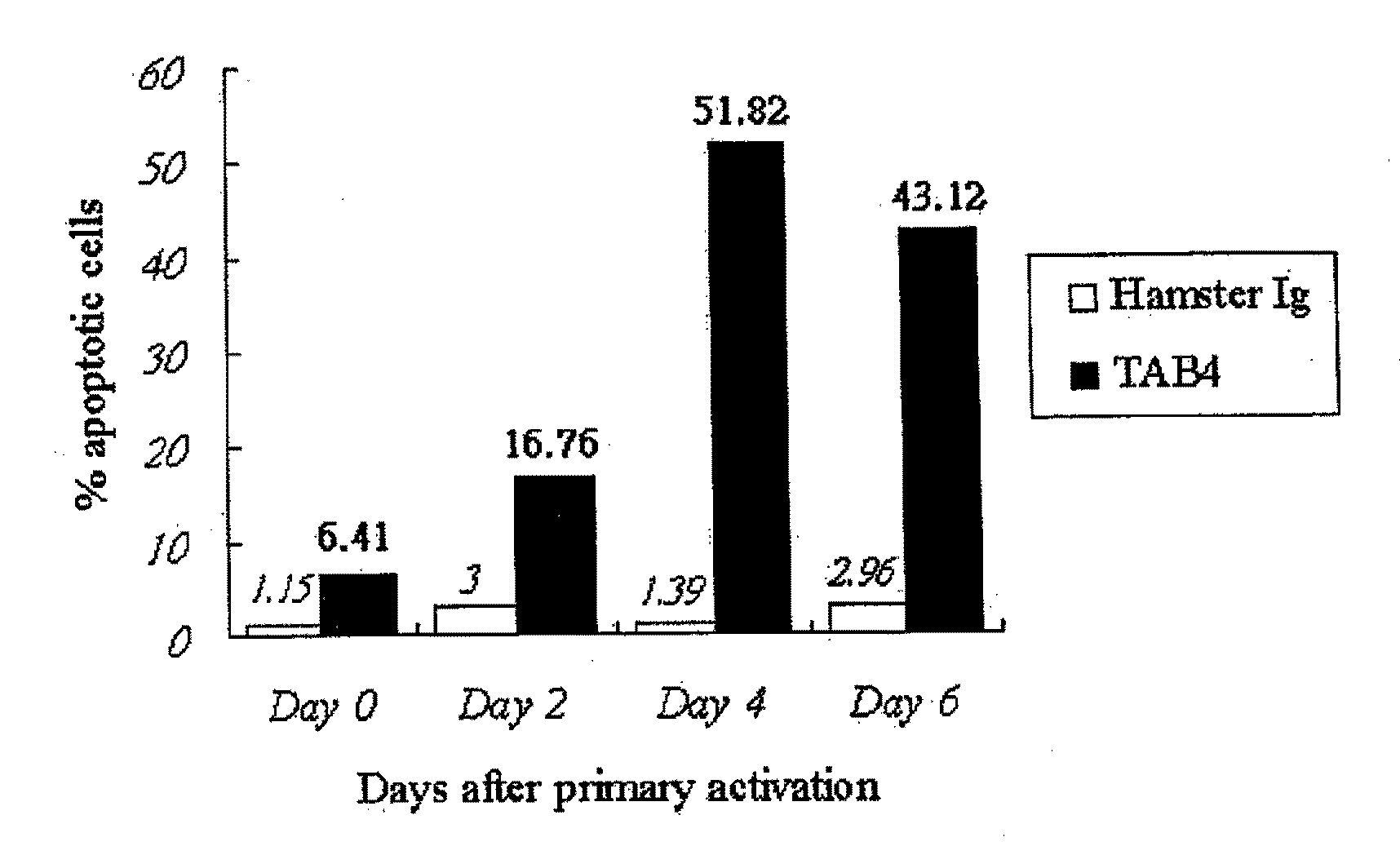
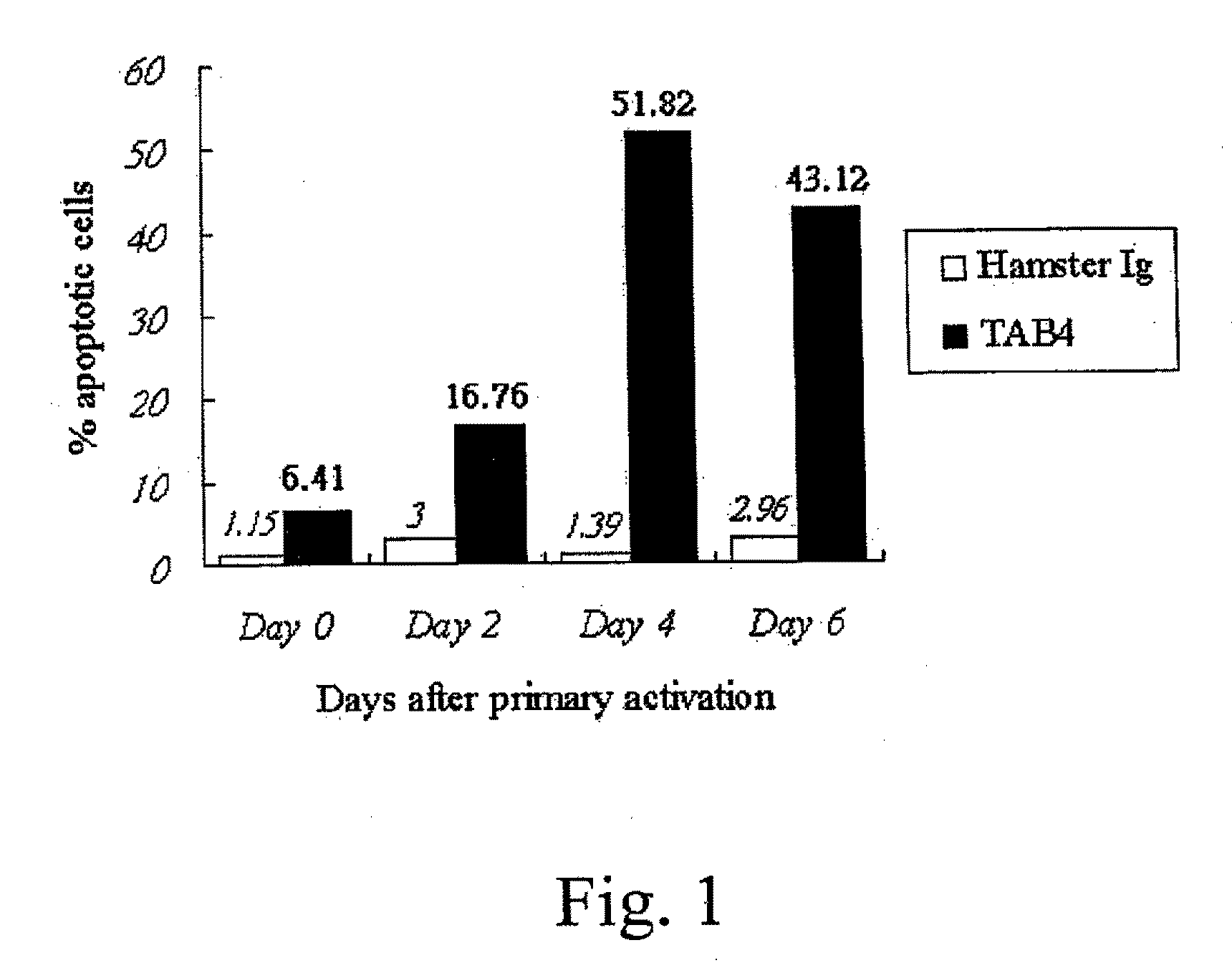
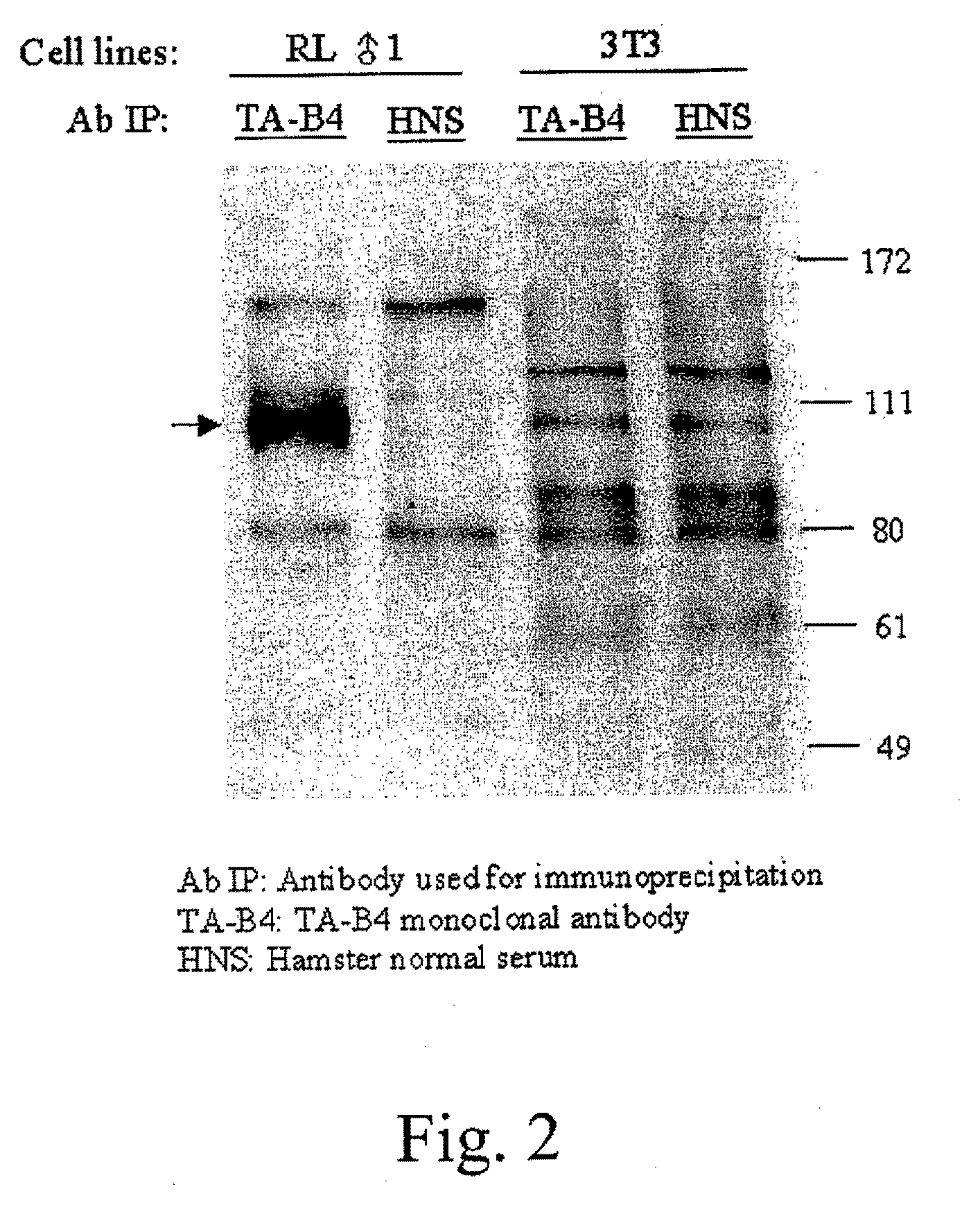
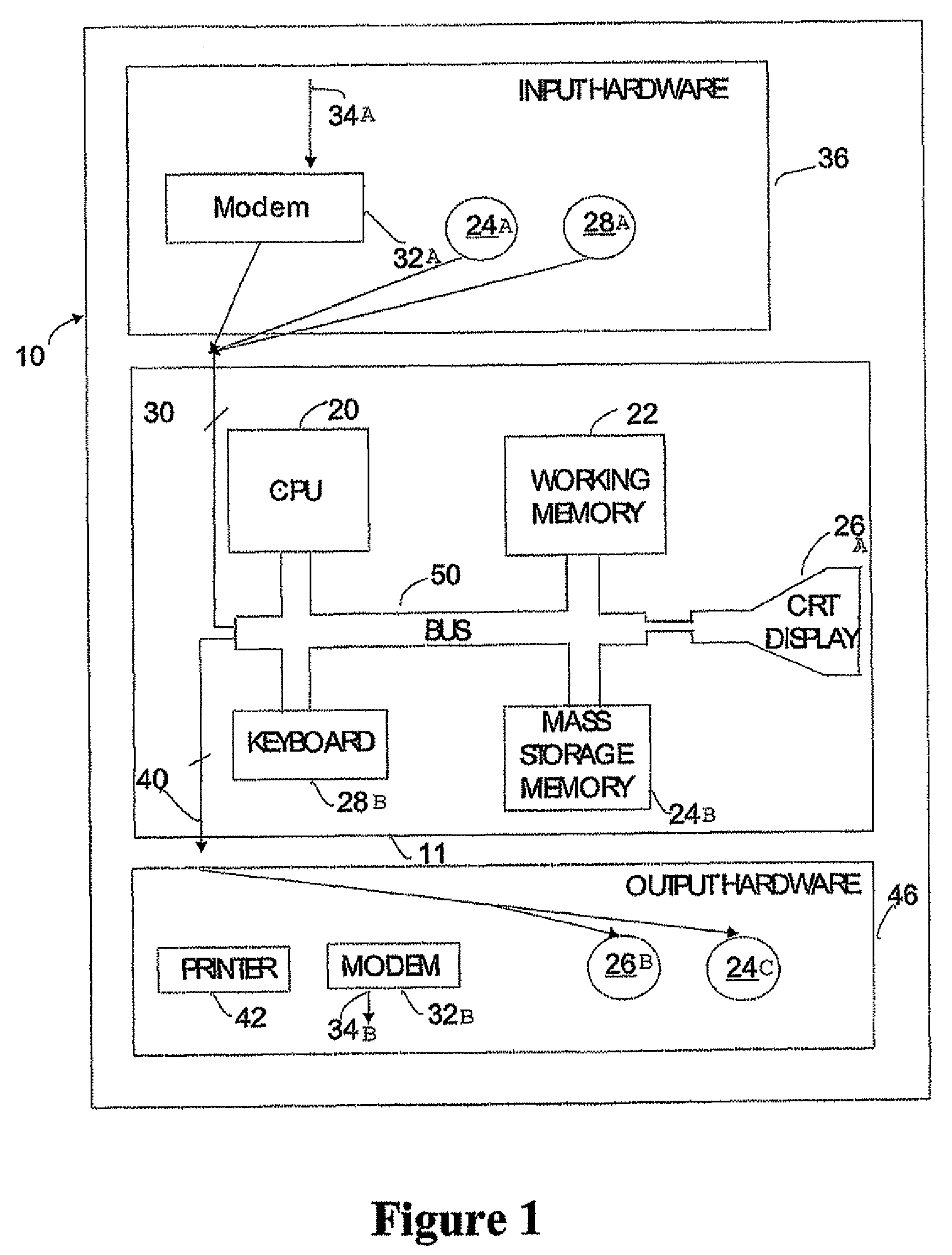
Patents
Literature
128 results about "Cell-mediated immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
