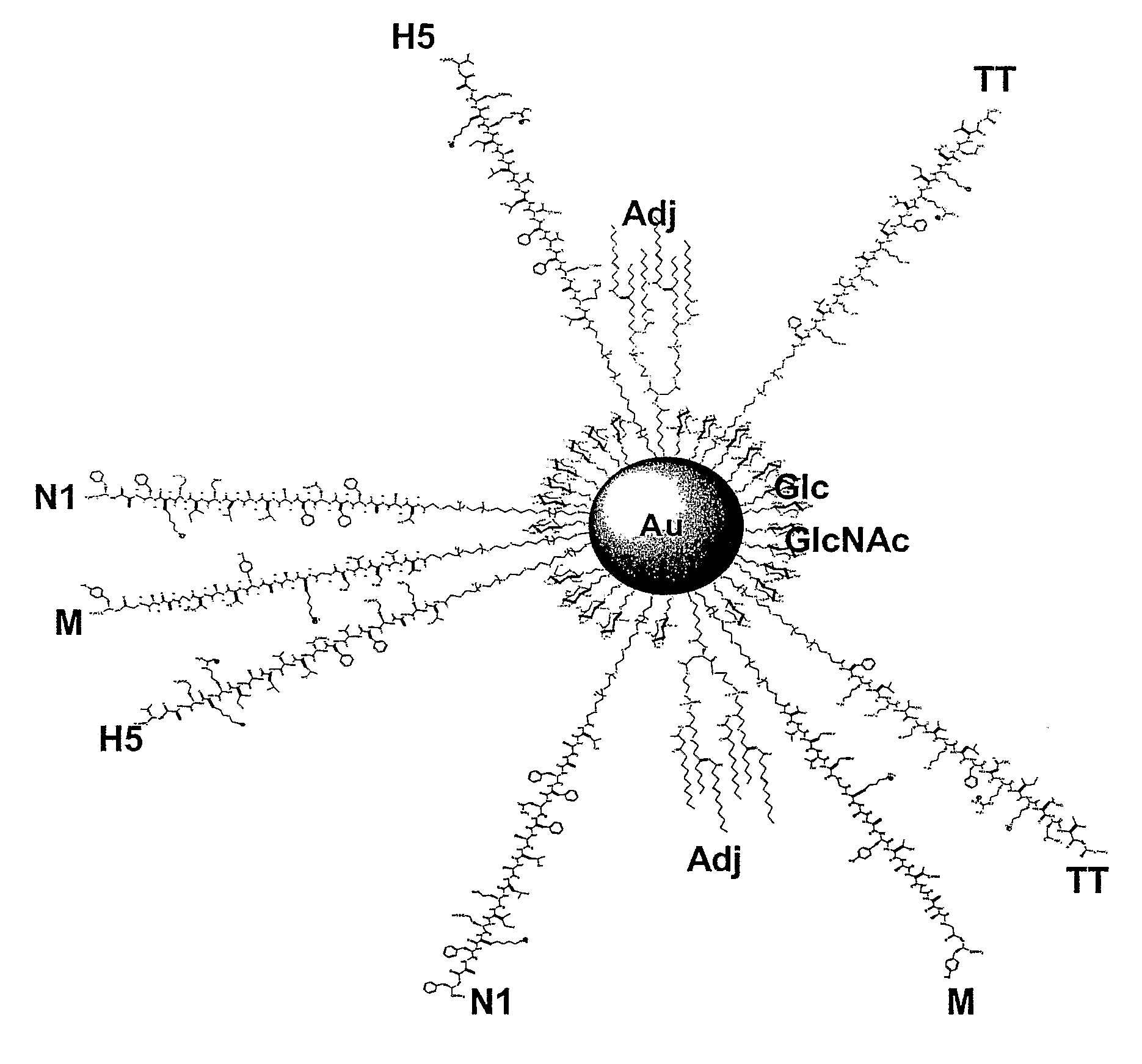
Nanoparticles for providing immune responses against infectious agents
ActiveUS20090297614A1Strong immune responseConvenience needsPowder deliveryHeavy metal active ingredientsNanoparticleT cell
Nanoparticles for providing immune responses for the treatment or prophylaxis of infection by infectious agents such as viruses, parasites, bacteria, prions and fungi are described which comprises a core including metal and / or semiconductor atoms, wherein the core is covalently linked to a plurality of ligands, the ligands including a carbohydrate residue capable of stimulating an innate immune response, a T cell helper peptide and a danger signal. This platform may then be adapted by including one or more further ligands capable of producing a specific response to a target infectious agent.
Owner:MIDATECH LTD
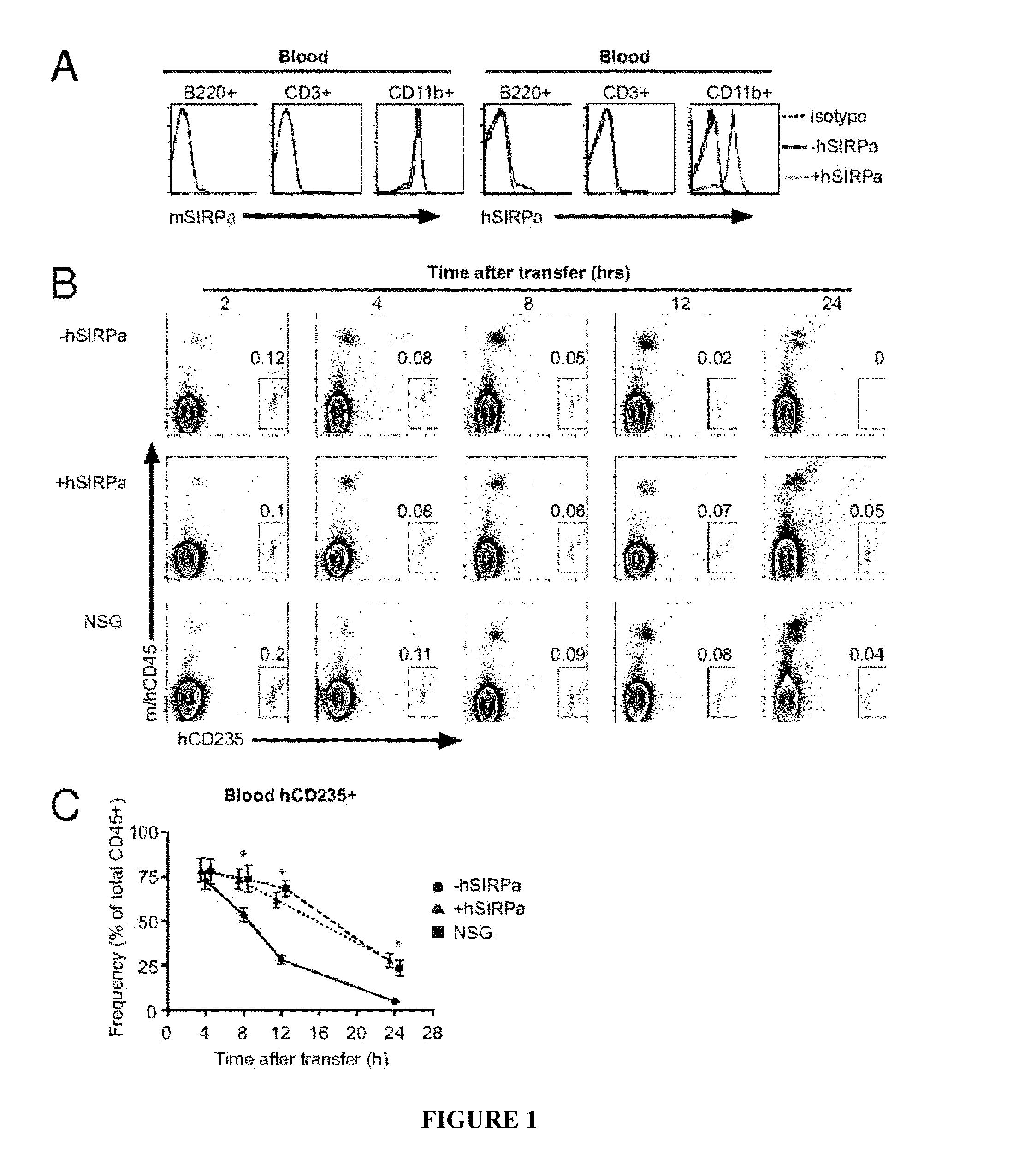
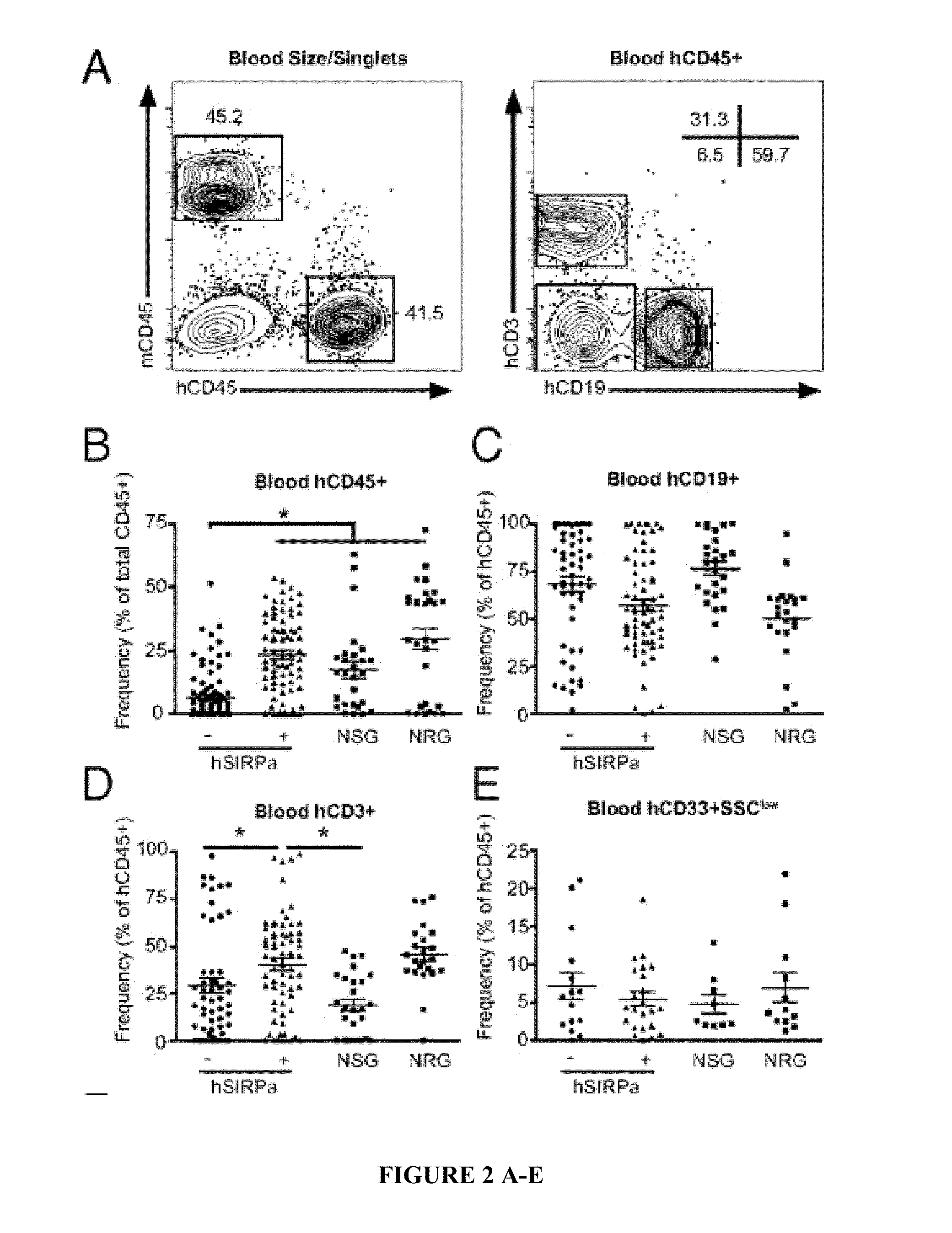