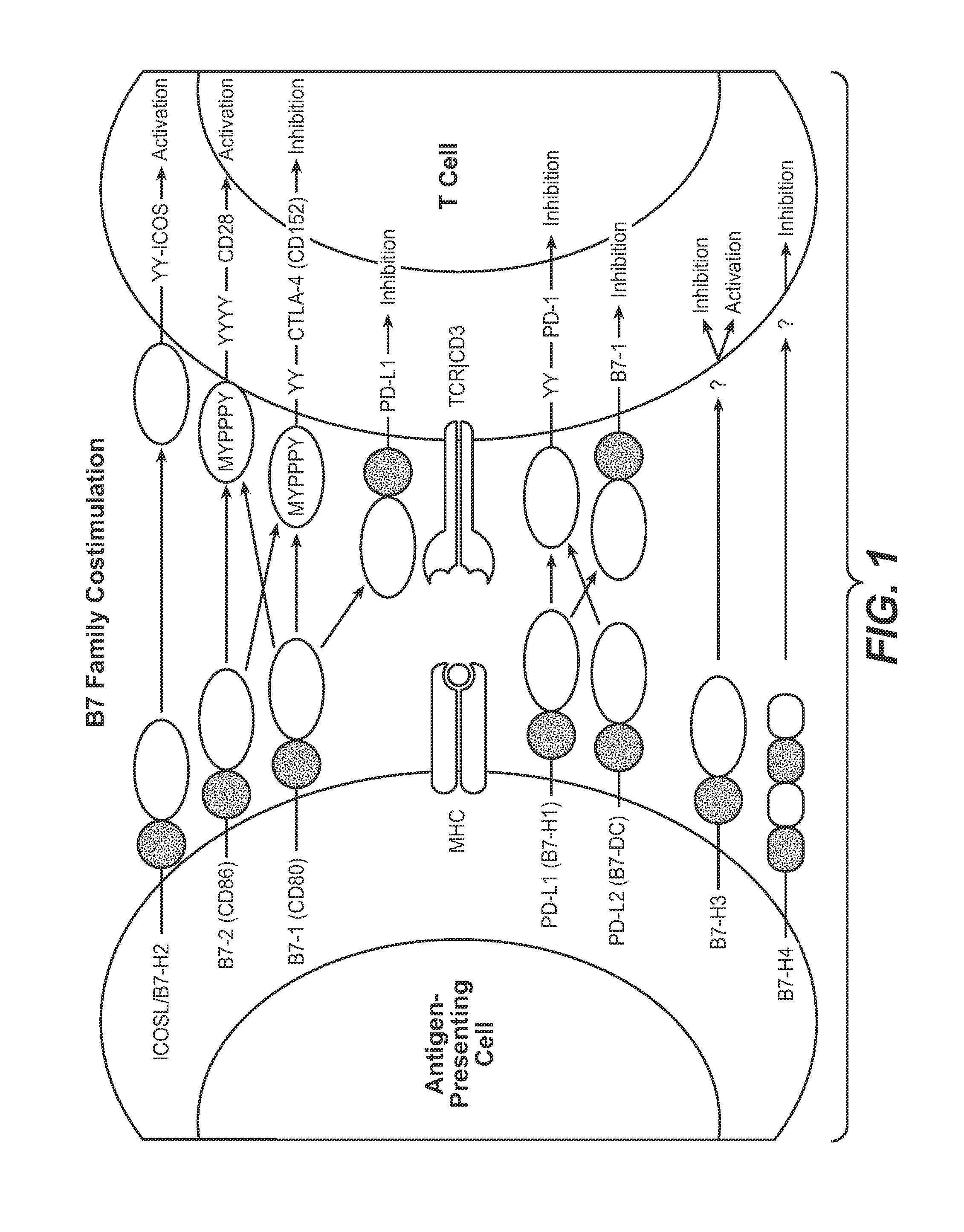
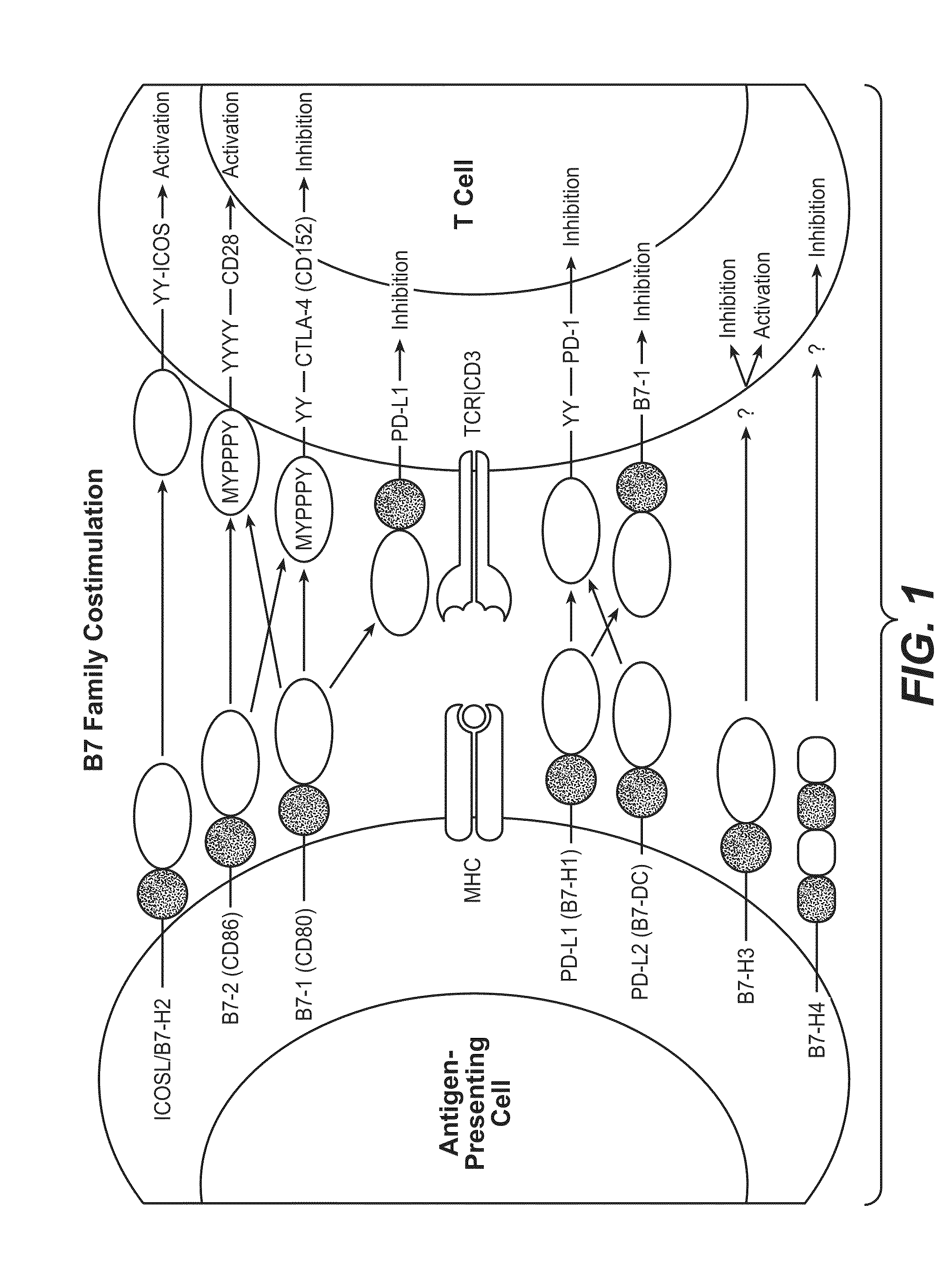
Cell-mediated immunity is an immune response that does not involve antibodies, but rather involves the activation of phagocytes, antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen.
Anti-PD-L1 antibodies, compositions and articles of manufacture
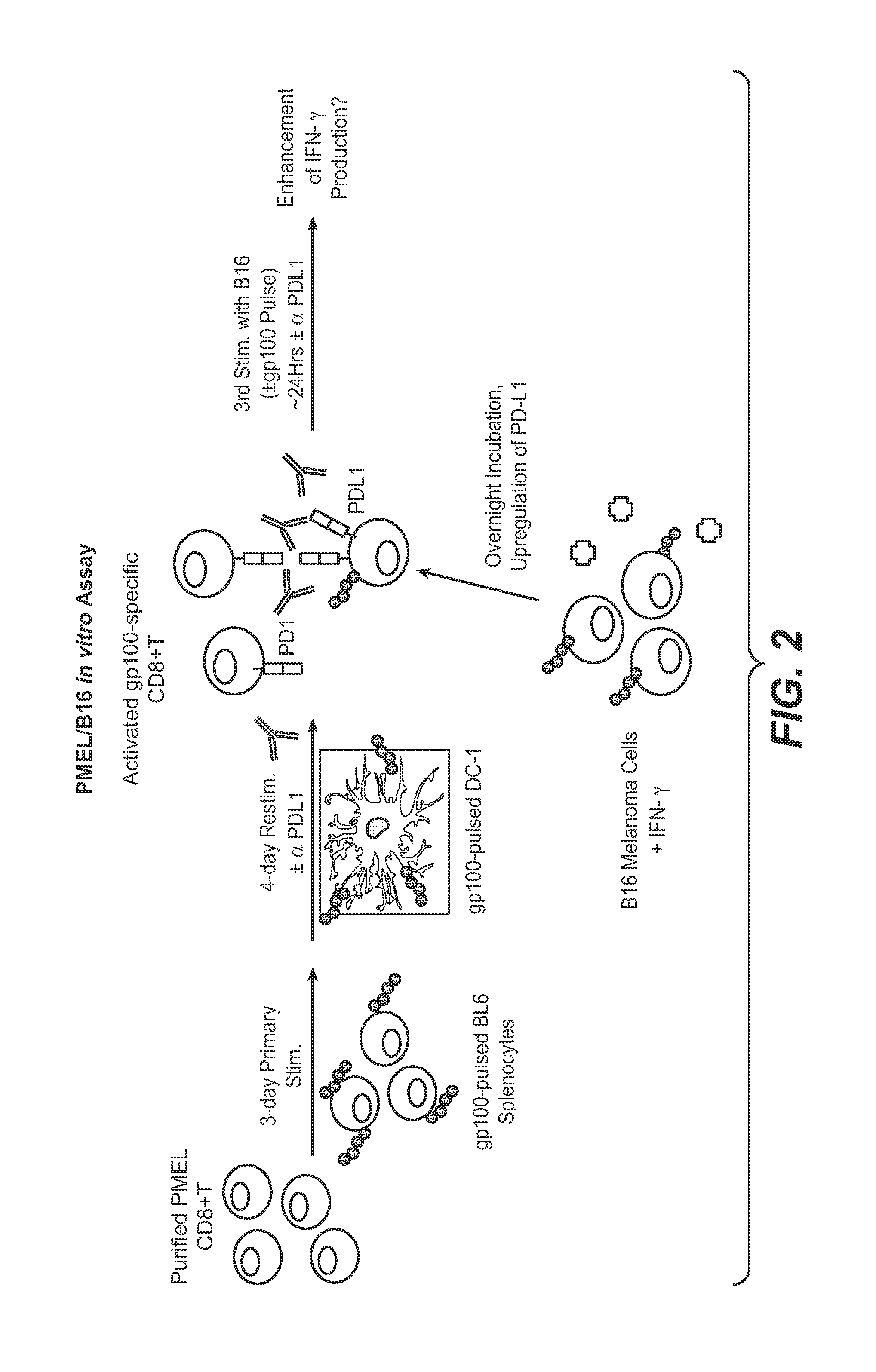
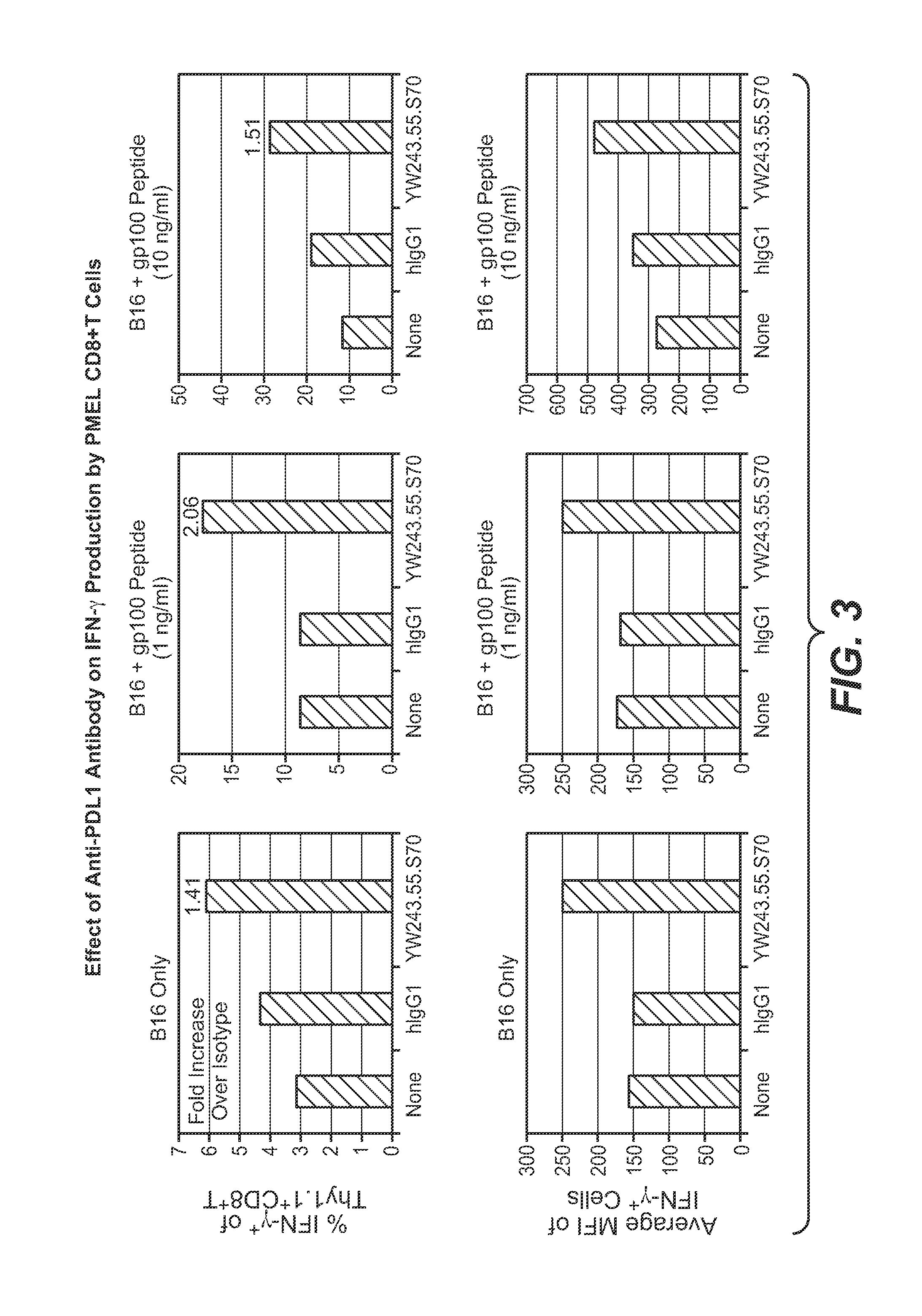
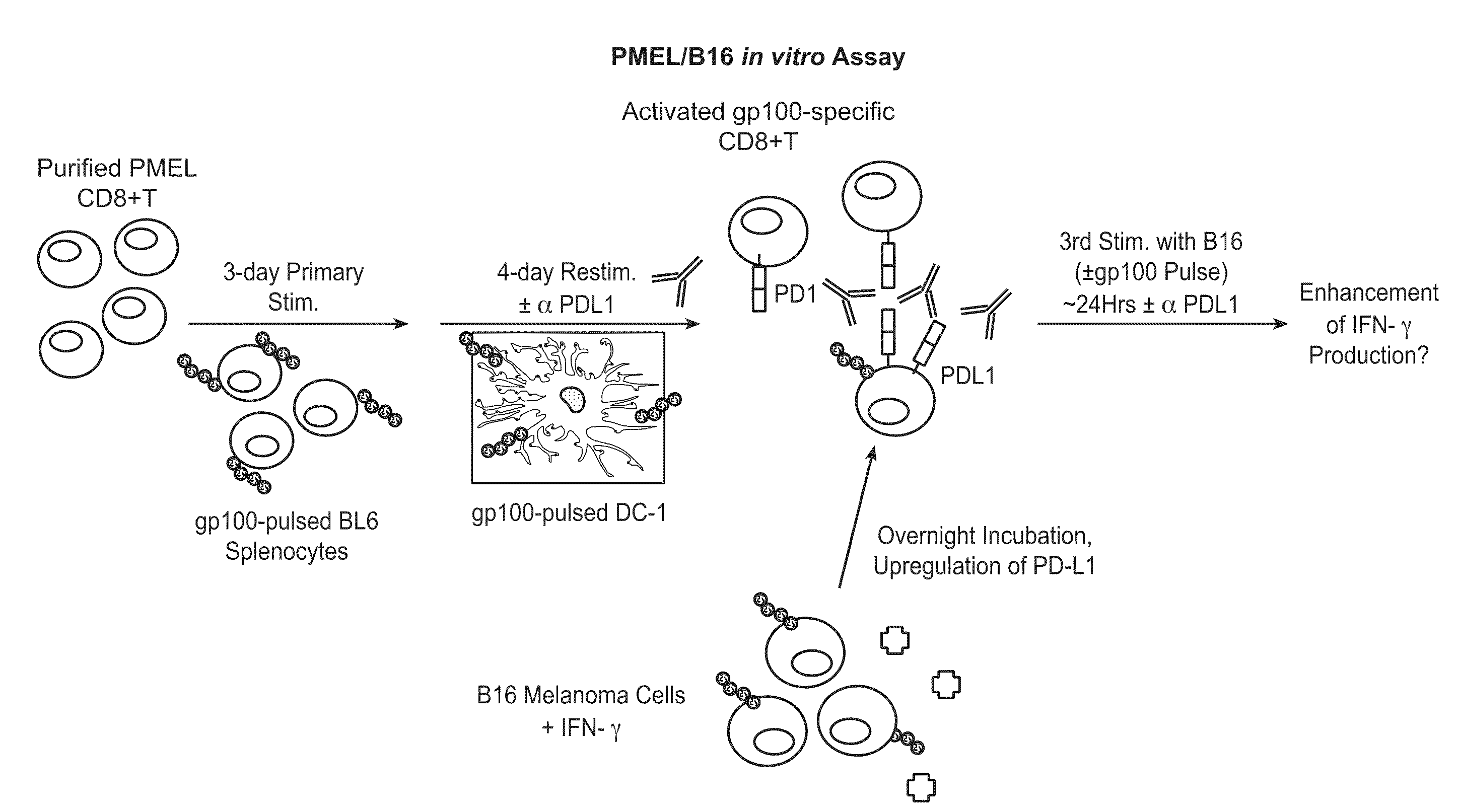
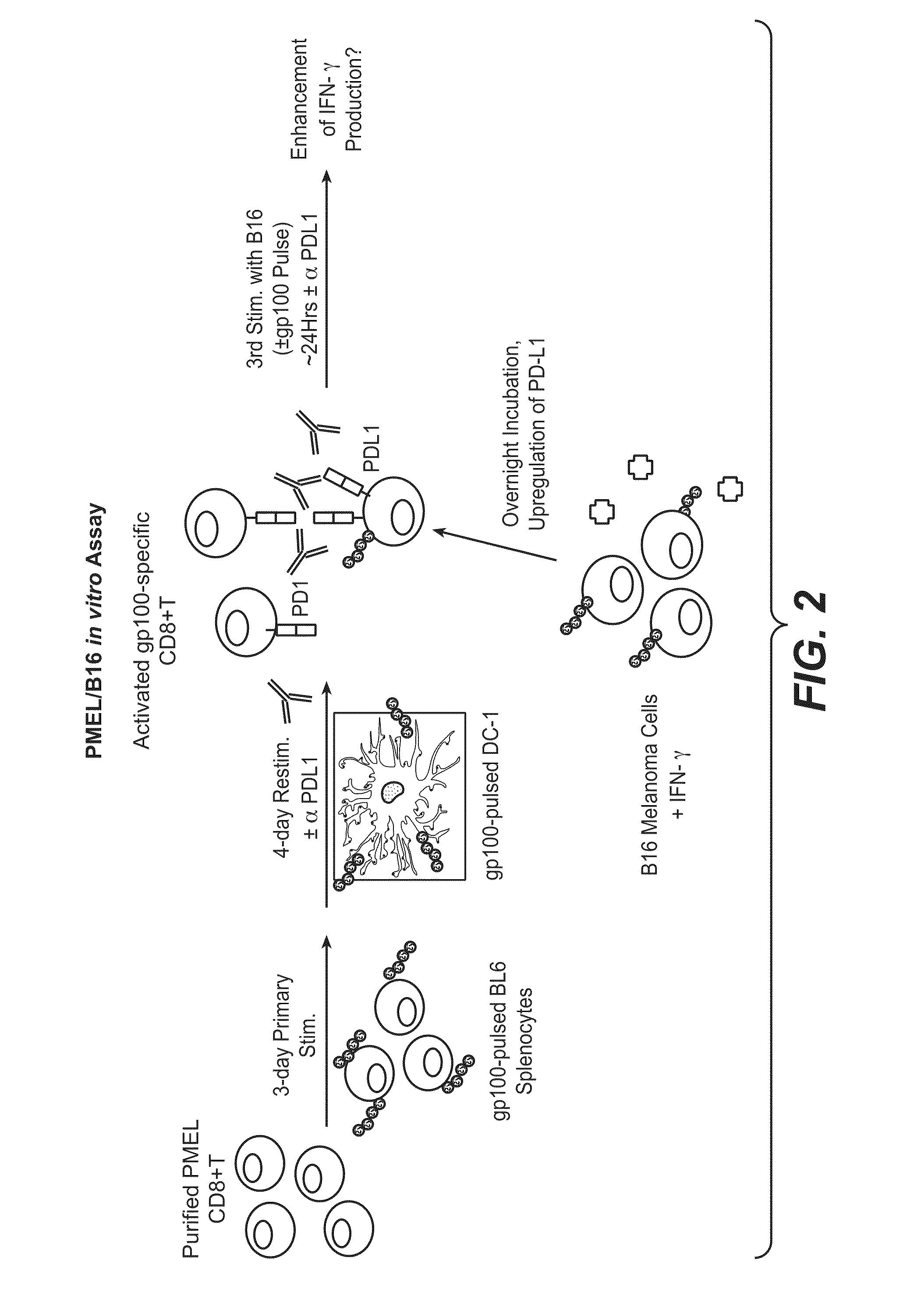
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
Anti-pd-l1 antibodies and their use to enhance t-cell function
ActiveUS20100203056A1Reduce level of pathogenChronic infectionAntibacterial agentsOrganic active ingredientsT-cell dysfunctionPD-L1
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
Anti-pd-l1 antibodies and uses thereof
ActiveUS20140341917A1Function increaseUpregulate cell-mediated immune responsesOrganic active ingredientsPeptide/protein ingredientsAntigen Binding FragmentAntigen binding
The present application relates to anti-PD-L1 antibodies or antigen binding fragments thereof, nucleic acid encoding the same, therapeutic compositions thereof, and their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity, for the treatment of and cancer.