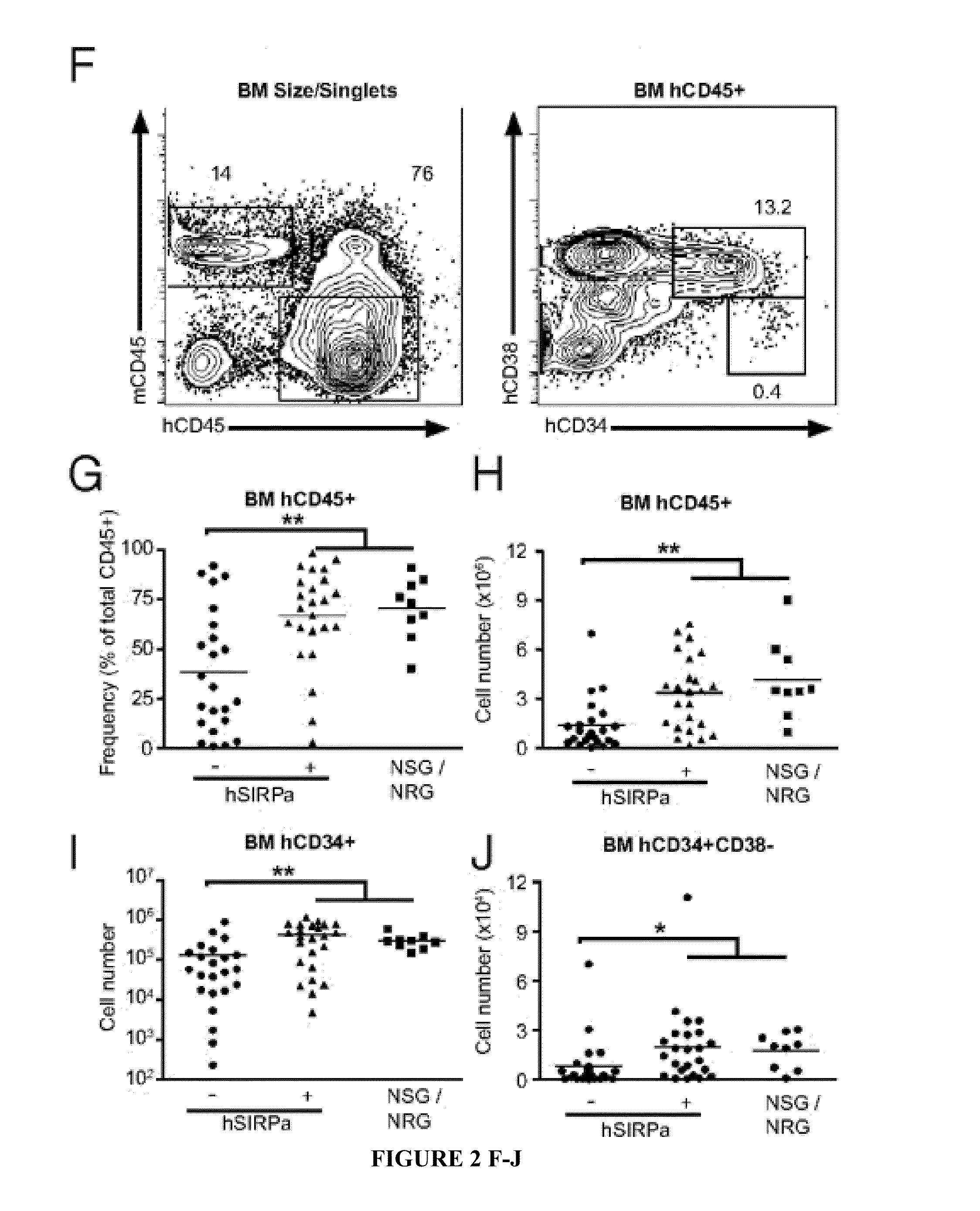
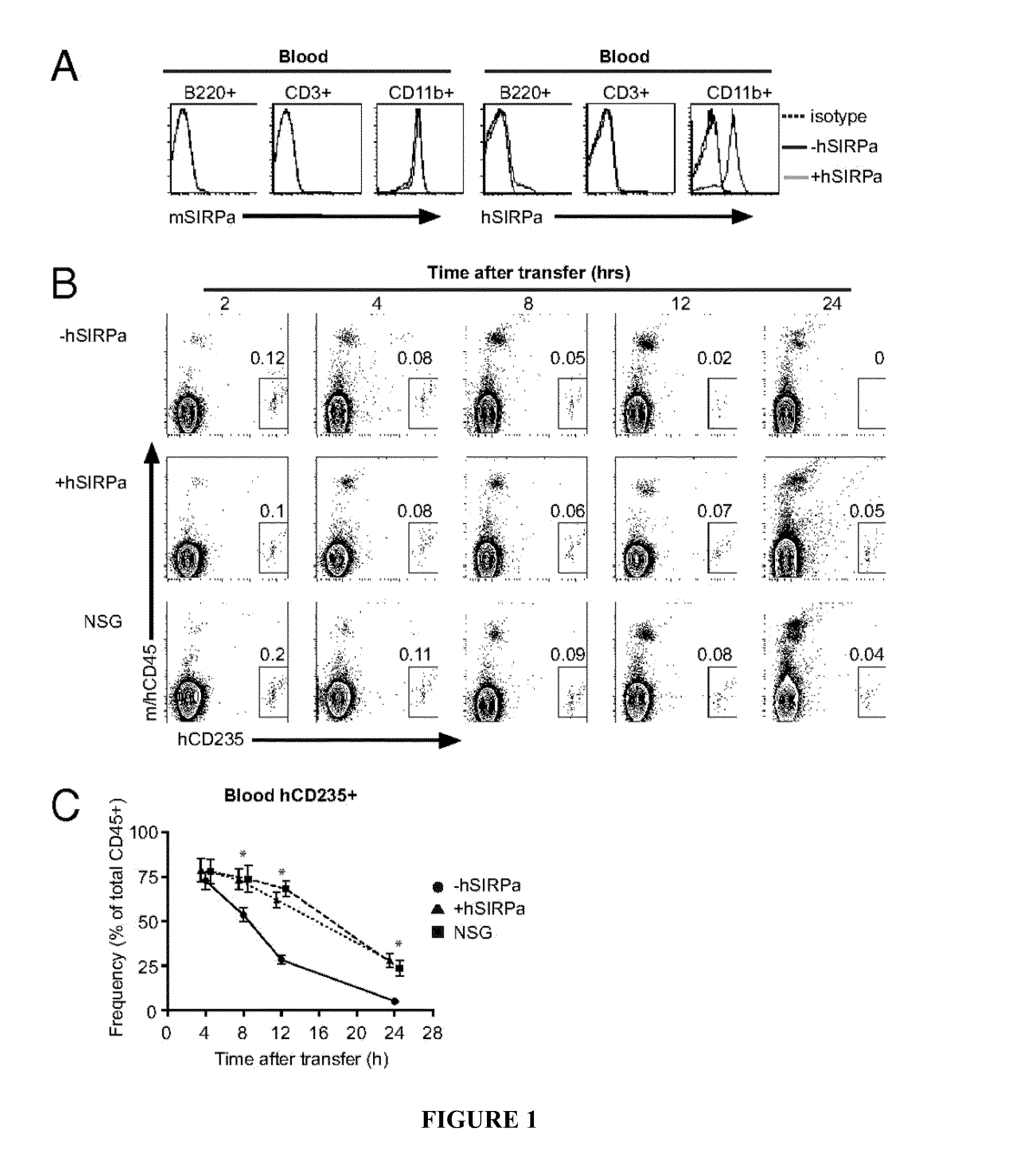
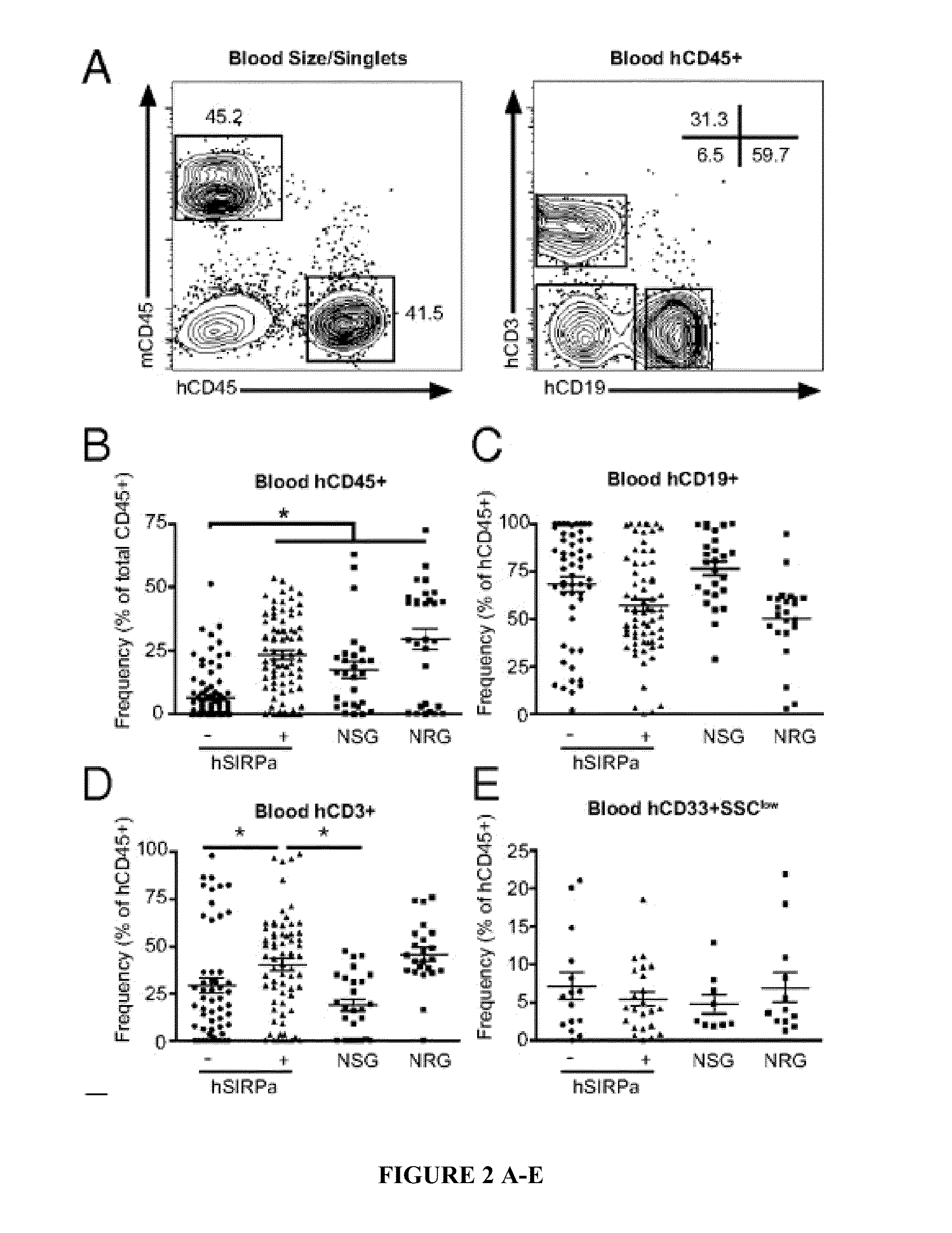
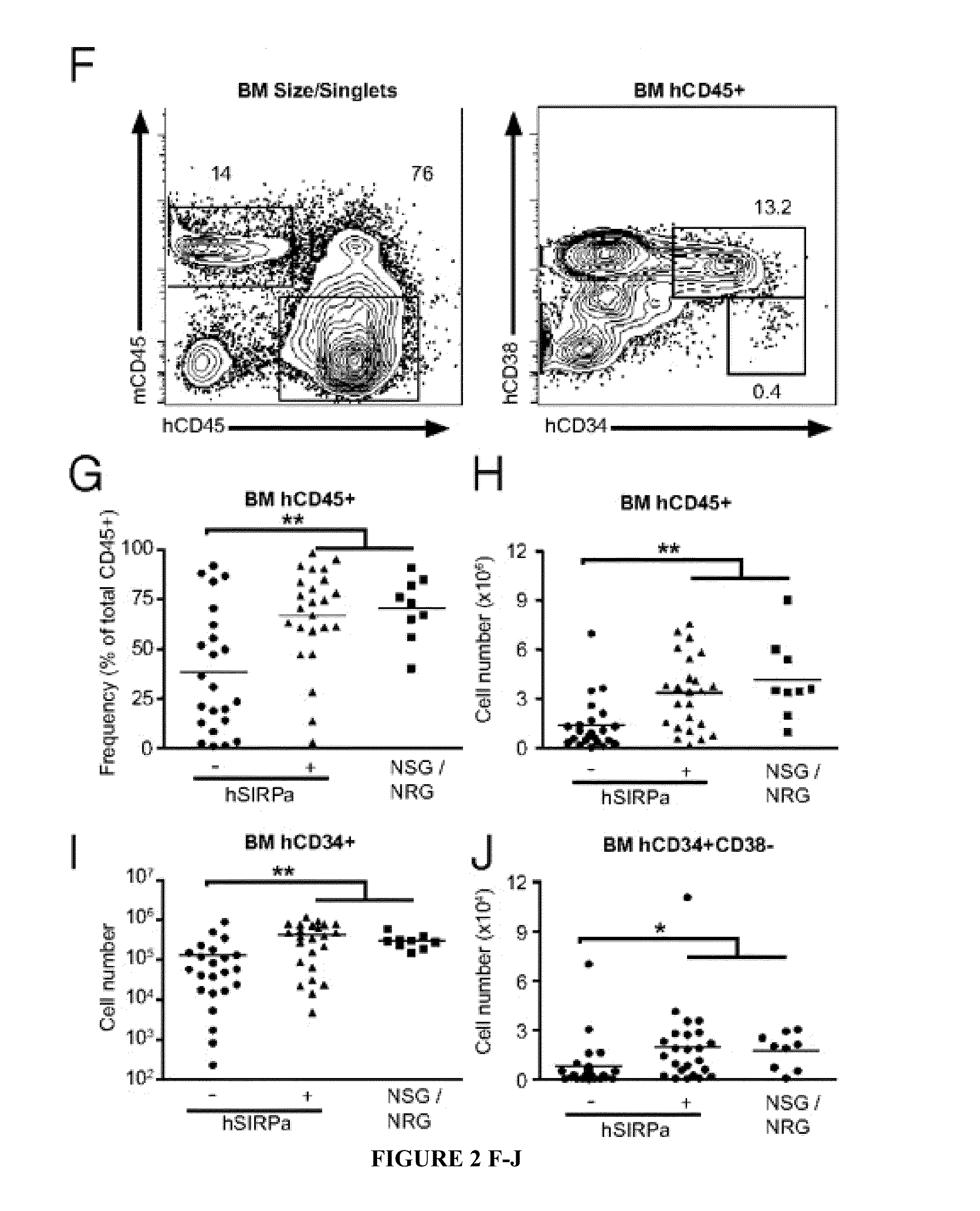
Human SIRPAalpha Transgenic Animals and Their Methods of Use
The invention relates generally to compositions and methods of using transgenic non-human animals expressing human SIRPα that are engrafted with a human hematopoietic system. In various embodiments, the human hematopoietic system engrafted, human SIRPα transgenic non-human animals of the invention are useful as systems for the in vivo evaluation of the growth and differentiation of hematopoietic and immune cells, for the in vivo assessment of an immune response, for the in vivo evaluation of vaccines and vaccination regimens, for in vivo production and collection of immune mediators, including human antibodies, and for use in testing the effect of agents that modulate hematopoietic and immune cell function.