Owner:MERCK PATENT GMBH
Methods of using Anti-pd-l1 antibodies and their use to enhance t-cell function to treat tumor immunity
InactiveUS20130045201A1Antibacterial agentsOrganic active ingredientsDiseaseAntiendomysial antibodies
The present application relates to methods of using anti-PD-L1 antibodies to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:GENENTECH INC
Modulation of the immune response
InactiveUS20120009222A1SsRNA viruses negative-senseOrganic active ingredientsCell mediated immunityImmune Stimulation
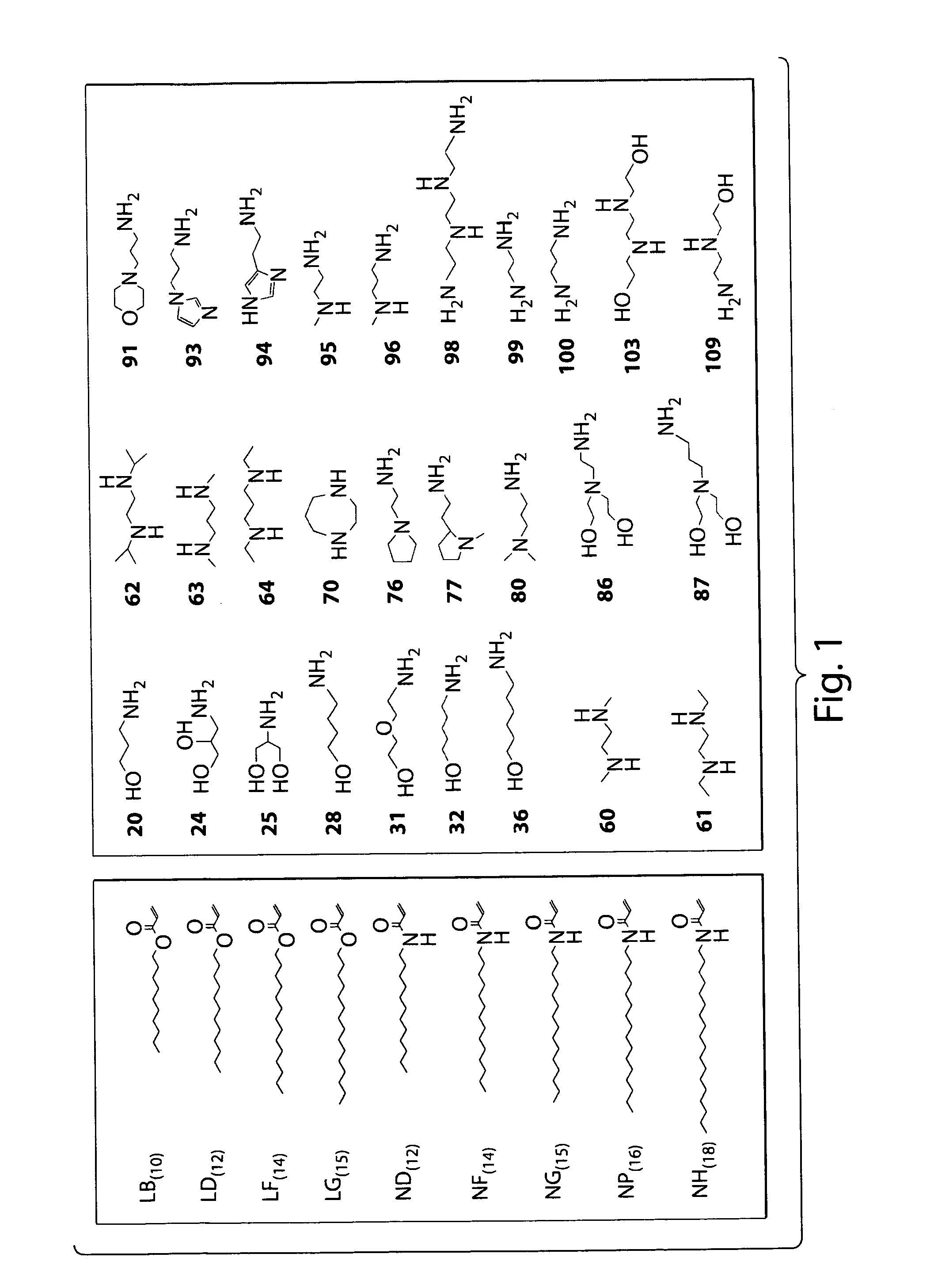
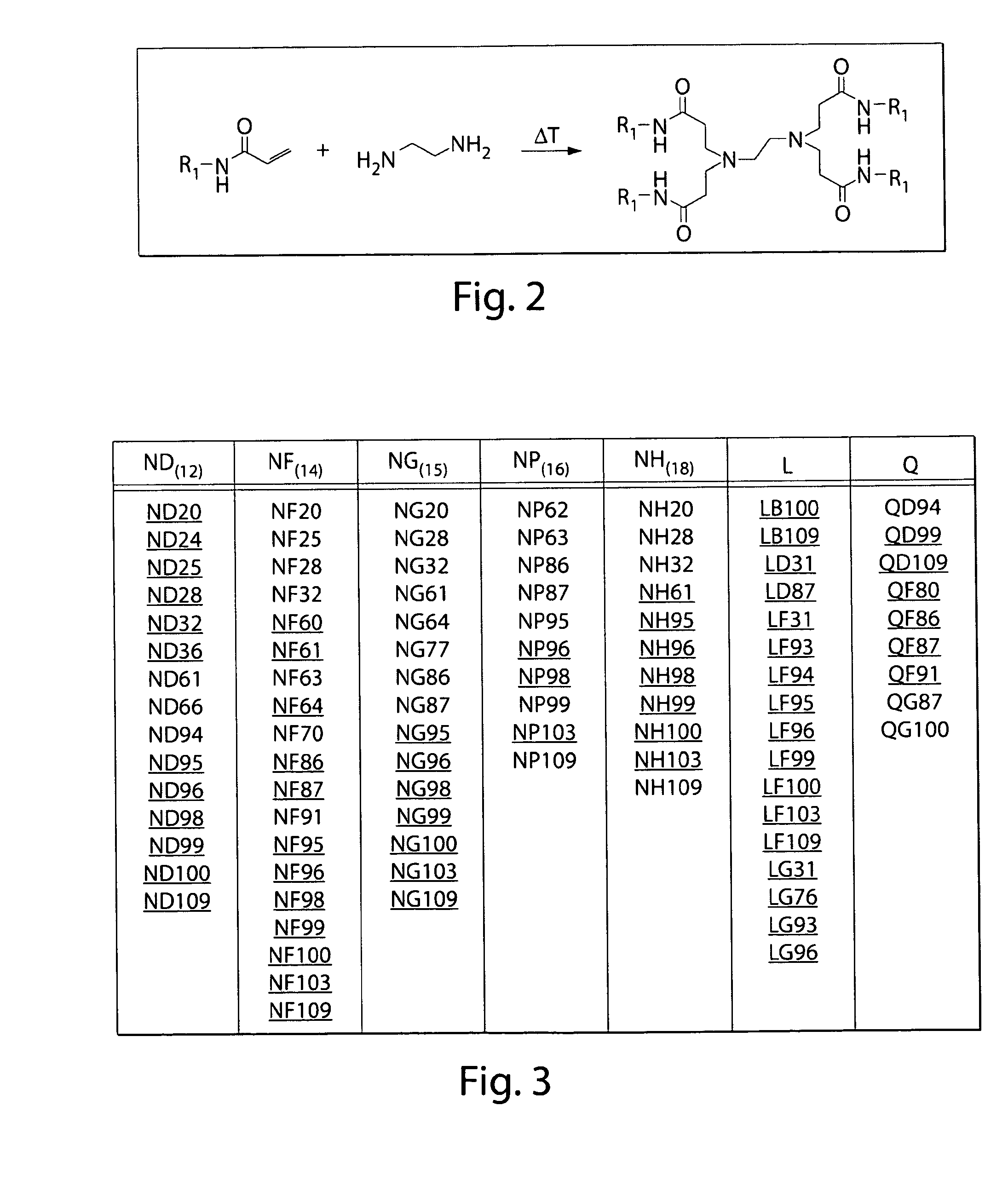
The present invention provides lipidoids that can be used to modulate the immune response in a subject. Lipidoids are prepared by the conjugate addition of an amine to an acrylate to acrylamide. The lipidoids form complexes or particles with an immunostimulatory polynucleotide, which are then administerd to a subject. Such compositions have been found to stimulate the production of cytokines and increase both humoral and cell-mediate immune response. The invention also provides pharmaceuti-cal compositions thereof and methods for using the same.
Owner:MASSACHUSETTS INST OF TECH
Anti-PD-L1 antibodies and uses thereof
ActiveUS9624298B2Function increaseUpregulate cell-mediated immune responsesNervous disorderPeptide/protein ingredientsAntigen Binding FragmentPD-L1
The present application relates to anti-PD-L1 antibodies or antigen binding fragments thereof, nucleic acid encoding the same, therapeutic compositions thereof, and their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity, for the treatment of and cancer.
Owner:MERCK PATENT GMBH
Methods and compositions for increased priming of t-cells through cross-presentation of exogenous antigens
InactiveUS20080171059A1Easy to demonstrateEffective vaccineTissue cultureCancer antigen ingredientsDiseaseVaccination
Methods for eliciting in an animal in need thereof a cell-mediated immune response specific to an antigen, the method comprising providing an antigen preparation comprising particles on the surface of which the antigen is attached, and administering the antigen preparation to the animal, wherein the particles are taken up by antigen presenting cells (APC) of the animal via phagocytosis, forming a phagosome inside the APC, wherein the antigen is attached to the surface of the particle in such a way that the antigen is released in the phagosome before the phagosome fuses with a late endosome or a lysosome, and wherein the antigen is cross-presented on a Class I MHC molecule. Also provided are particulate antigen preparations or particulate vaccines that can be delivered to an animal in need thereof for vaccination against, for preventing or treating, a disease related to the antigen, such as cancer and a viral infection.
Owner:LUDWIG INST FOR CANCER RES +1
Adjuvant combinations of liposomes and mycobacterial lipids for immunization compositions and vaccines
ActiveUS8241610B2Good auxiliary effectEnhance immune responseAntibacterial agentsBiocideLiposomeMycobacterium
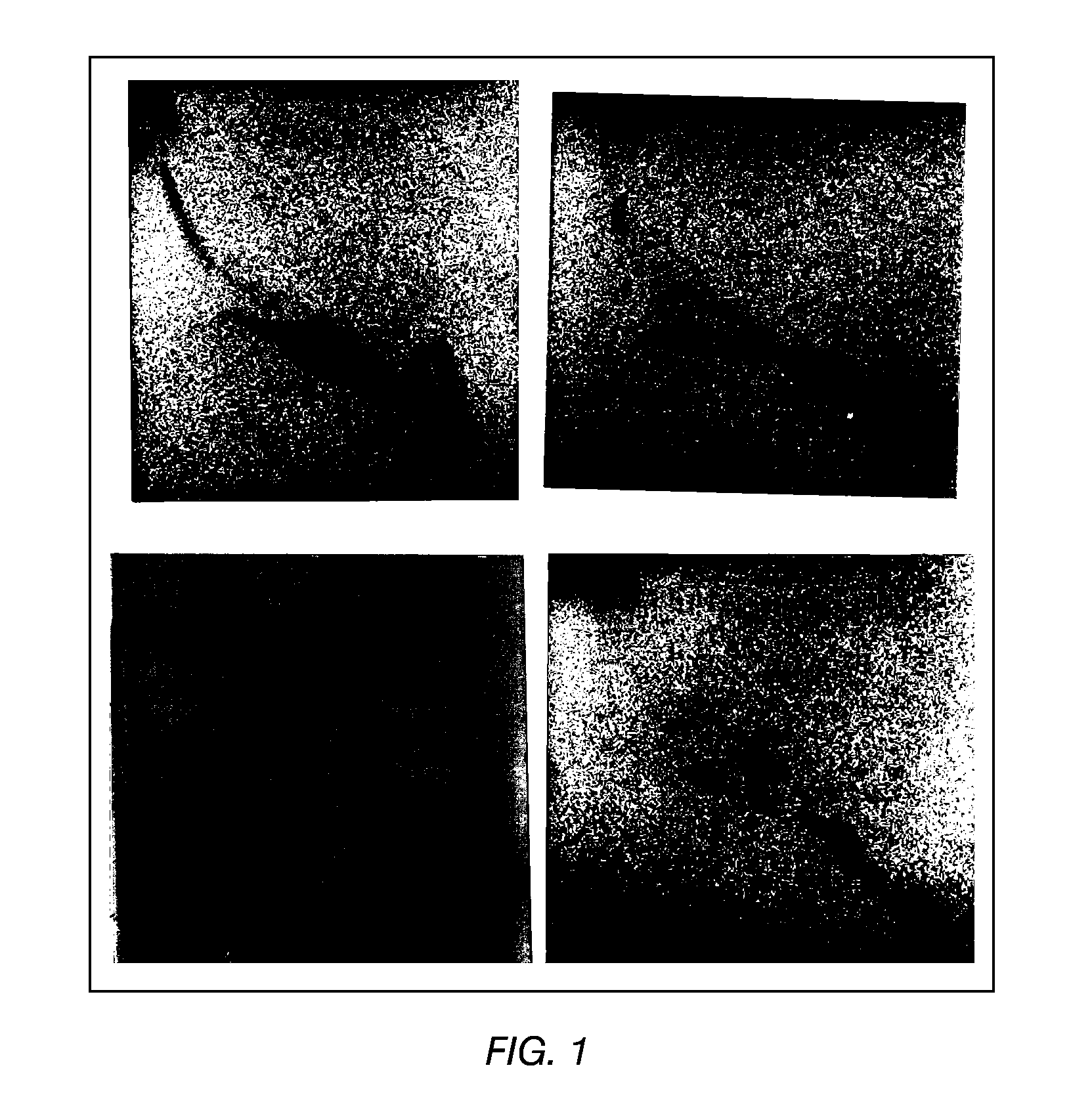
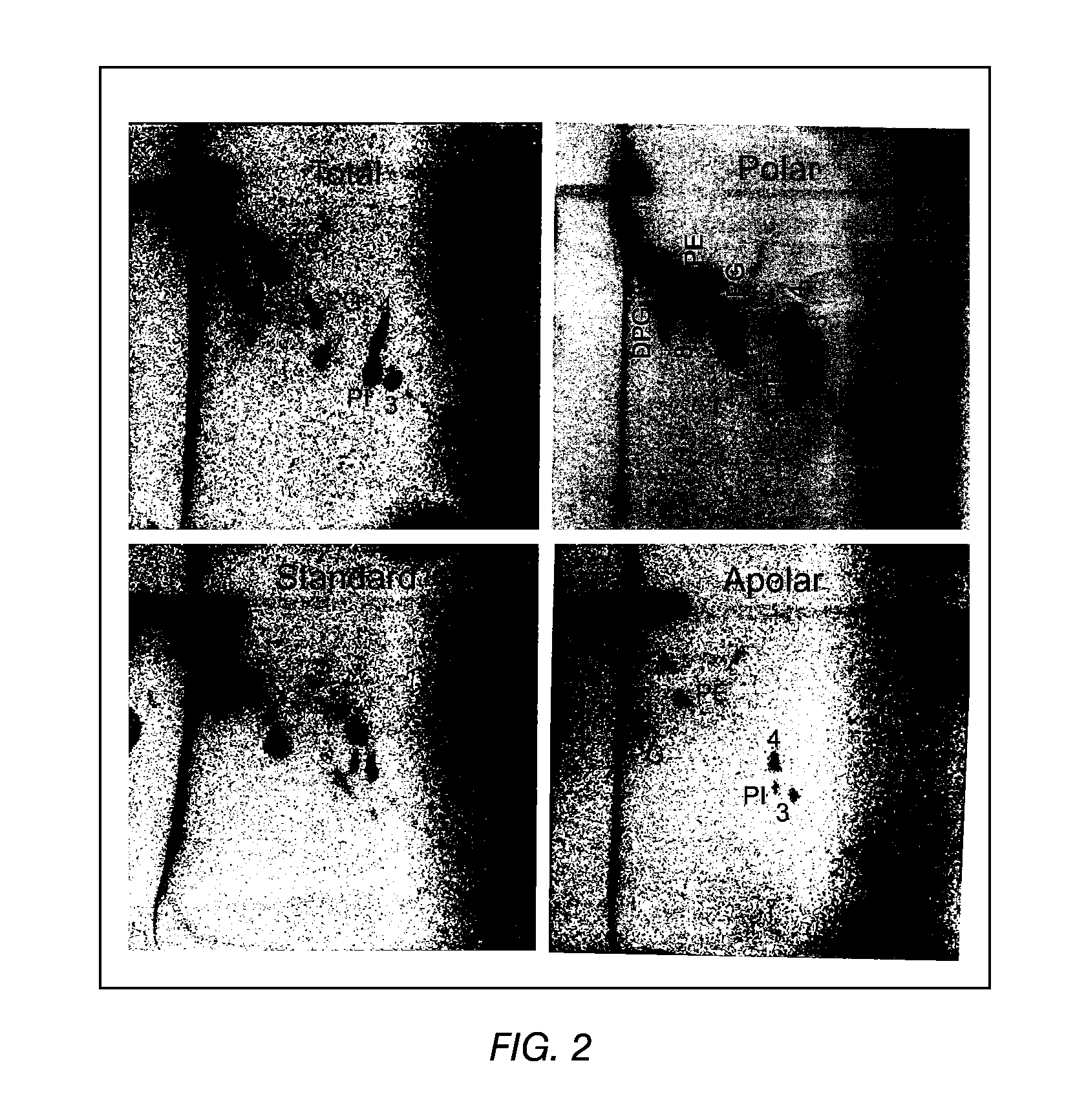
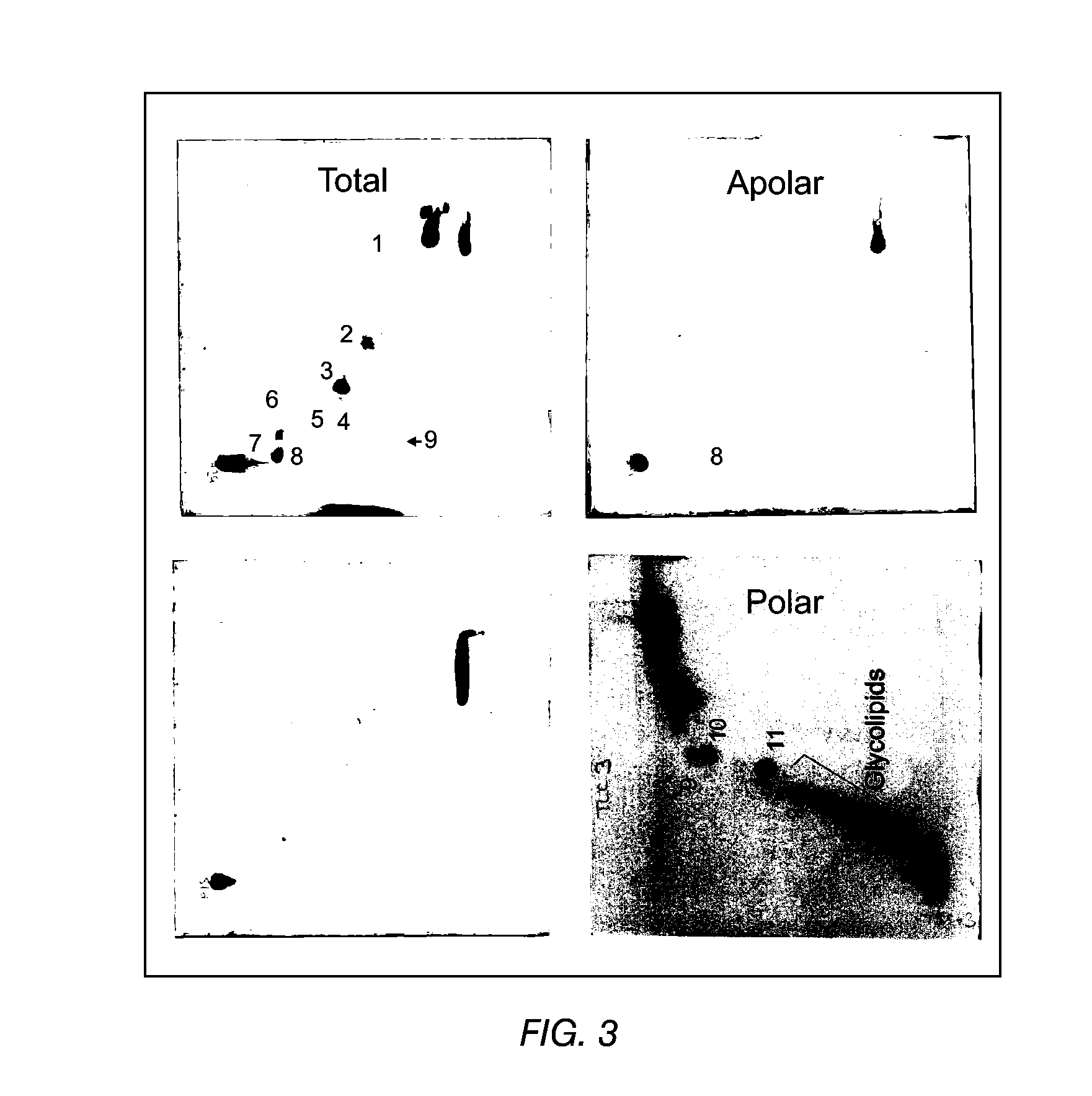
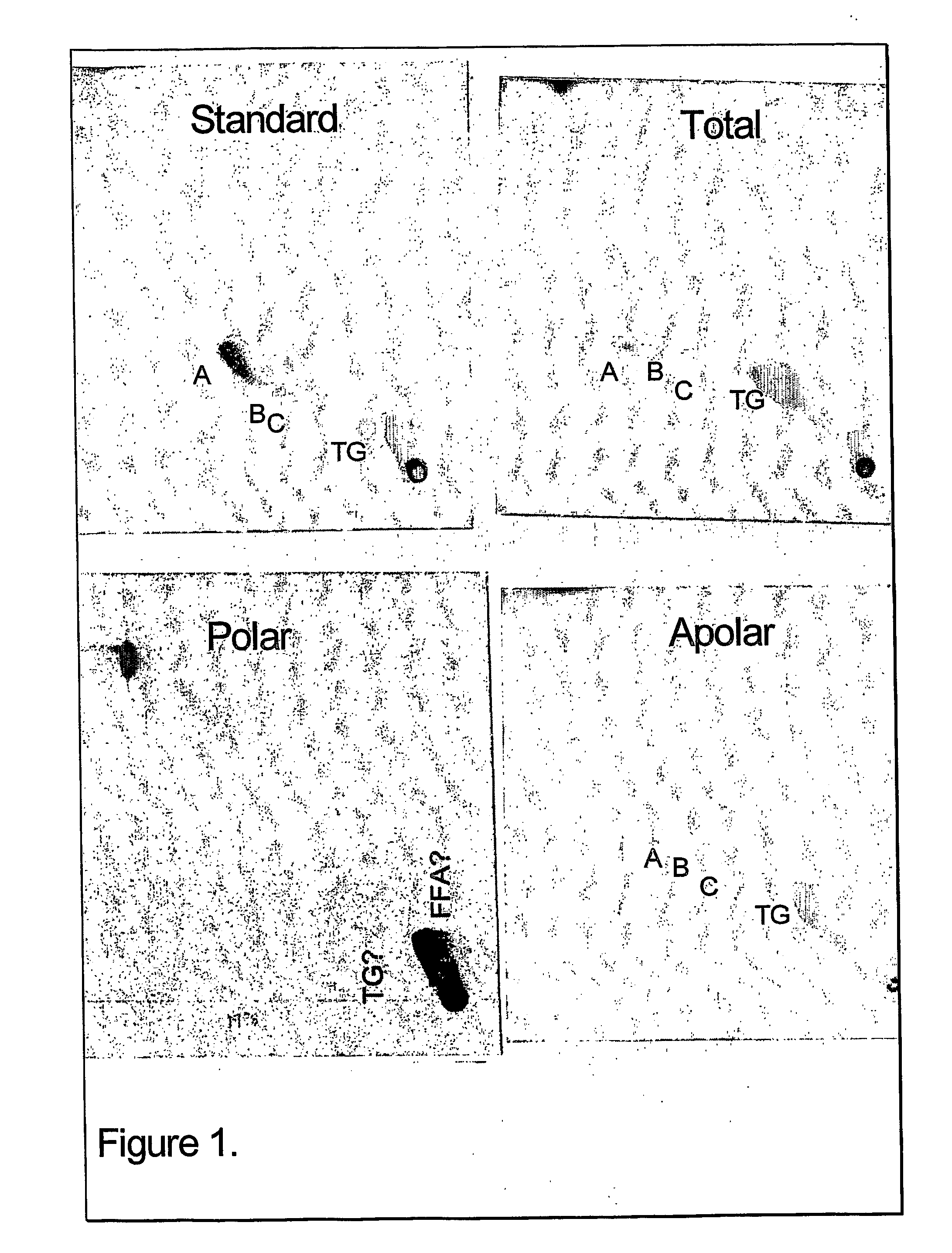
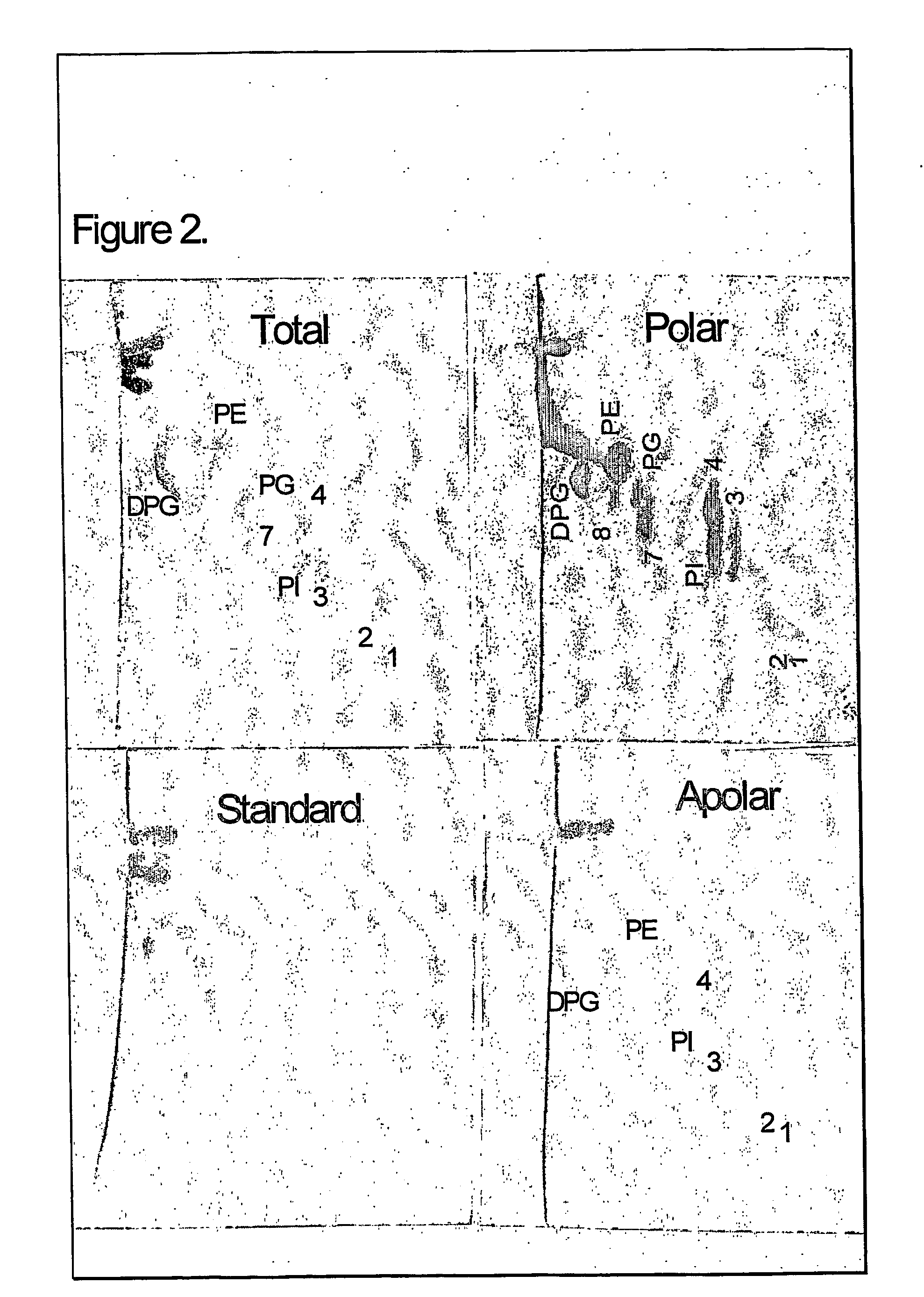
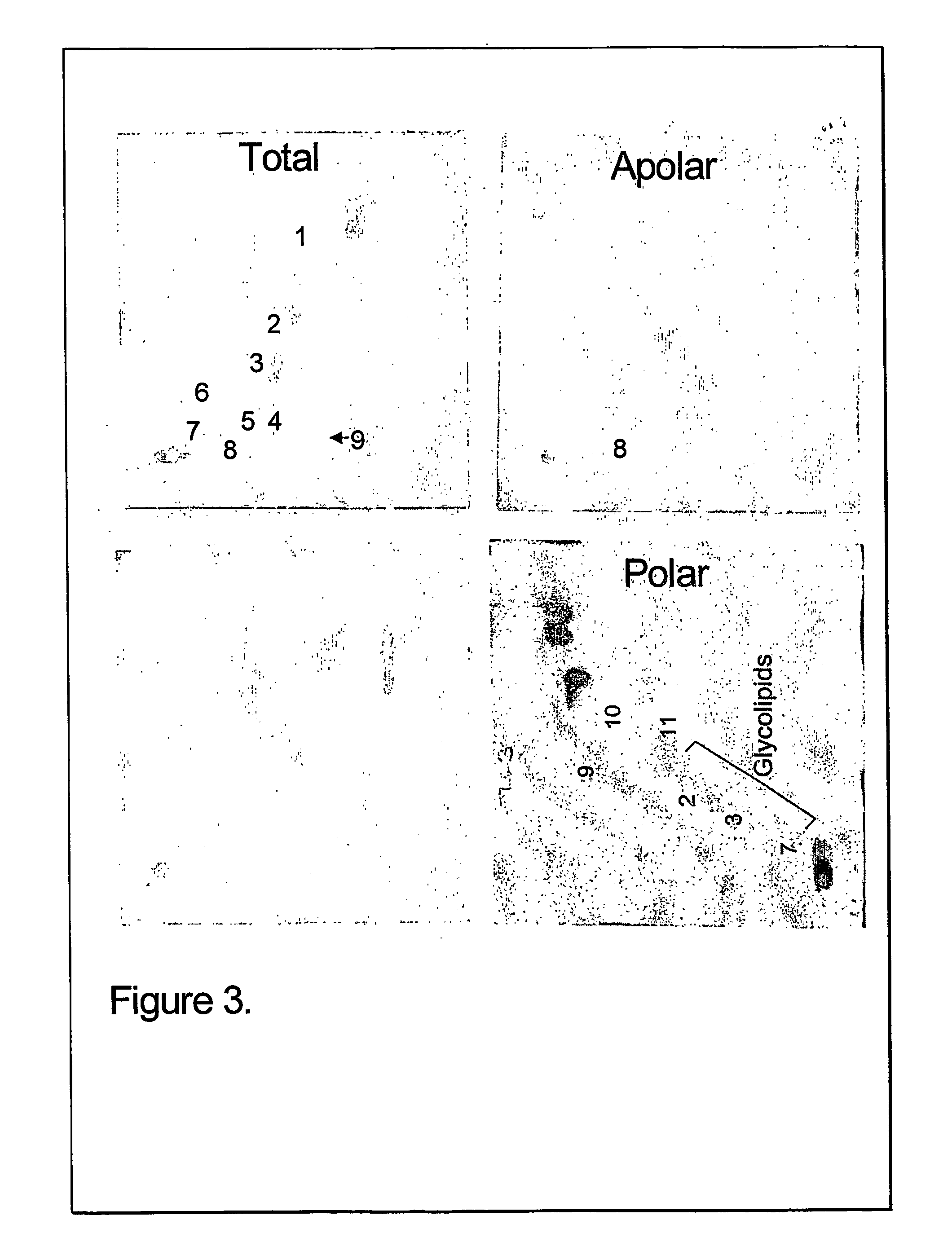
The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis BCG. The total lipid extract contains both apolar lipids, polar lipids, and lipids of intermediate polarity of which the apolar lipids were found to induce the most powerful immune responses. The total lipids may be extracted with chloroform / methanol and re-dissolved in water before the addition of surfactant. This preparation may be used to induce prominent cell-mediated immune responses in a mammal in order to combat pathogens, or as a treatment for cancer.