Owner:YALE UNIV +1
Human SIRPAalpha transgenic animals and their methods of use
The invention relates generally to compositions and methods of using transgenic non-human animals expressing human SIRPα that are engrafted with a human hematopoietic system. In various embodiments, the human hematopoietic system engrafted, human SIRPα transgenic non-human animals of the invention are useful as systems for the in vivo evaluation of the growth and differentiation of hematopoietic and immune cells, for the in vivo assessment of an immune response, for the in vivo evaluation of vaccines and vaccination regimens, for in vivo production and collection of immune mediators, including human antibodies, and for use in testing the effect of agents that modulate hematopoietic and immune cell function.
Owner:YALE UNIV +1
Targeting of innate immune response to tumor site
The invention provides microparticles or nanoparticles for treatment of tumors comprising: (i) a targeting agent to the tumor or the tumor environment; and (ii) at least one inducer that stimulates a desired immune response in the tumor environment, leading to tumor apoptosis, wherein components (i) and (ii) are non-covalently or covalently attached to the surface of said microparticles or nanoparticles. The targeting agent is an agent that recognizes and binds to an antigen, a receptor or other molecules found on the surface of tumor cells or in the tumor environment and are preferably antibodies.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT +1
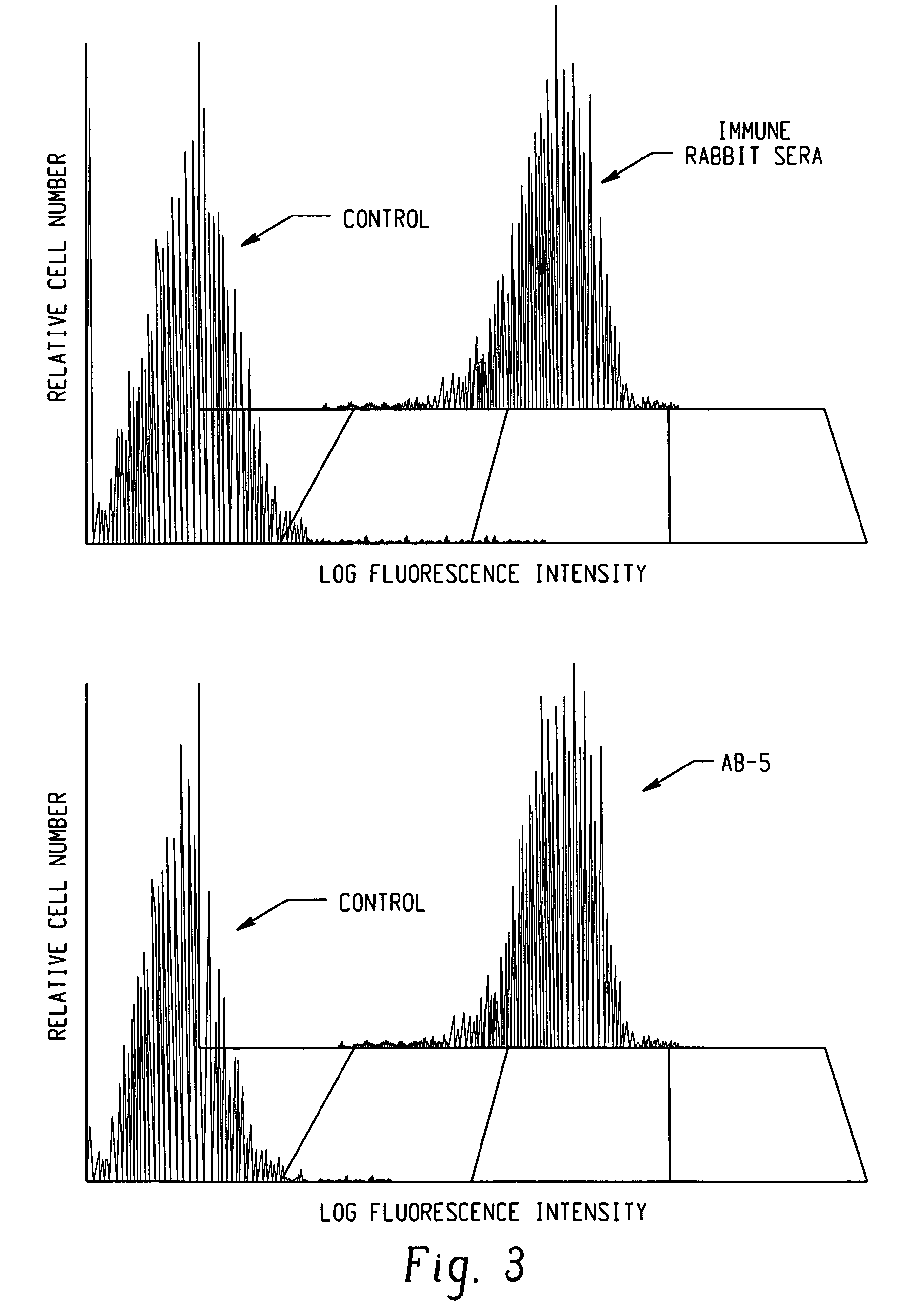
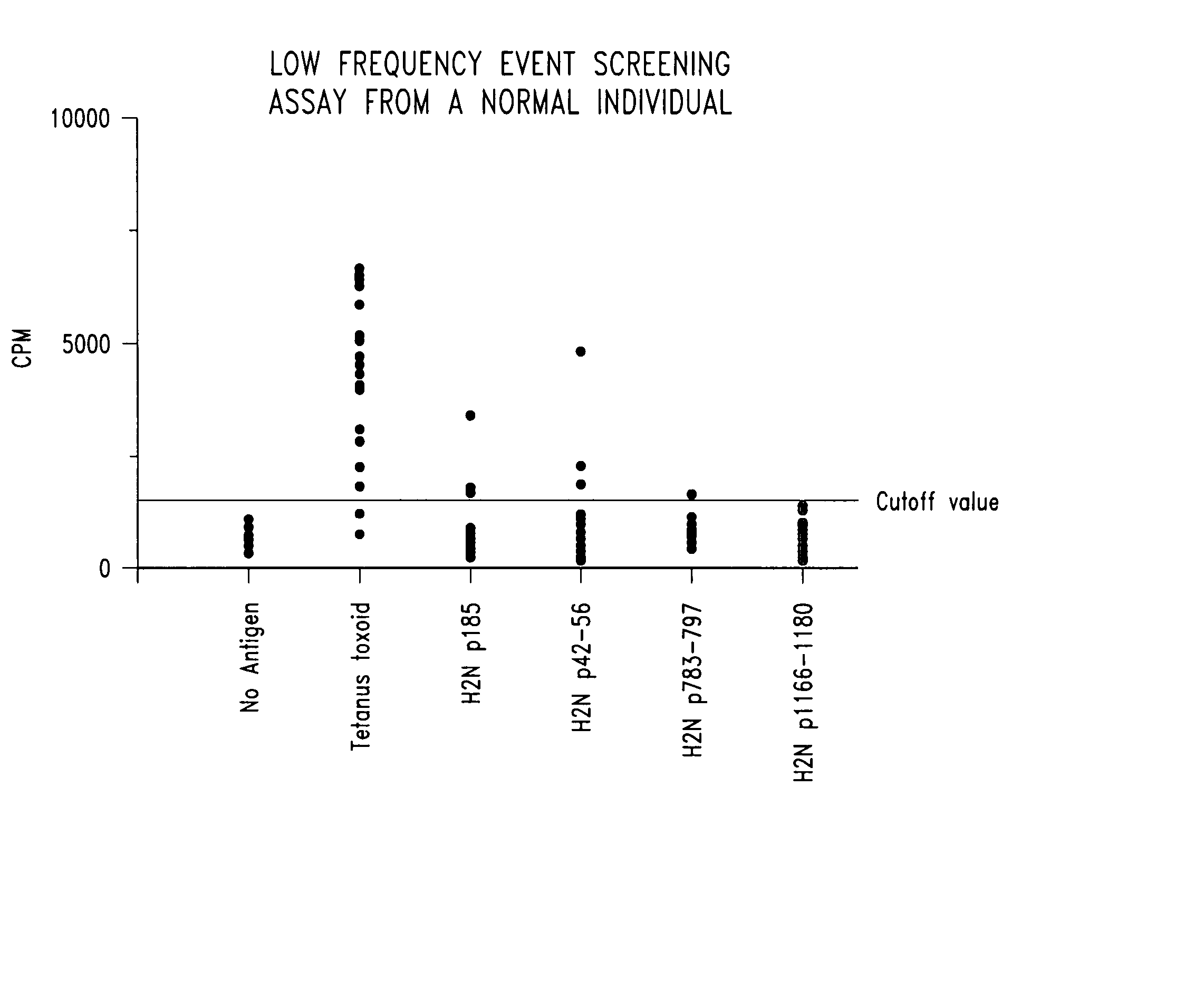
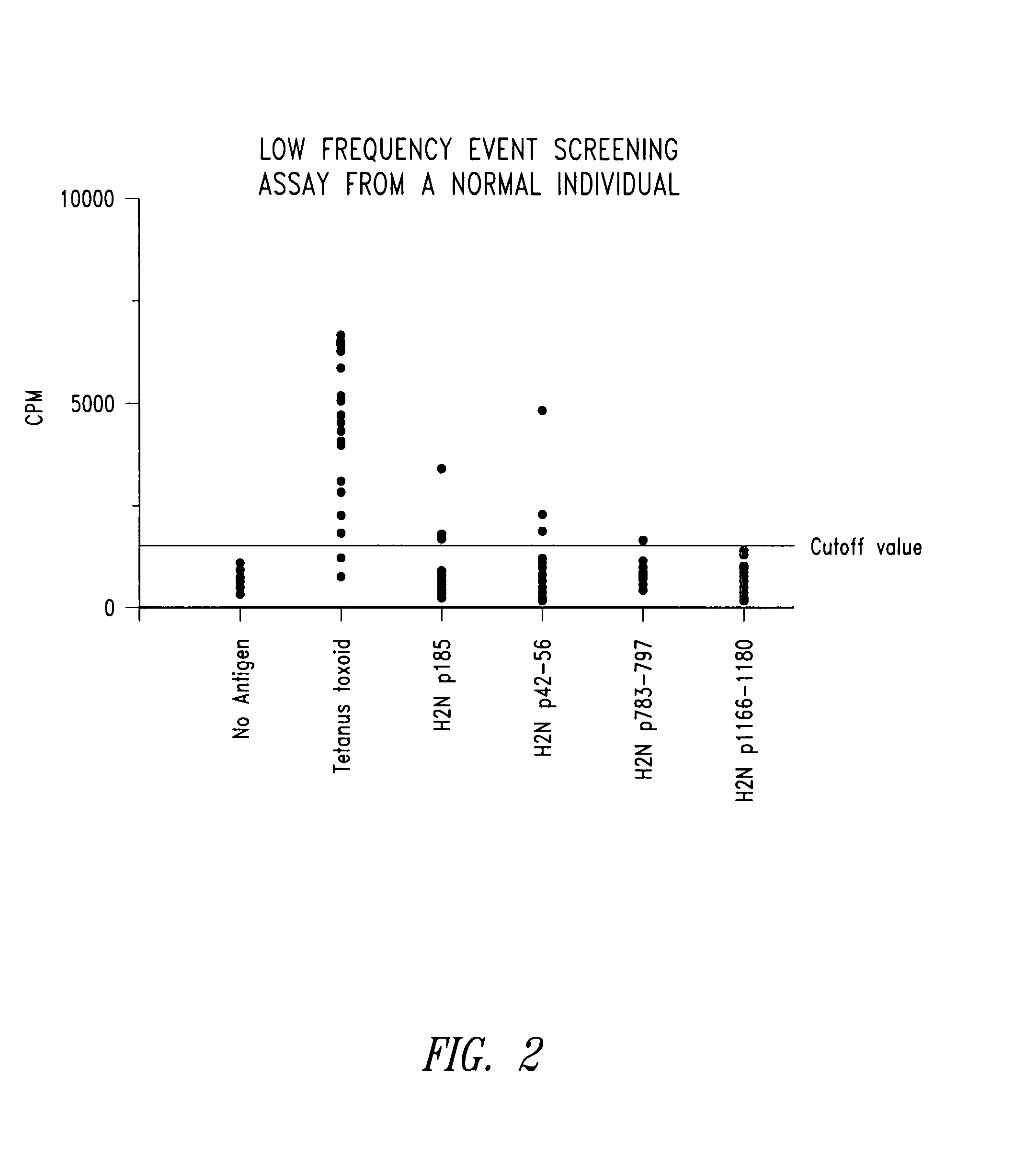
Diagnostic assay for measuring a cell mediated immune response
ActiveUS20050014205A1Reduce handling costsReduce exposureMicrobiological testing/measurementBiological testingWild lifeCell-mediated immune response
The present invention relates generally to a diagnostic assay and, more particularly, an assay for measuring cell-mediated immune reactivity. Even more particularly, the present invention provides an assay and a kit for measuring a cell-mediated response to an antigen using whole blood or other suitable biological sample. The assay may be conducted using ligands to immune effector molecules or at the nucleic acid level, screening for expression of genes encoding the immune effector molecules. The assay is useful in therapeutic and diagnostic protocols for human, livestock and veterinary and wild life applications.
Owner:CELLESTIS
Modified Foot-And-Mouth Disease Virus 3C Proteases, Compositions And Methods Thereof
ActiveUS20180066235A1Poor recombinant yieldLow toxicitySsRNA viruses positive-senseViral antigen ingredientsNucleotideVirus-like particle
This application is directed generally to foot-and-mouth disease virus (FMDV) 3C proteases that have been modified by mutating a polynucleotide sequence coding for the FMDV 3C protease. The modified FMDV proteases exhibit proteolytic activity on FMDV P1 precursor protein and exhibit a reduction in one or more toxic or inhibitory properties associated with an unmodified FMDV 3C protease on a host cell used to recombinantly produce it. Vectors carrying polynucleotides encoding modified FMDV 3C protease sequences can induce production of FMDV virus-like particles in a host cell when expressed in the host cell. The modified FMDV 3C proteases can generally be used to produce immunogenic FMDV preparations capable of inducing an immune response against FMDV.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF HOMELAND SECURITY
Pharmaceutical composition for treatment and/or prevention of cancer
ActiveUS20150050283A1Reduce usageUseful in treatmentSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenic proteinActive ingredient
The invention provides an antibody targeting a cancer antigenic protein specifically expressed on the surface of cancer cells and use thereof in a therapeutic and / or preventive agent for cancer. Specifically, this invention provides an antibody or a fragment thereof which has immunological reactivity with a partial CAPRIN-1 polypeptide consisting of the amino acid sequence shown in SEQ ID NO: 5 or an amino acid sequence having 80% or higher sequence identity to the amino acid sequence, and a pharmaceutical composition for treatment and / or prevention of cancer, comprising the antibody or fragment thereof as an active ingredient.
Owner:TORAY IND INC
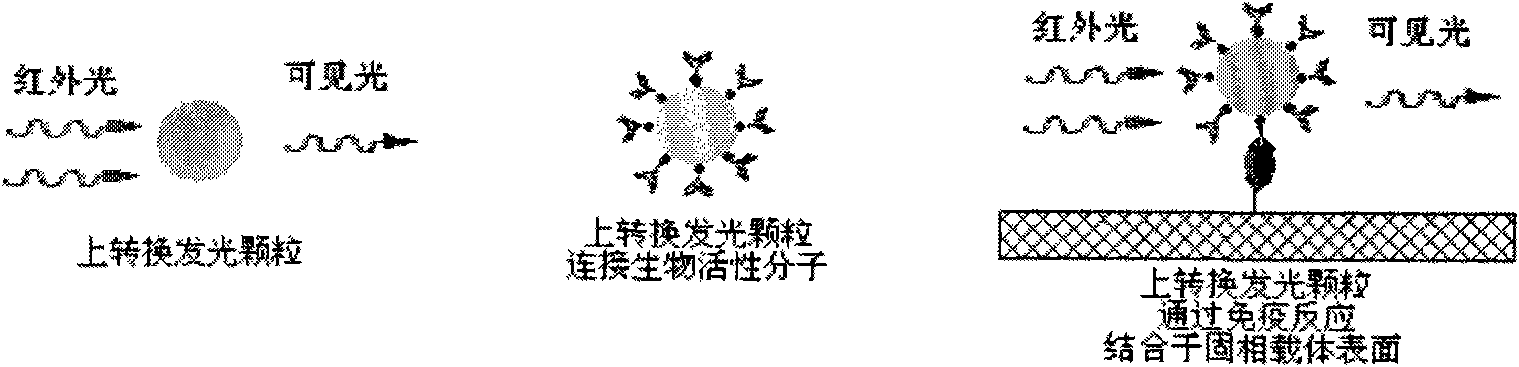
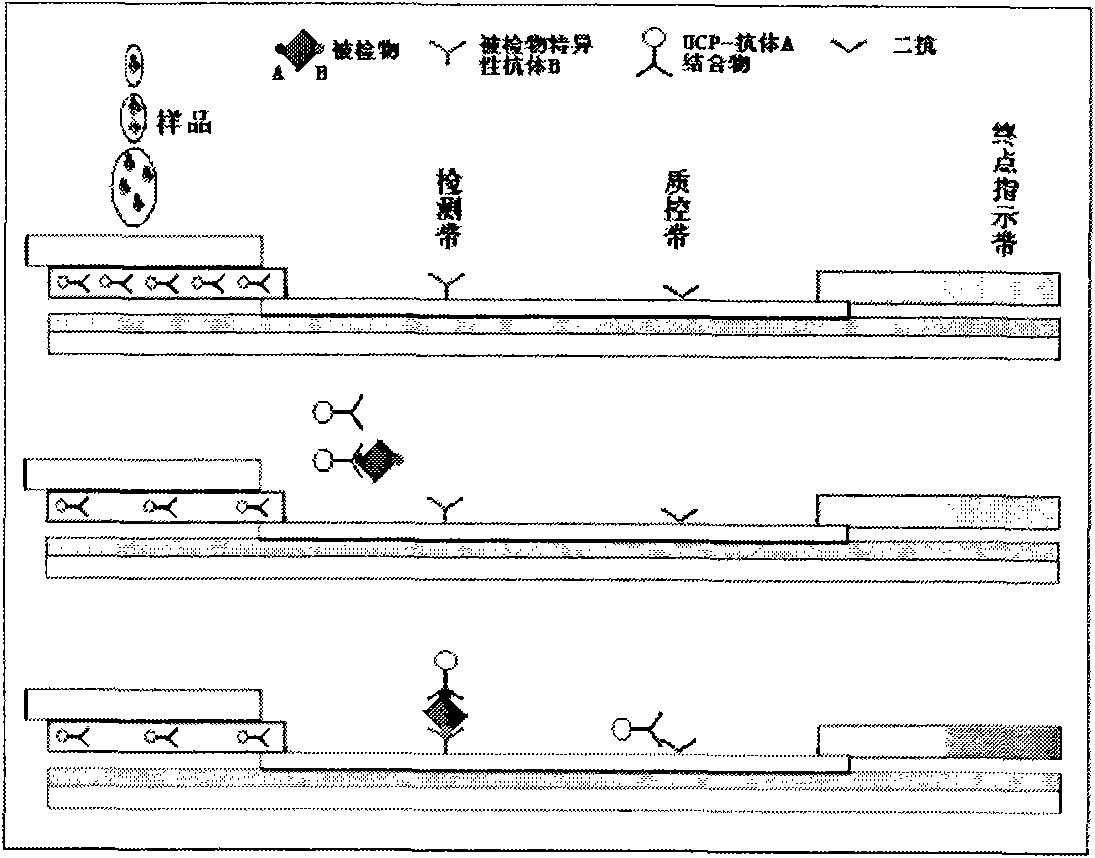
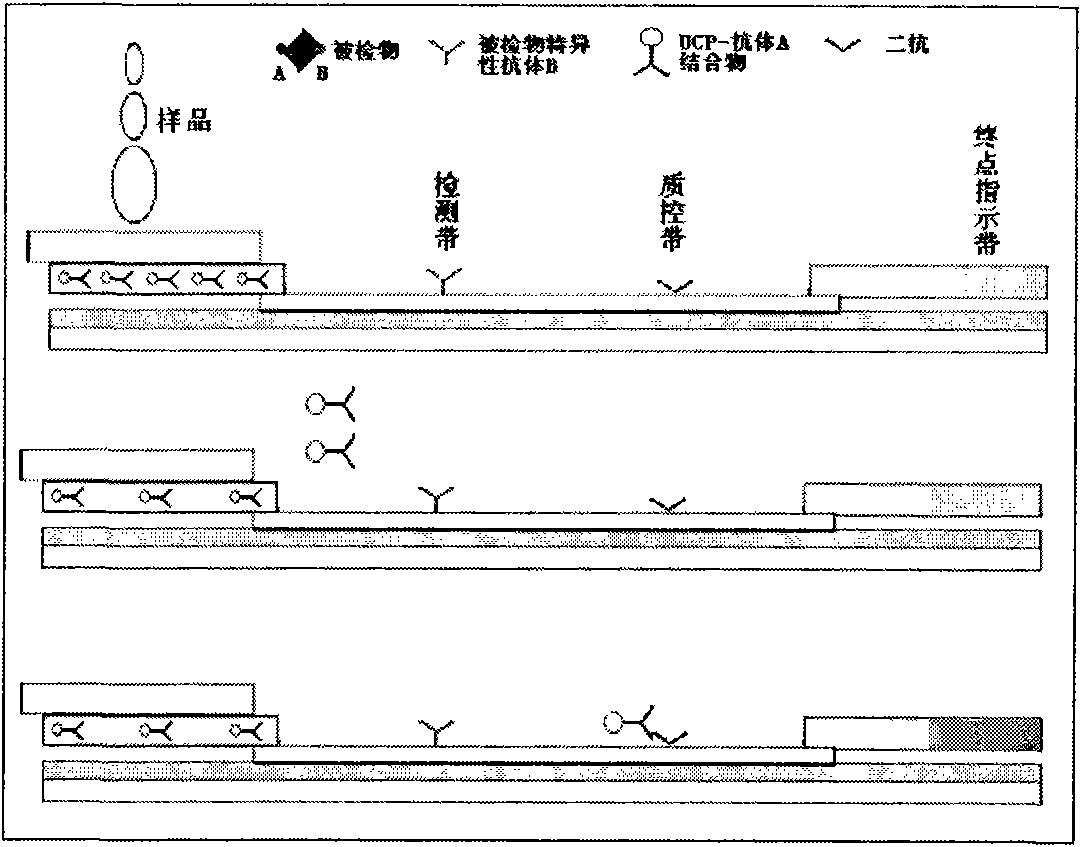
Immune chromatography test paper strip based on up-conversion luminescence technology
InactiveCN101788559AAuxiliary diagnosisImprove stabilityBiological testingUpconversion luminescenceLight signal
The invention discloses an immune chromatography test paper strip based on an up-conversion luminescence technology. The up-conversion luminescence material, i.e. UCP, is used as a biological marker, and the result can be presented in a visible light signal form under the radiation of infrared light and can be interpreted by an instrument, thereby quantitative detection on the target detected object is realized. The test paper strip mainly comprises a sample pad, a binding pad or a binder release pad, an analyzing membrane, a water absorbing pad and a plastic back plate. The invention also discloses a method for preparing the test paper strip, and application in the biological sample quantitative detection. According to different immune reaction modes of the substances to be detected, the test paper strip is classified according to a sandwich mode, a competition mode and an indirection mode, and various modes of the test paper strips can carry out quick and sensitive qualitative and quantitative detection and analysis on different substances to be detected in the sample.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Method for obtaining tumor specific T cell receptor
InactiveCN109485721AHigh killing efficiencyImmunoglobulin superfamilyGenetically modified cellsT-Cell Receptor GeneWilms' tumor
The invention discloses a method for obtaining a tumor specific T cell receptor. The method comprises the following steps: firstly, obtaining a T cell receptor gene sequence in an antigen peptide specific T cell which comes from an immunoreactive positive and / or tumor symptom relieved tumor patient after antigen peptide treatment; then, preparing an antigen specific T cell receptor according to the antigen specific T cell receptor gene sequence; introducing the antigen peptide specific T cell receptor gene into the T cell, and expressing the antigen peptide specific T cell receptor gene to obtain the specific TCR-T cell. The specific TCR-T cell can be used for successfully killing gene mutation corresponding to antigen peptide and HLA typed tumor cell, and has high killing efficiency and agood application prospect.