Owner:STATENS SERUM INST
Vaccine compositions and methods of treating coronavirus infection
InactiveUS20060286124A1Elicit immune responseSsRNA viruses positive-sensePeptide/protein ingredientsAdjuvantSARS coronavirus
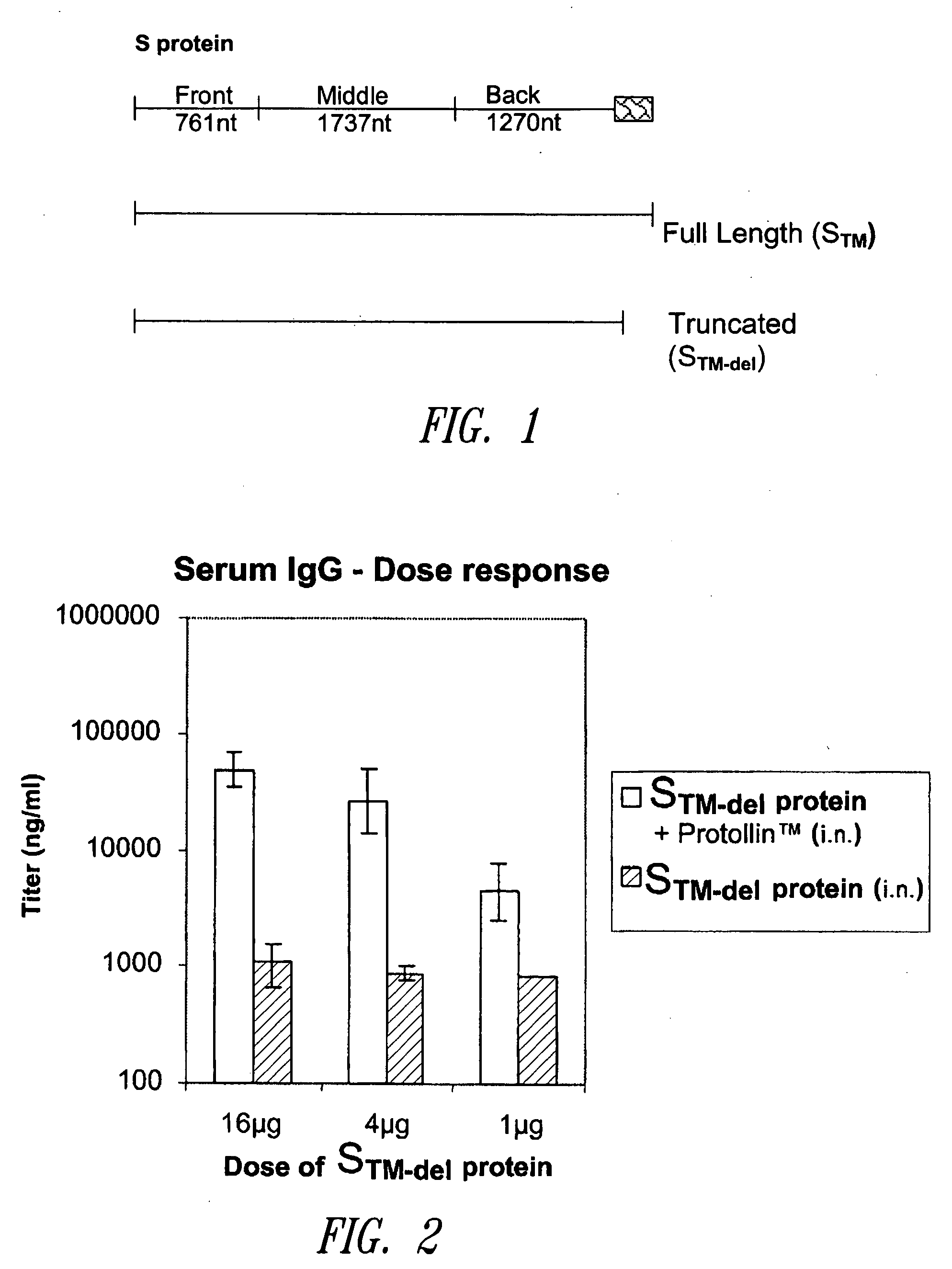
The present disclosure relates to compositions and methods for treating or preventing coronavirus infections. For example, compositions are provided that comprise a coronavirus S protein or N protein, fragment, or variant thereof, capable of eliciting a protective humoral and / or cell-mediated immune response, which compositions are useful for treating or preventing infection by coronavirus, such as the causative agent of SARS. Also, coronavirus S protein and N protein immunogen compositions are provided that include an adjuvant, such as Proteosome or Protollin, which may be used for treating or preventing infection caused by a coronavirus, such as a SARS coronavirus.