Owner:杜学明
Pharmaceutical composition for treatment and/or prevention of cancer
ActiveUS20150017172A1Reduce usageUseful in treatmentSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsDrugAntigenic protein
This invention provides an antibody targeting a cancer antigenic protein specifically expressed on the surface of cancer cells and use thereof in a therapeutic and / or preventive agent for cancer. Specifically, this invention provides an antibody or a fragment thereof which has immunological reactivity with a partial CAPRIN-1 polypeptide consisting of the amino acid sequence set forth in SEQ ID NO: 5 or an amino acid sequence having 80% or higher sequence identity to the amino acid sequence, and a pharmaceutical composition for treatment and / or prevention of cancer, comprising the antibody or fragment thereof as an active ingredient.
Owner:TORAY IND INC
Immune response assessment method
The present invention includes compositions and methods for identifying T-cell epitopes independent of the subject's HLS type by simultaneously analyzing the culture for multiple parameters of immune reactivity to identify at least one epitope wherein the epitopes that elicit immune reactivity of interest in the subset are identified as targeting agents for the subset.
Owner:BAYLOR RES INST
Pharmaceutical composition for treatment and/or prophylaxis of cancer
ActiveUS20140199311A1Treatment and/or prevention of cancerReduce usageSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsCancer preventionComplementarity determining region
This invention provides an antibody targeting a cancer antigenic protein specifically expressed on the surface of cancer cells and use thereof as a therapeutic and / or preventive agent for cancer. More specifically, the present invention provides an antibody, or a fragment thereof which has immunological reactivity with a CAPRIN-1 protein, the antibody comprising a heavy chain variable region comprising complementarity determining regions of SEQ ID NOs: 5, 6, and 7 and a light chain variable region comprising complementarity determining regions of SEQ ID NOs: 9, 10, and 11, and a pharmaceutical composition for treatment and / or prevention of cancer, comprising the same as an active ingredient.
Owner:TORAY IND INC
Immune reactivity to HER-2/neu protein for diagnosis and treatment of malignancies in which the HER-2/neu oncogene is associated
Methods for the detection, monitoring and treatment of malignancies in which the HER-2 / neu oncogene is associated are disclosed. Detection of specific T cell activation (e.g., by measuring the proliferation of T cells) in response to in vitro exposure to the HER-2 / neu protein, or detection of immunocomplexes formed between the HER-2 / neu protein and antibodies in body fluid, allows the diagnosis of the presence of a malignancy in which the HER-2 / neu oncogene is associated. The present invention also discloses methods and compositions, including peptides, for treating such malignancies.
Owner:UNIV OF WASHINGTON
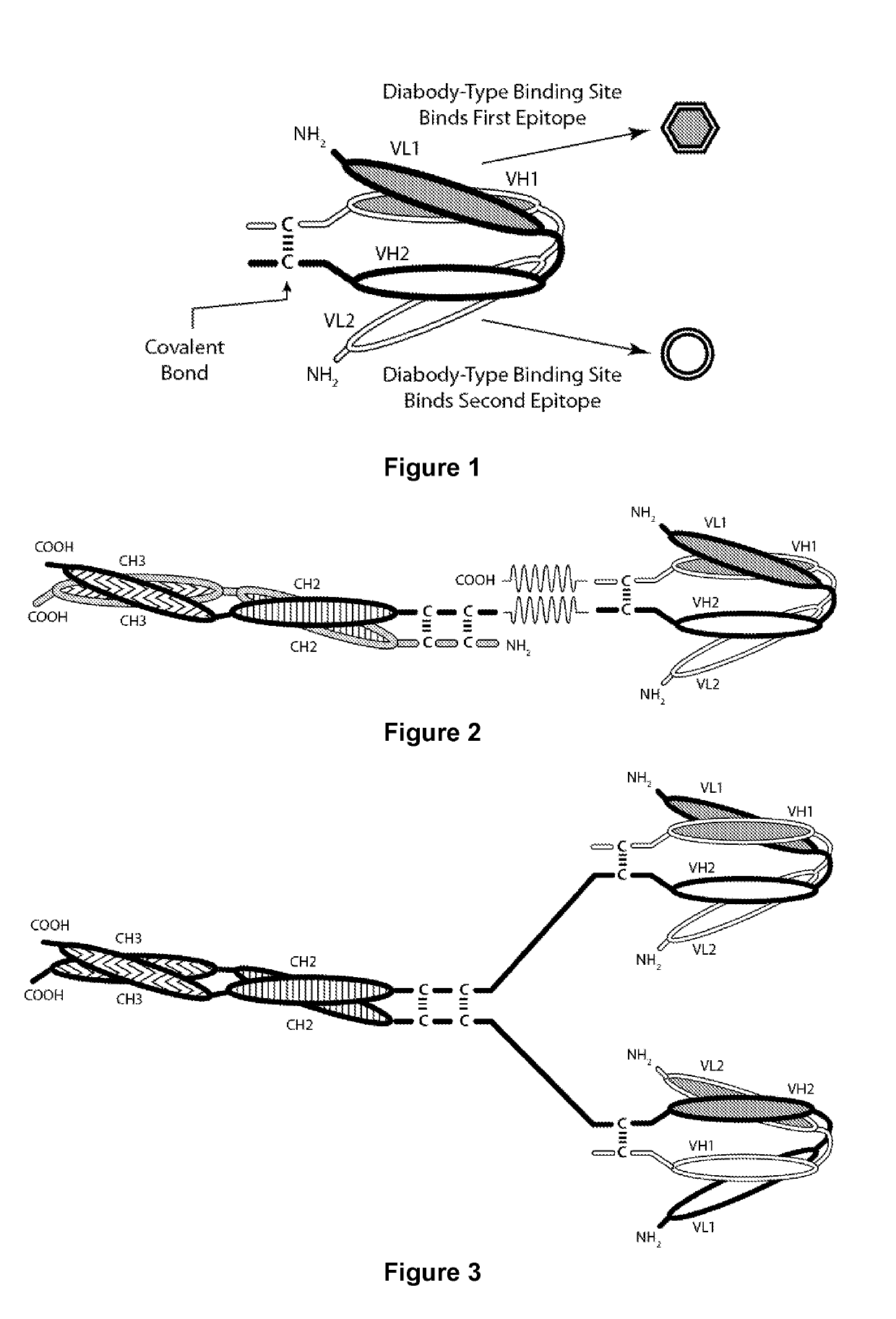
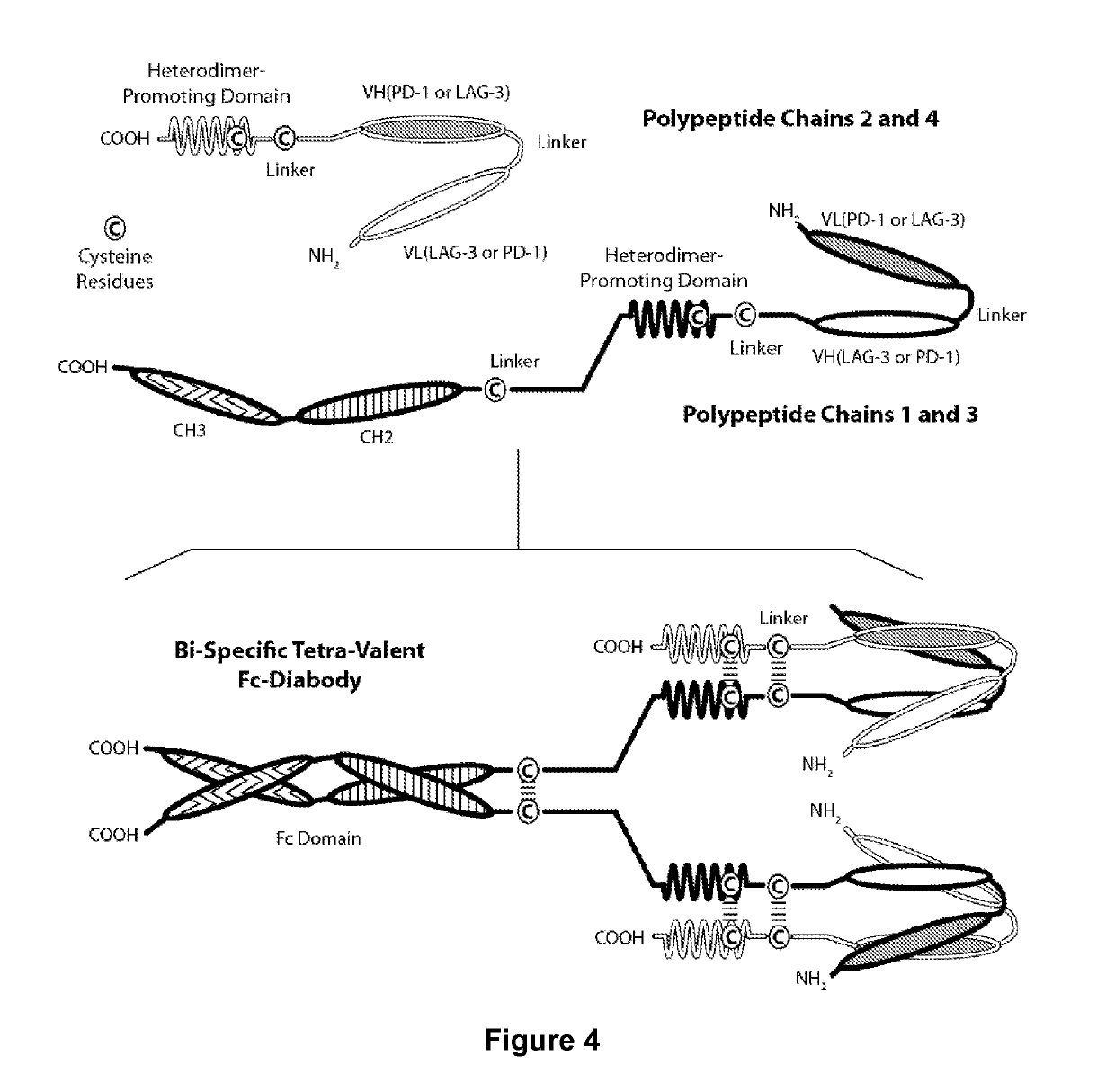
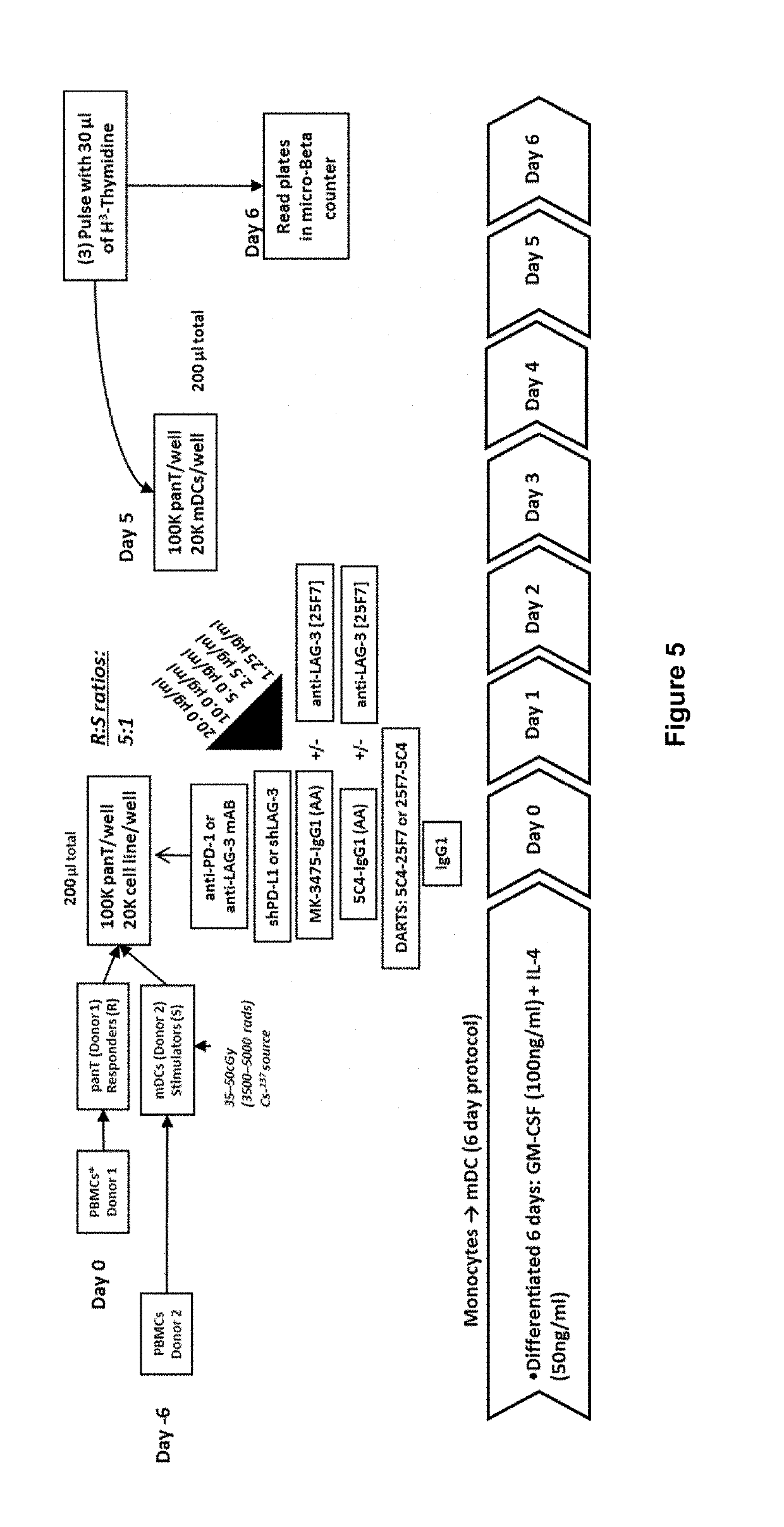
Covalently Bonded Diabodies Having Immunoreactivity with PD-1 and LAG-3, and Methods of Use Thereof
The present invention is directed to bi-specific diabodies that comprise two or more polypeptide chains and which possess at least one Epitope-Binding Site that is immunospecific for an epitope of PD-1 and at least one Epitope-Binding Site that is immunospecific for an epitope of LAG-3 (i.e., a “PD-1×LAG-3 bi-specific diabody”). More preferably, the present invention is directed to bi-specific diabodies that comprise four polypeptide chains and which possess two Epitope-Binding Sites that are immunospecific for one (or two) epitope(s) of PD-1 and two Epitope-Binding Site that are immunospecific for one (or two) epitope(s) of LAG-3 (i.e., a “PD-1×LAG-3 bi-specific, tetra-valent diabody”). The present invention also is directed to such diabodies that additionally comprise an immunoglobulin Fc Domain (“bi-specific Fc diabodies and bi-specific, tetra-valent, Fc diabodies”). The diabodies of the present invention are capable of simultaneously binding to PD-1 and to LAG-3, particularly as such molecules are arrayed on the surfaces of human cells. The invention is directed to pharmaceutical compositions that contain such diabodies, and to methods involving the use of such diabodies in the treatment of cancer and other diseases and conditions.
Owner:MACROGENICS INC
Diagnostic assay for measuring a cell mediated immune response
ActiveUS7608392B2Reduce exposureAmenable to analysisMicrobiological testing/measurementBiological testingImmune effectsWild life
The present invention relates generally to a diagnostic assay and, more particularly, an assay for measuring cell-mediated immune reactivity. Even more particularly, the present invention provides an assay and a kit for measuring a cell-mediated response to an antigen using whole blood or other suitable biological sample. The assay may be conducted using ligands to immune effector molecules or at the nucleic acid level, screening for expression of genes encoding the immune effector molecules. The assay is useful in therapeutic and diagnostic protocols for human, livestock and veterinary and wild life applications.
Owner:CELLESTIS
Pharmaceutical composition for treatment and/or prophylaxis of cancer
ActiveUS9175074B2Treatment and/or prevention of cancerReduce usageImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCancer cellBULK ACTIVE INGREDIENT
Owner:TORAY IND INC
Pharmaceutical composition for treatment and/or prophylaxis of cancer
ActiveUS9273128B2Treatment and/or prevention of cancerReduce usageImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCancer cellBULK ACTIVE INGREDIENT
This invention provides an antibody targeting a cancer antigenic protein specifically expressed on the surface of cancer cells and use thereof as a therapeutic and / or preventive agent for cancer. More specifically, the present invention provides an antibody, or a fragment thereof which has immunological reactivity with a partial CAPRIN-1 polypeptide consisting of the amino acid sequence shown by SEQ ID NO: 5 or an amino acid sequence having 80% or higher sequence identity to the amino acid sequence, and a pharmaceutical composition for treatment and / or prevention of cancer comprising the same as an active ingredient.
Owner:TORAY IND INC
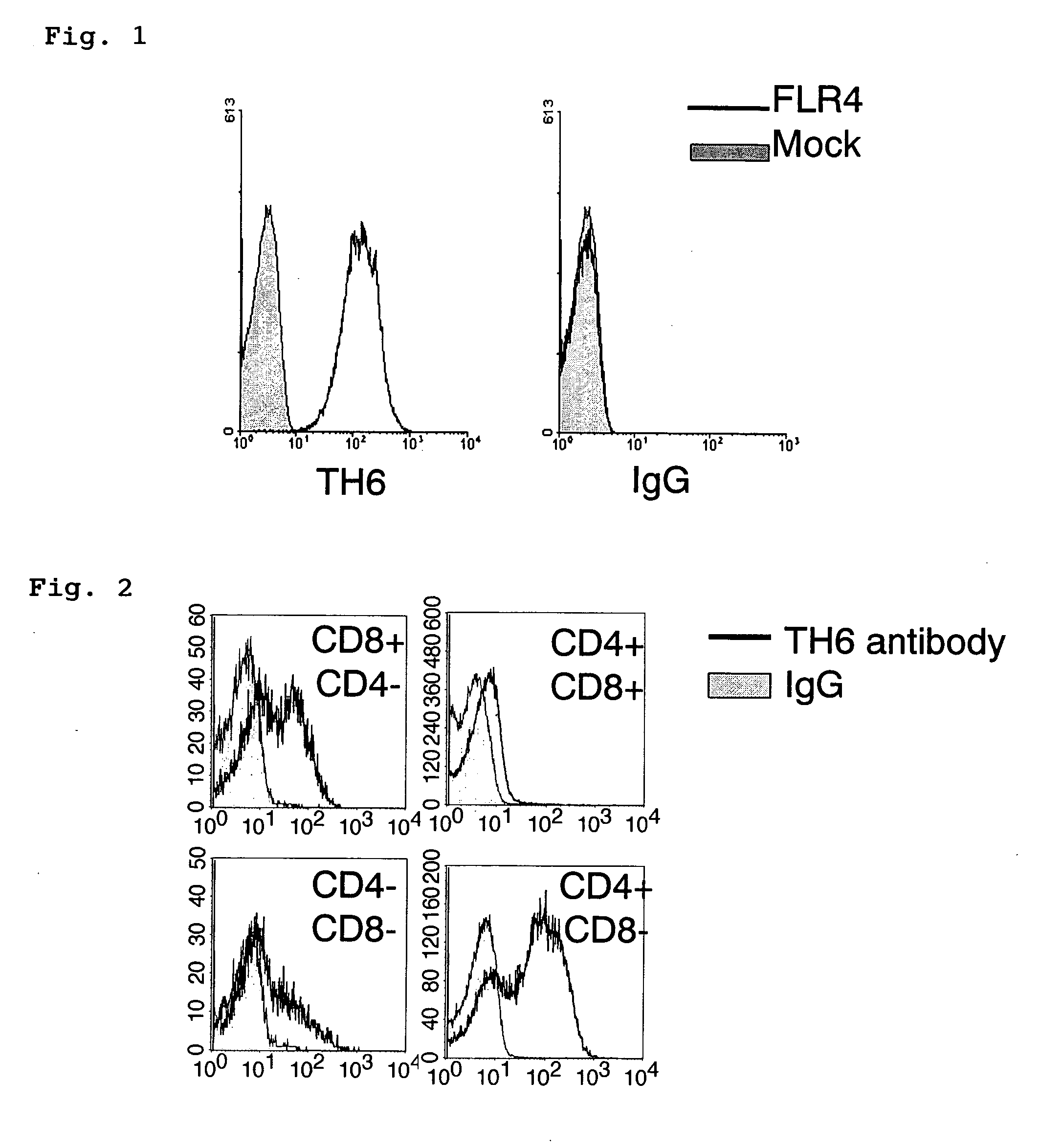
Method for detecting regulatory T cells using expression of folate receptor 4 as indicator, method for treating diseases using the detection method, pharmaceutical composition for immunostimulation, and method for treating diseases using the composition
ActiveUS20060246063A1Effectively expresses immune responseReduce in quantityBiocideMicrobiological testing/measurementDrugFolate receptor
An object of the present invention is to provide a technique to distinguish between Treg cells and activated T cells in a live state. Another object of the present invention is to provide a pharmaceutical composition for immunostimulation that can reduce the number of Treg cells in vivo and effectively express the immune response of activated T cells. In the method of the present invention, Treg cells are detected from test cells containing (i) regulatory T cells and (ii) at least one type of cell selected from the group consisting of naive T cells and activated T cells, wherein expressions of folate receptor 4 on the surfaces of cells are measured and Treg cells are detected using the expressions as an indicator. The present invention uses anti-folate receptor 4 antibody or folate receptor 4-binding fragment as an active ingredient contained in a pharmaceutical composition for immunostimulation.
Owner:KYOTO UNIV +1
Polypeptides and Polynucleotides for Enhancing Immune Reactivity to HER-2 Protein
Owner:THE OHIO STATES UNIV
Method of biological and medical diagnostics using immune patterns obtained with arrays of peptide probes
InactiveUS20110275537A1Promote generationEasy to convertLibrary screeningBiostatisticsBiological bodyBiological studies
Immune-chips, which are arrays of peptides probes are used to obtain a pattern which characterizes the global immune reactivity status of the human or other organism, are described. The peptide probes participate in immune reactions with antibodies and immune receptors of the investigated organisms to generate an immune pattern on the chip, which are detected and stored as patterns in databases. The patterns are then compared with other patterns observed with the same array and obtained under physiological, pathological and experimental conditions from the same or other organisms. The comparison is used to classify the state of the investigated organisms based on similarity to other observed states. The immune chips and the obtained patterns can be used for clinical diagnosis and biological studies, such as the investigation of similarities between physiological, pathological or experimental processes.
Owner:RYCHLEWSKI LESZEK +1
Compounds for eliciting or enhancing immune reactivity to HER-2/neu protein for prevention or treatment of malignancies in which the HER-2/neu oncogene is associated
Compounds and compositions for eliciting or enhancing immune reactivity to HER-2 / neu protein are disclosed. The compounds include polypeptides and nucleic acid molecules encoding such peptides. The compounds may be used for the prevention or treatment of malignancies in which the HER-2 / neu oncogene is associated.