Owner:ID BIOMEDICAL CORP LAVAL
Gelled immunomodulating topical compositions and a method of treating warts and other human papilloma virus skin infections
Topical drug compositions of this invention contain delayed type contact sensitizing haptens in a unique non-flowable, non-toxic, non-volatile, anhydrous gel composition to achieve retained site application on warts and other human papilloma virus (HPV) skin infections. The preferred gelled compositions contain, but are not limited to, the sensitizing haptens, squaric acid dibutylester and diphenylcyclopropenone in optimized blends of Polysorbate 80, Isopropyl myristate uniquely gelled with Polyoxyl 40 stearate to form a penetrant of keratinized epitheliiuim of warts for direct application wherein virucidal pharlacologic action is induced by Th-1 cell mediated immune responses with resultant releases of CD4 helper T cells, CD8 killer T cells and cytokines to attack the human papilloma viruses. The commonly used vehicles with these contact sensitizers are acetone, petrolatum, or water containing emulsion creams which do not have the capacity to penetrate the keratinized wart surfaces and are therefore minimally effective in treating warts.
Owner:HAPTEN PHARMA
Immunostimulation methods for providing disease protection in poultry
InactiveUS6019985ACause painCause stressBacterial antigen ingredientsProtozoa antigen ingredientsIn ovoCoccidiosis
Methods are provided for improved immunization against coccidiosis and other bacterial, viral, or parasitic diseases in poultry. One method includes administering a solution of Propionibacterium acnes suspended in normal saline to a chick at age day one, following hatching. An anticoccidial vaccine and / or other vaccine is then administered to the chick such as by oral administration. Alternatively the method includes the steps of aseptically injecting Propionibacterium acnes in ovo at about day 18 in development, followed by post-hatching vaccination with an anticoccidial vaccine and / or other vaccine. Alternatively, either method can be utilized without the subsequent vaccination step for stimulating non-specific cell-mediated immune responses in poultry.
Owner:NEOGEN CORP
Recombinant proteins from filoviruses and their use
ActiveUS20070082011A1Decrease cell surface expressionLower immune responseSsRNA viruses negative-senseViral antigen ingredientsCell-mediated immune responseImmunogenicity
Filovirus subunit protein immunogens are produced using a recombinant expression system and combined with one or more adjuvants in immunogenic formulations. The subunit proteins include GP95, GP-FL, VP40, VP24, and NP derived from Ebola Virus and Marburg Virus. Adjuvants include saponins, emulsions, alum, and dipeptidyl peptidase inhibitors. The disclosed immunogenic formulations are effective in inducing strong antibody responses directed against individual Filovirus proteins and intact Filovirus particles as well as stimulating cell-mediated immune responses to the Filoviruses.
Owner:PANTHERA BIOPHARMA
Immune cell compositions and methods of use
ActiveUS20180273601A1Restores effector functionEnhances tumor burden controlPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorT cell mediated immunity