Owner:UNIV OF WASHINGTON
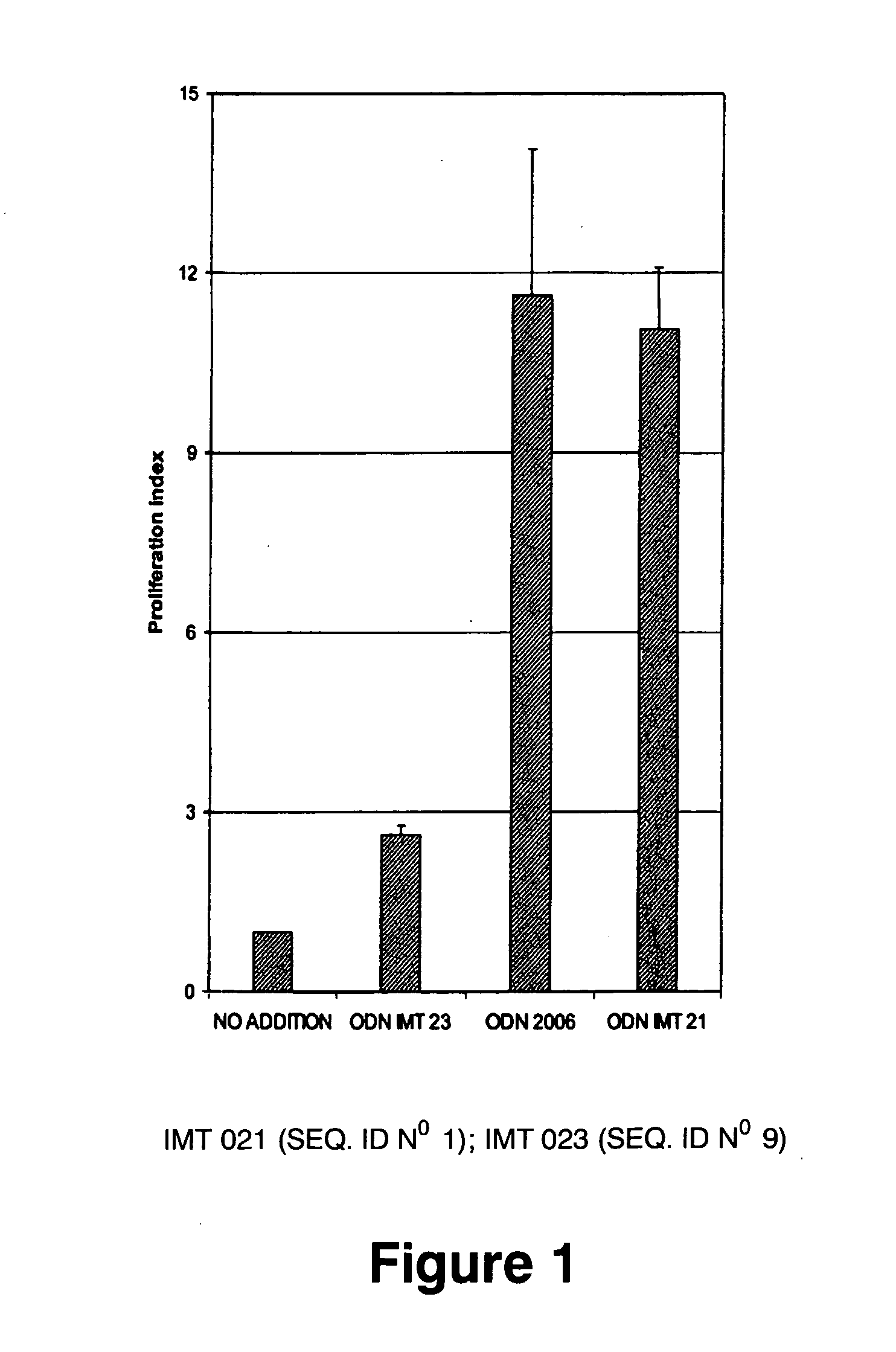
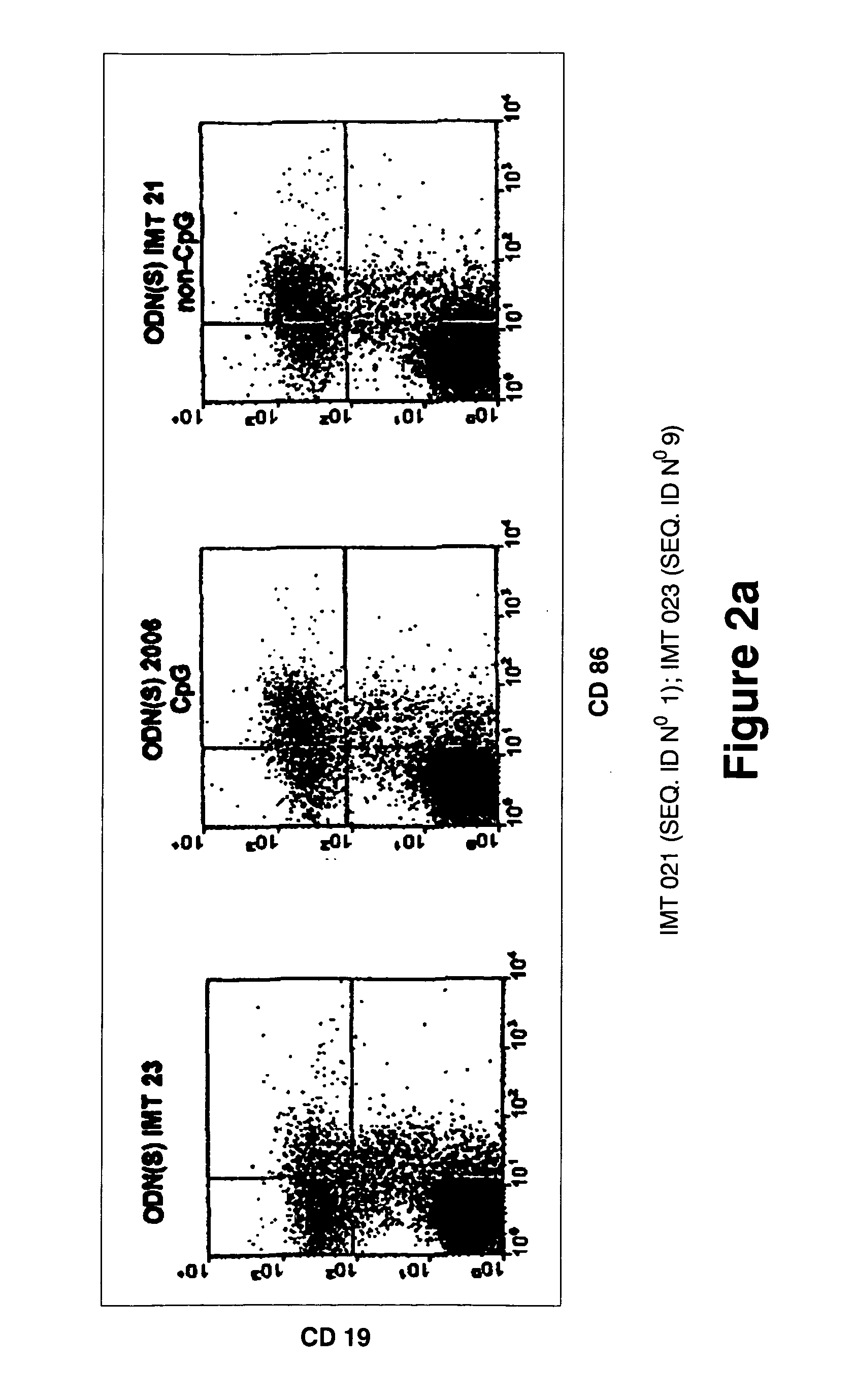
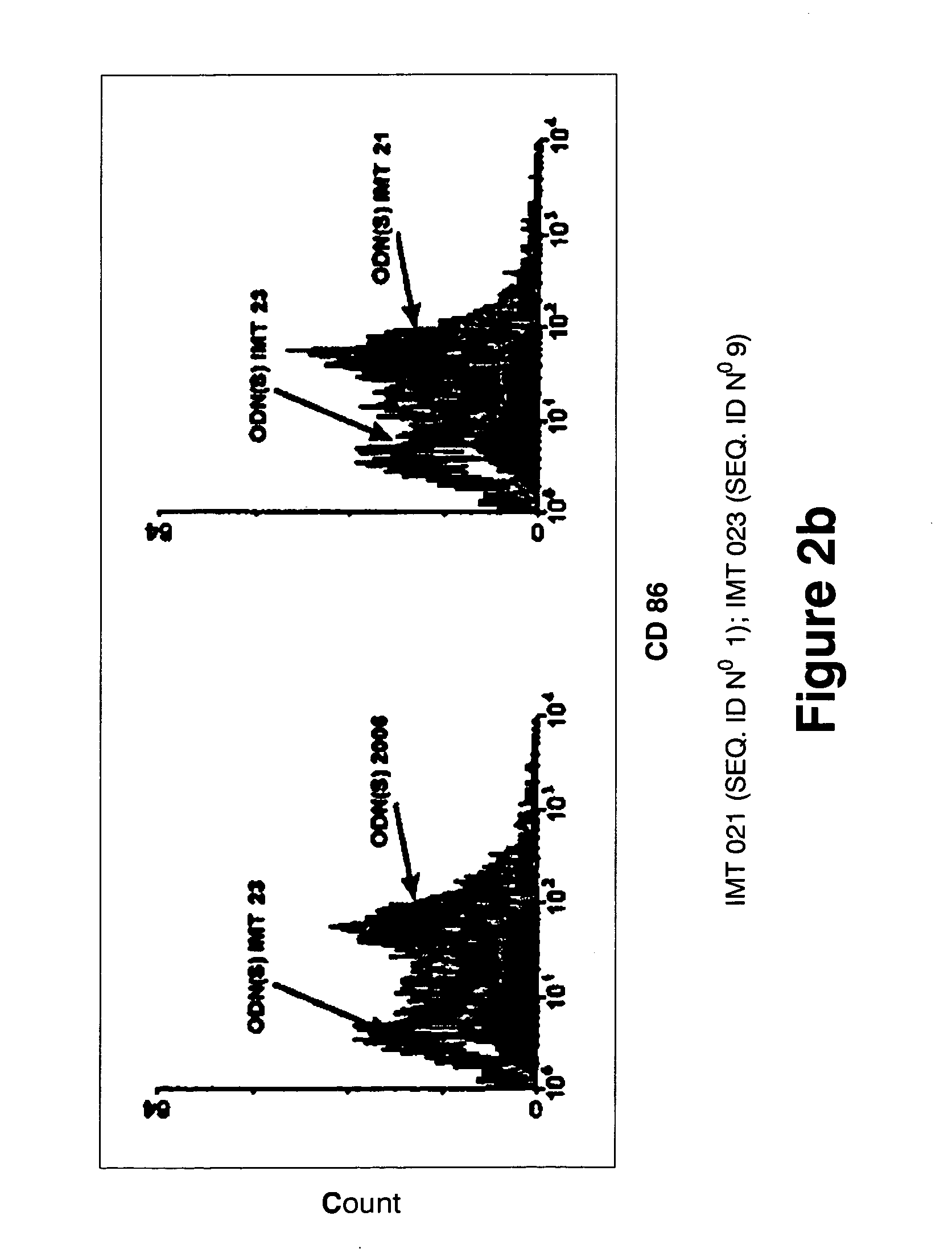
Immunostimulatory oligonucleotides and uses thereof
Oligonucleotides containing the non-palindromic sequence motif: X1X2X3X4X5X6X7X8, wherein X1 is C,T,G or A (preferably T or C); wherein X2 is C,T,G or A; wherein X7 is C,T,G or A (preferably G); at least three, and preferably all, of X3, X4, X5, X6 and X8 are T; and with the proviso that, in the motif, a C does not precede a G (in other terms, the nucleic acid motif does not consist of a CpG oligonucleotide), that modulate the immune response of animals of the order Primate, including humans, are disclosed. This immune modulation is characterized by stimulation of proliferation, differentiation, cytokine production and antibody production on B-cells and cell differentiation on plasmacytoid dendritic cells.
Owner:DAVID HORN LLC
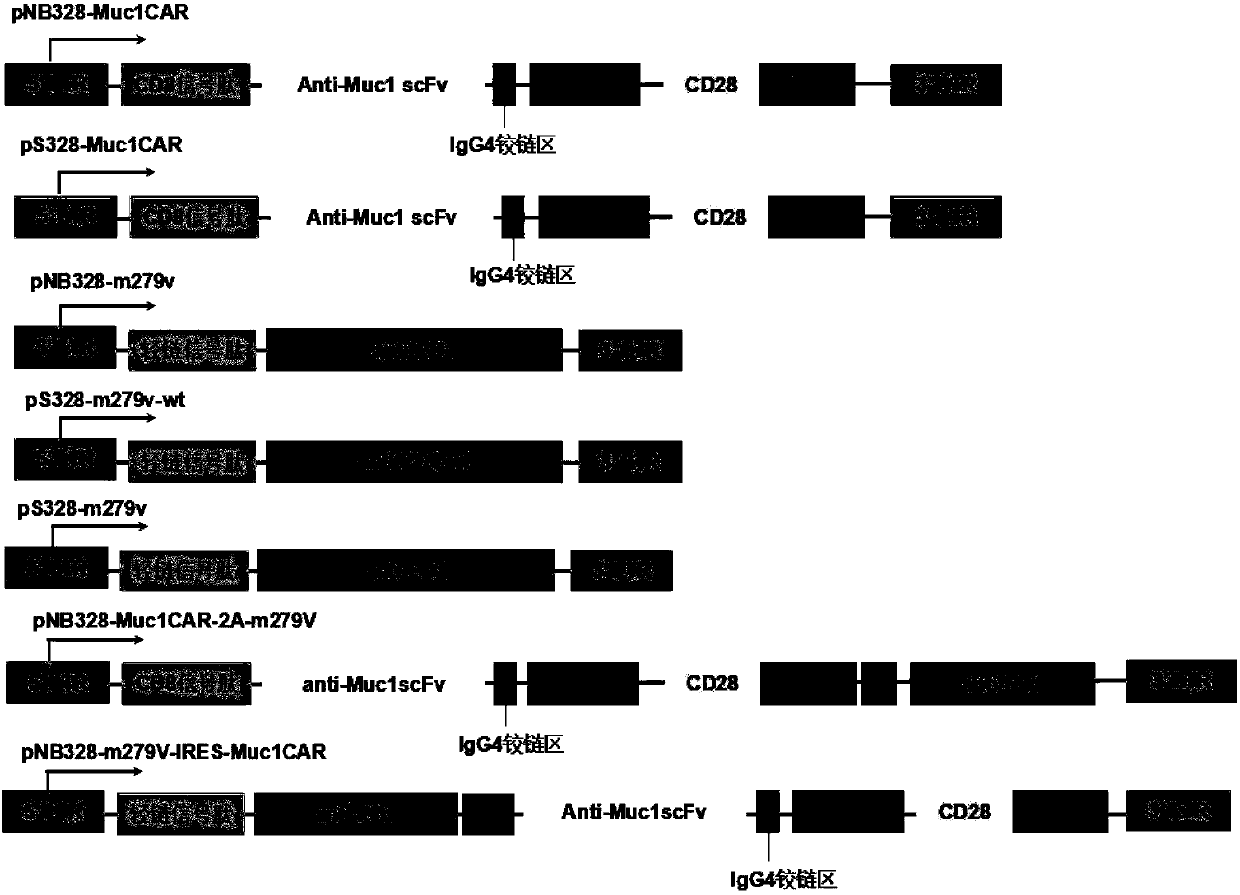
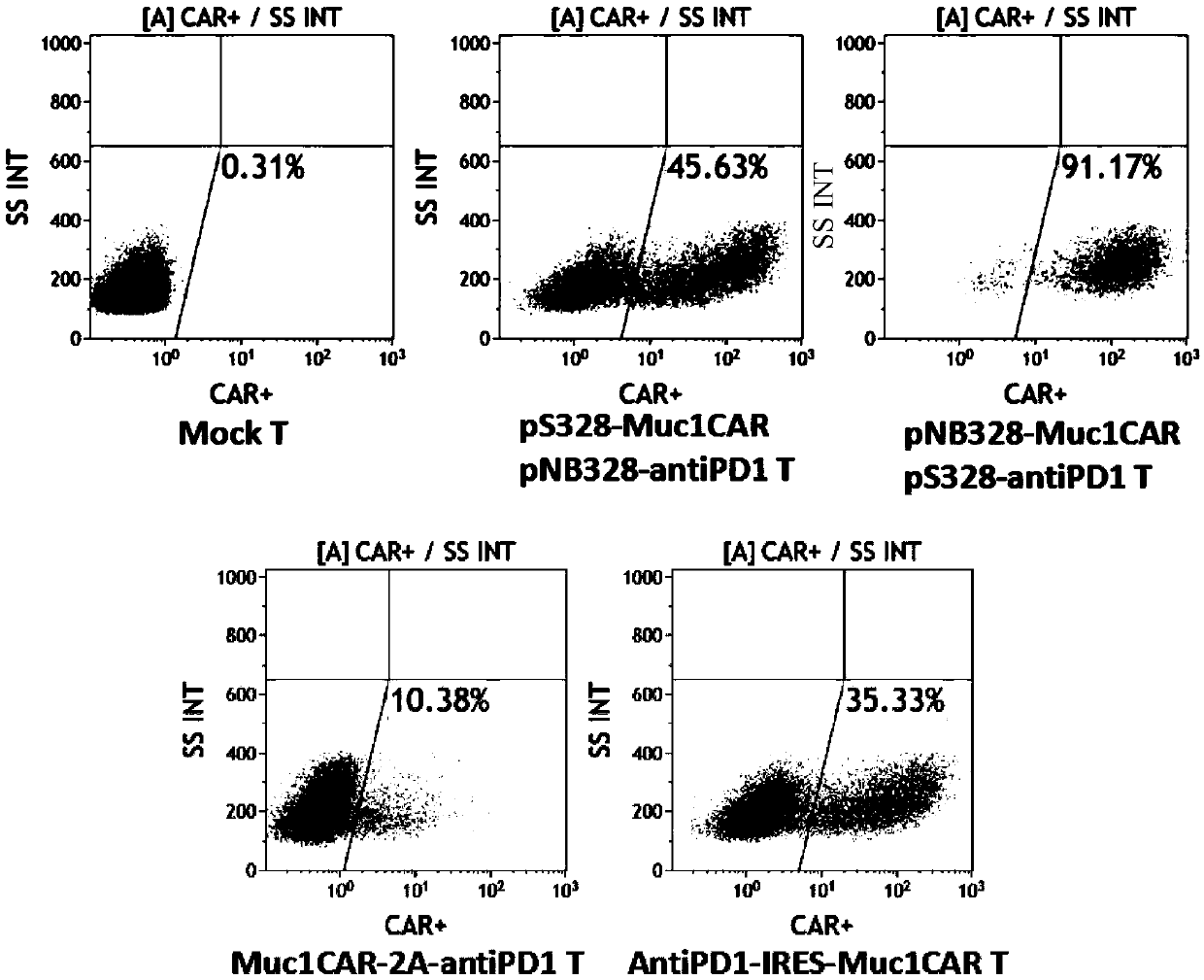
Muc1-specific CAR-T cells capable of stably expressing PD-1 antibodies and usage thereof
ActiveCN109971713APolypeptide with localisation/targeting motifImmunoglobulin superfamilySequence signalSingle-Chain Antibodies
The invention relates to CAR-T cells with specifically targeted Muc1 antigens capable of stably expressing PD-1 antibodies at high levels, as well as usage thereof. Specifically, the CAR-T cells provided by the invention have a coding sequence of a chimeric antigen receptor expressing and recognizing Muc1 antigens and a coding sequence of PD1 antibodies; and / or the CAR-T cells comprise chimeric antigen receptors expressing and recognizing the Muc1 antigens, as well as PD-1 antibodies. The chimeric antigen receptors provided by the invention sequentially comprise, from N-terminals to C-terminals, membrane protein signal peptides, anti-Muc1 membrane proximal single-chain antibodies, hinge regions of which the lengths are 50 amino acid residues or above, transmembrane regions, intracellular domains of costimulatory signaling molecules and immunoreceptor tyrosine-based activation motifs. The CAR-T cells provided by the invention are capable of overcoming inhibition of immune micro-environment, promoting apoptosis of tumor cells, and exerting anti-tumor immune response; and thus, the CAR-T cells provided by the invention can be used for treating a multiple kinds of Muc1-positive malignant tumors.
Owner:SHANGHAI CELL THERAPY RES INST +1
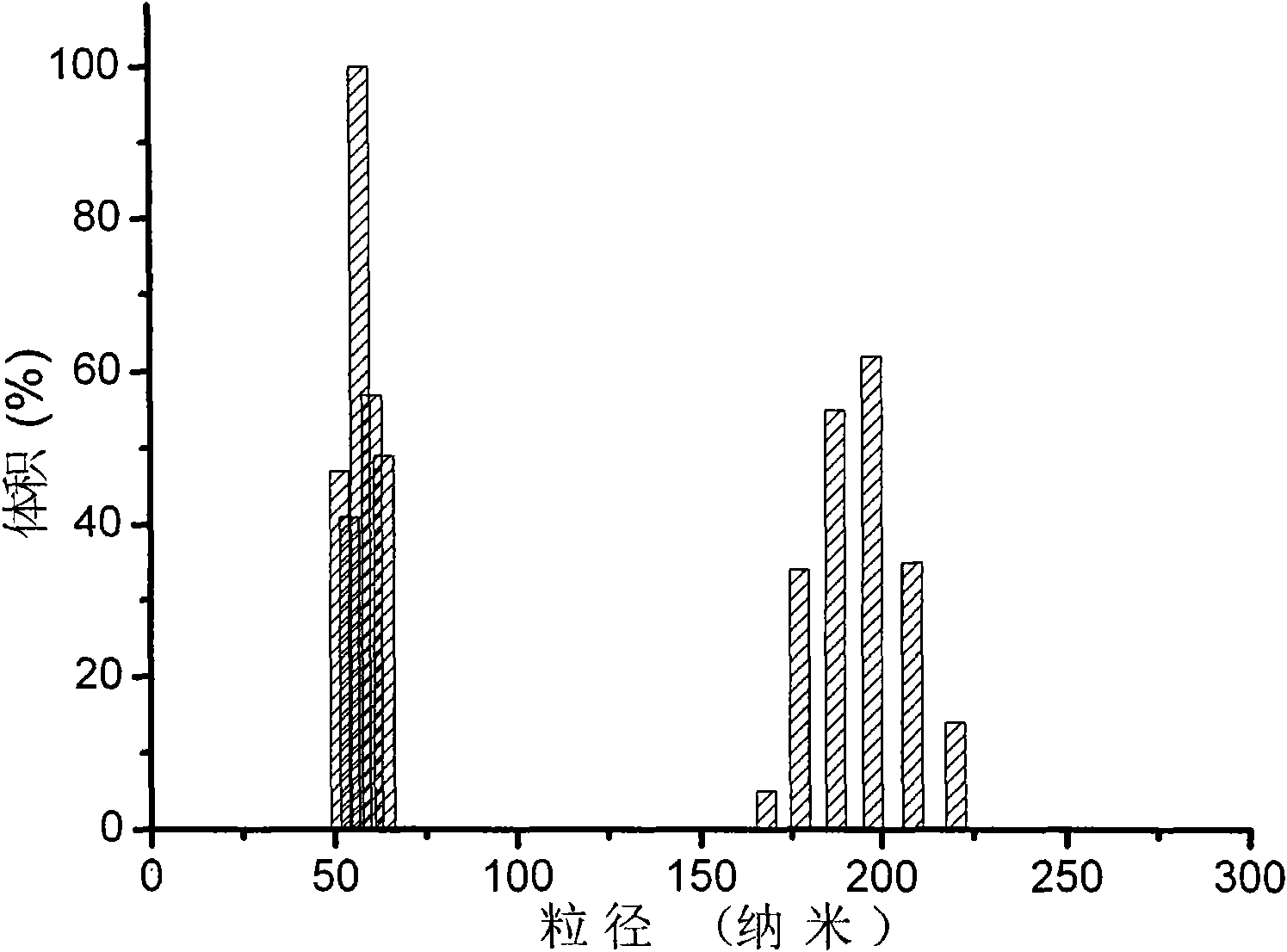
Nanometer microsphere or hollow sphere of casein or casein-polyacrylic acid compound and preparation method and application thereof
InactiveCN101648022AGood biocompatibilityPromote degradationPowder deliveryMacromolecular non-active ingredientsCross-linkMicrosphere
The invention relates to a nanometer microsphere or a nanometer hollow sphere of casein or casein-polyacrylic acid compound, and a nanometer microsphere or a nanometer hollow sphere of cross-linking casein, wherein the nanometer microsphere or the nanometer hollow sphere of the casein or casein-polyacrylic acid compound consists of a compound of casein and polyacrylic acid, and the average grain diameter of the nanometer microsphere or the nanometer hollow sphere is 170-370nm; the nanometer microsphere or the nanometer hollow sphere of cross-linking casein consists of cross-linking casein, andthe average grain diameter of the nanometer microsphere or the nanometer hollow sphere is 240-500nm. The nanometer microsphere or the nanometer hollow sphere of cross-linking casein has good biocompatibility and biodegradability without causing organism immunoreaction; degradation products of the nanometer microsphere or the nanometer hollow sphere of cross-linking casein can be absorbed and metabolized by a human body and has the treatment action, nutritive efficiency and the like on the human body, and therefore, the nanometer microsphere or the nanometer hollow sphere of cross-linking casein can be used as a carrier of drugs. The invention discloses a preparation method of the nanometer microsphere or the nanometer hollow sphere of the casein or casein-polyacrylic acid compound, and apreparation method of the nanometer microsphere or the nanometer hollow sphere of cross-linking casein.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com