Disclosed herein are cells that are immune cells or precursor cells thereof, which cells recombinantly express a chimeric antigen receptor (CAR), and a dominant negative form of an inhibitor of a cell-mediated immune response of the immune cell, wherein the CAR binds to a cancer antigen. Also disclosed herein are T cells that recognize and are sensitized to a cancer antigen, which T cells recombinantly express a dominant negative form of an inhibitor of a T cell-mediated immune response. Additionally provided are methods of using such cells to treat cancer in a subject in need thereof.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Diagnostic assay for measuring a cell mediated immune response
ActiveUS20050014205A1Reduce handling costsReduce exposureMicrobiological testing/measurementBiological testingWild lifeCell-mediated immune response
The present invention relates generally to a diagnostic assay and, more particularly, an assay for measuring cell-mediated immune reactivity. Even more particularly, the present invention provides an assay and a kit for measuring a cell-mediated response to an antigen using whole blood or other suitable biological sample. The assay may be conducted using ligands to immune effector molecules or at the nucleic acid level, screening for expression of genes encoding the immune effector molecules. The assay is useful in therapeutic and diagnostic protocols for human, livestock and veterinary and wild life applications.
Owner:CELLESTIS
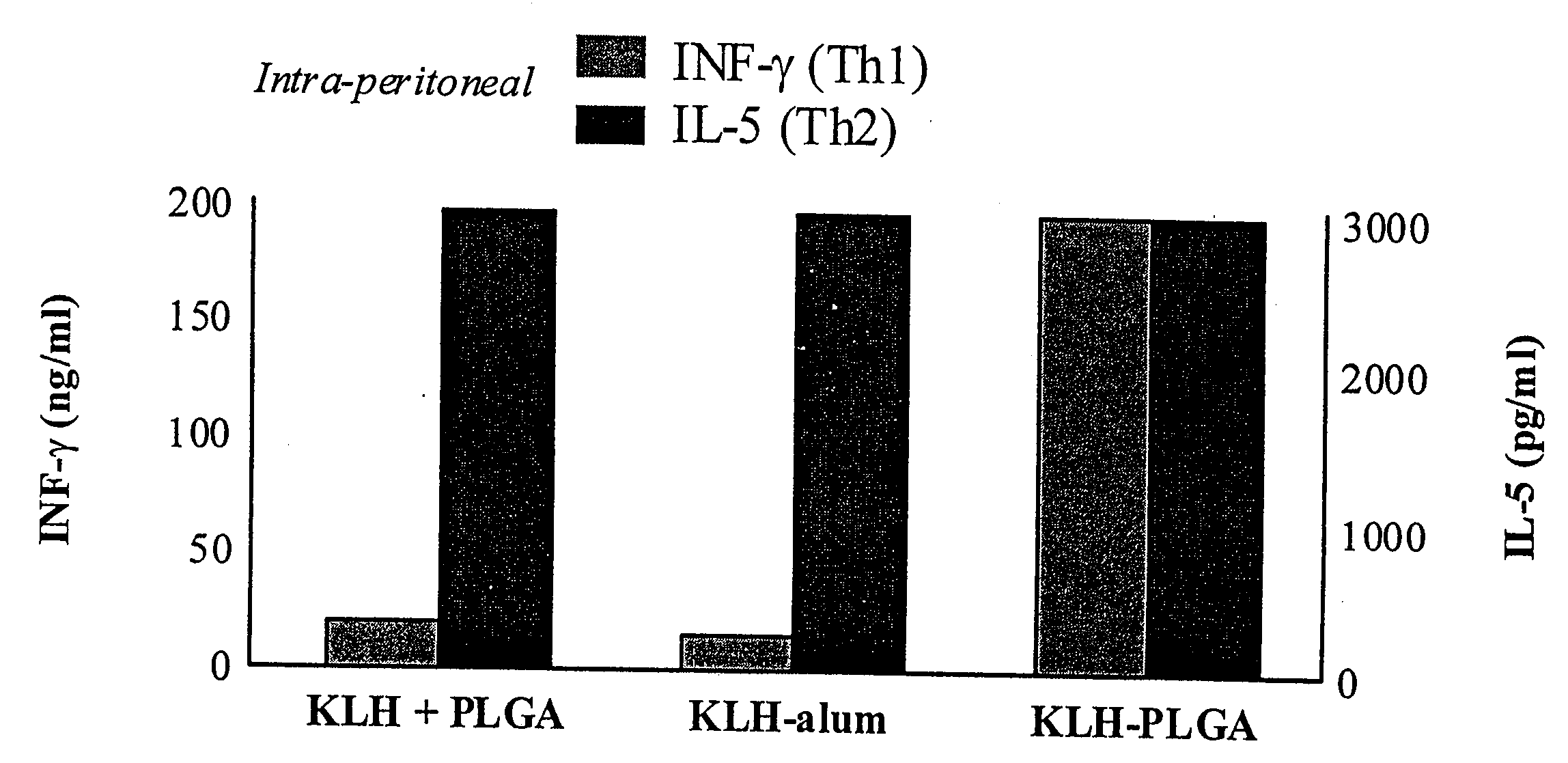
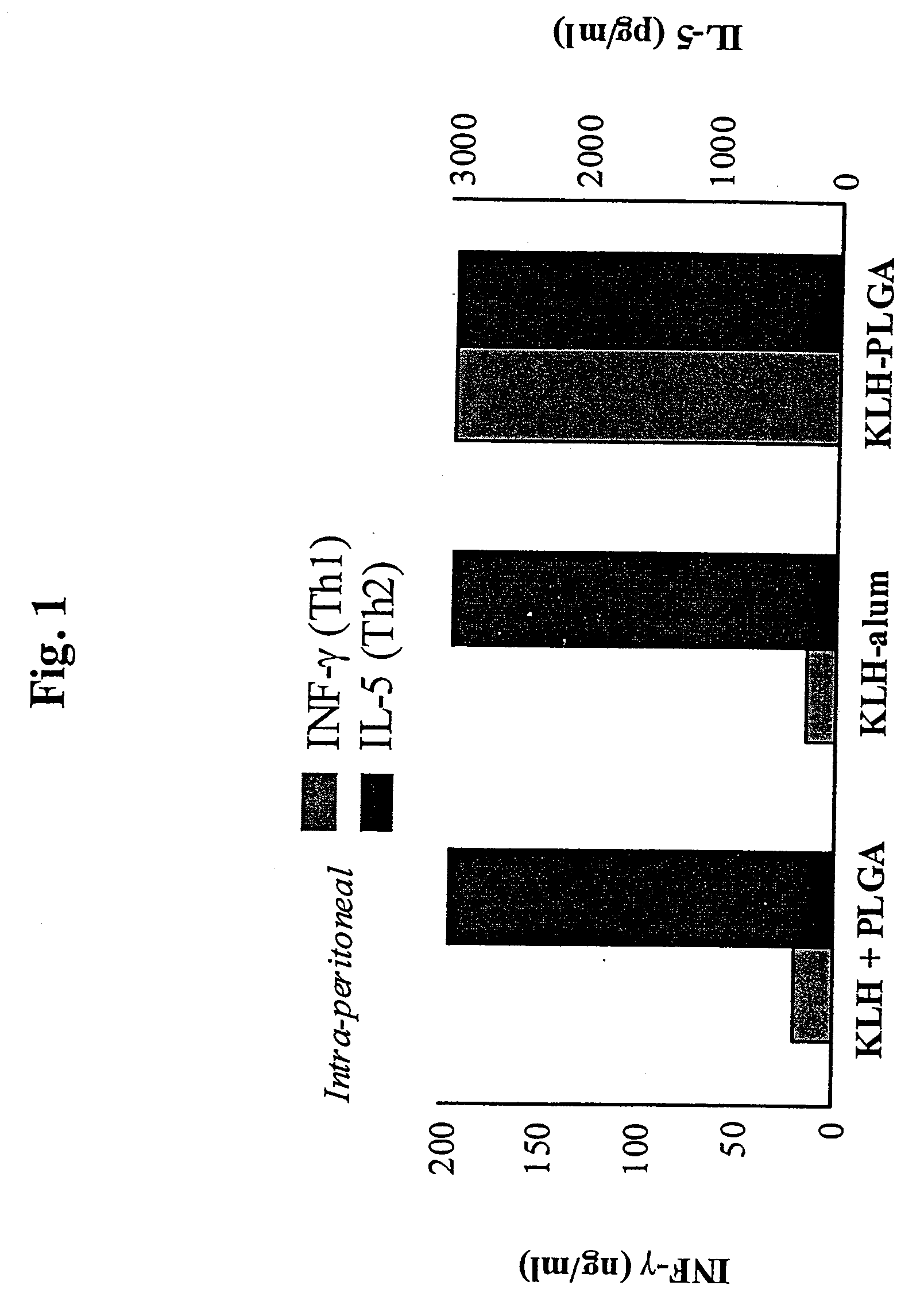
Method for inducing a cell-mediated immune response and improved parenteral vaccine formulations thereof
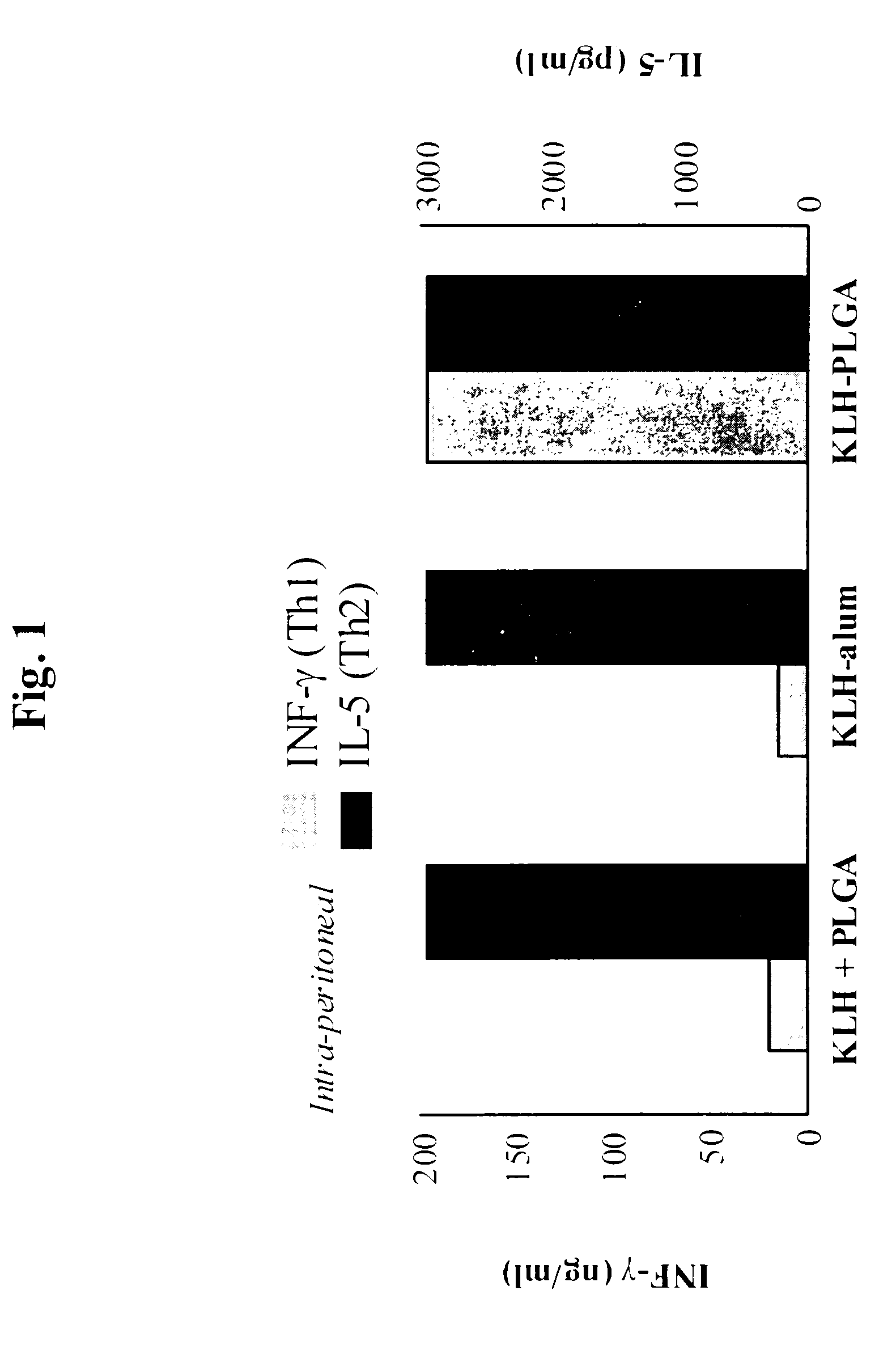
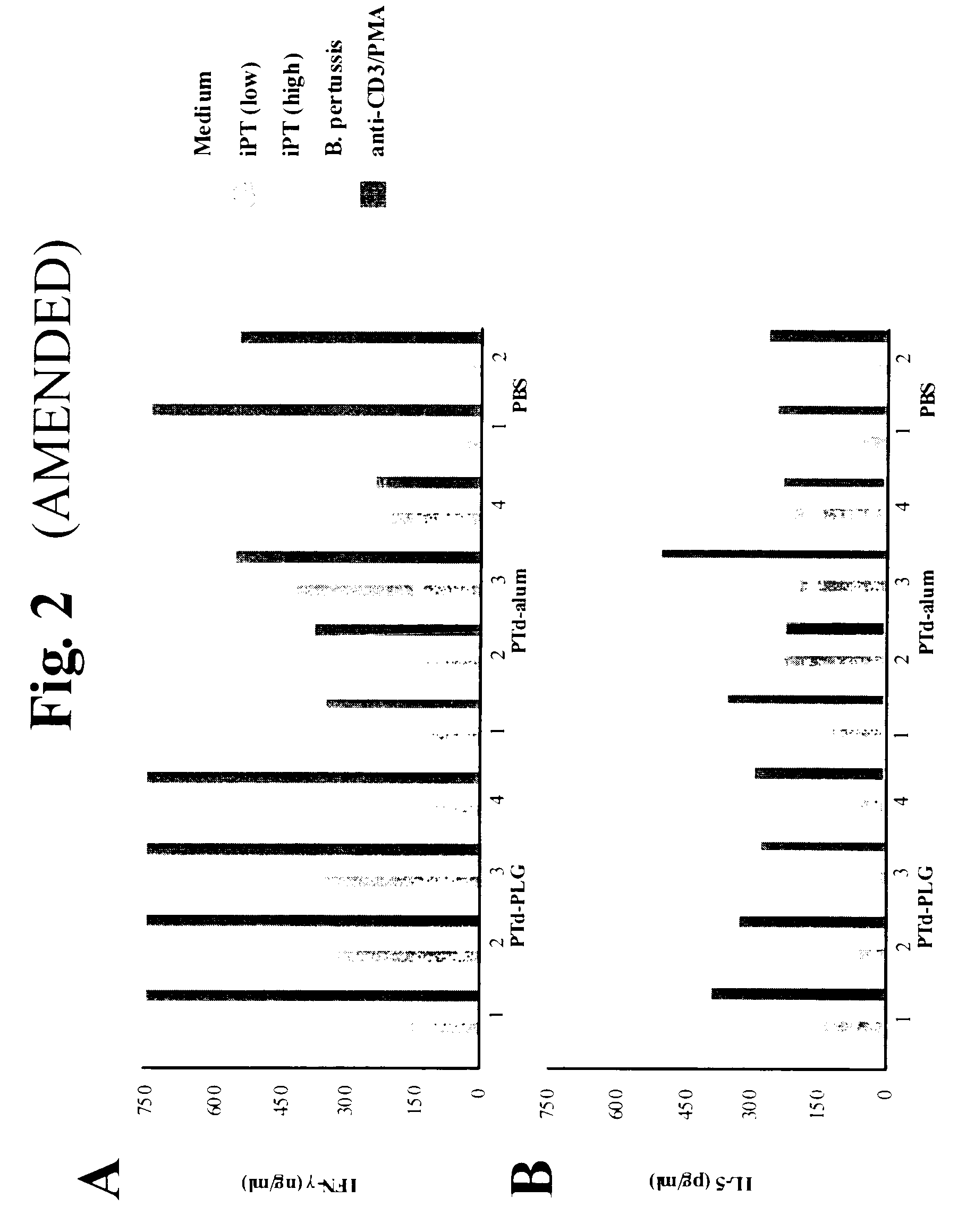
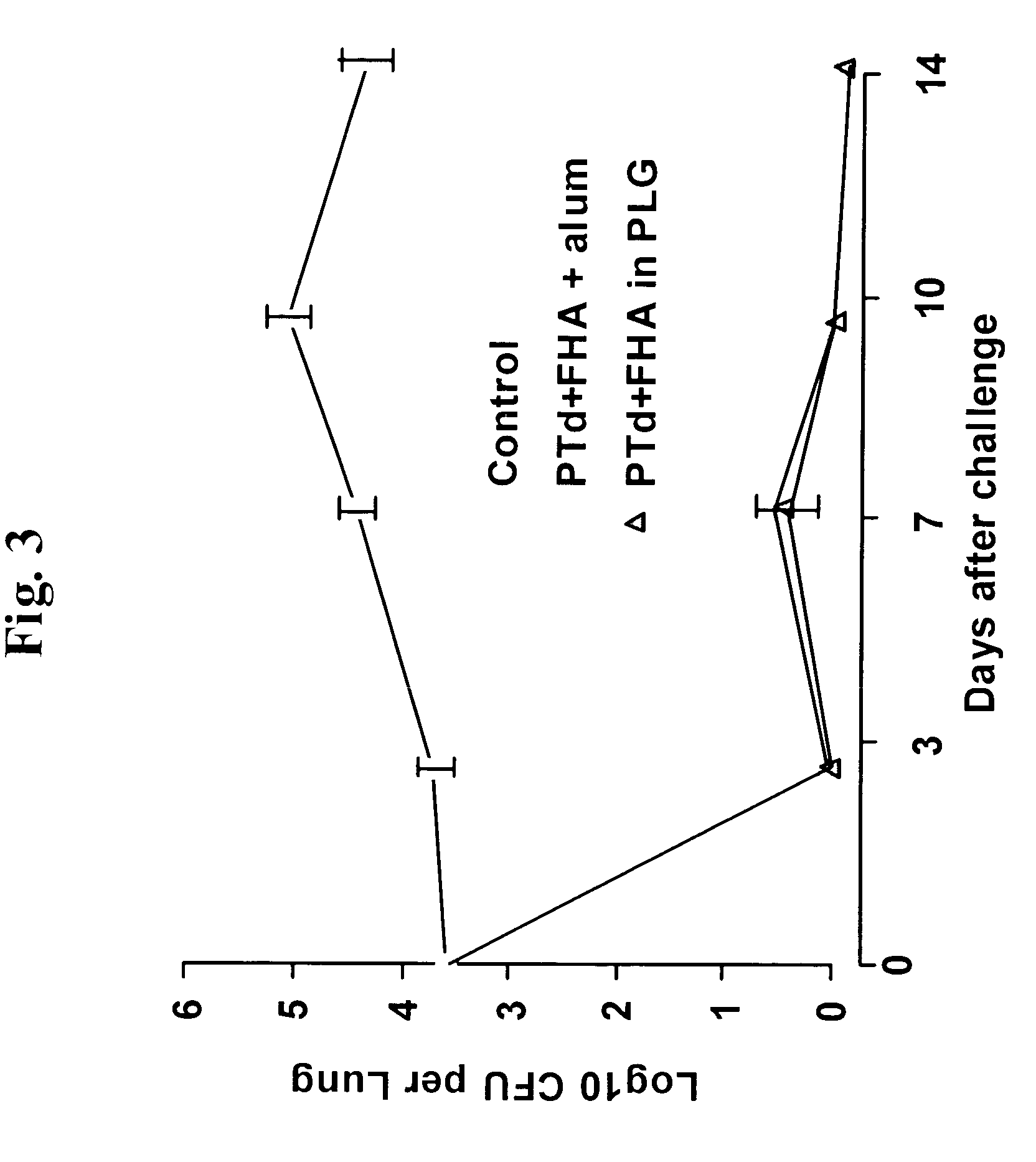
A method of inducing either a TH1 polarized immune response, a TH2 polarized immune response or a combined TH1 and TH2 response to an antigen and associated vaccine formulations are disclosed. A method is provided for inducing a polarized TH1 response by parenteral administration of microparticles sized such that at least 50% of the microparticles are less than 5 μm. The microparticles containing antigen entrapped or encapsulated by a biodegradable polymer. Additionally, a method is provided for inducing a polarized TH2 response by parenteral administration of nanoparticles sized such that at least 50% of the nanoparticles are less than 600 nm, the nanoparticles containing antigen entrapped or encapsulated by a biodegradable polymer. Vaccine formulations containing the B. pertussis antigens PTd, FHA or a combination of PTd and FHA are provided.
Owner:NOVO NORDISK AS
T-cell vaccination with viral vectors via mechanical epidermal disruption
ActiveUS8691502B2Strong immune responseProtected growthAntibacterial agentsAntimycoticsAdjuvantCuticle
Attenuated, replication-deficient viruses such as vaccinia viruses are used to deliver an exogenous viral, bacterial, parasitic or tumor antigen to an epidermal tissue such as the skin, lungs or gastrointestinal tract, which has been mechanically disrupted, in an amount effective to elicit or stimulate a cell mediated immune response. The epidermal tissue may be mechanically disrupted by a device such as a scarification needle or an abrader device. The epidermis may be mechanically disrupted prior to, at the same time, or immediately after the administration of the vaccine. The vaccine can be used to induce immunity against a pathogen, such as a virus, bacteria, or parasite, or against a cancer in a subject that has or is at risk of developing cancer. In some embodiments a co-stimulatory molecule, a growth factor, an adjuvant and / or a cytokine is administered before, with or after the viral vaccine. In some embodiments, the co-stimulatory molecule is co-expressed with the antigen by the virus.
Owner:TREMRX
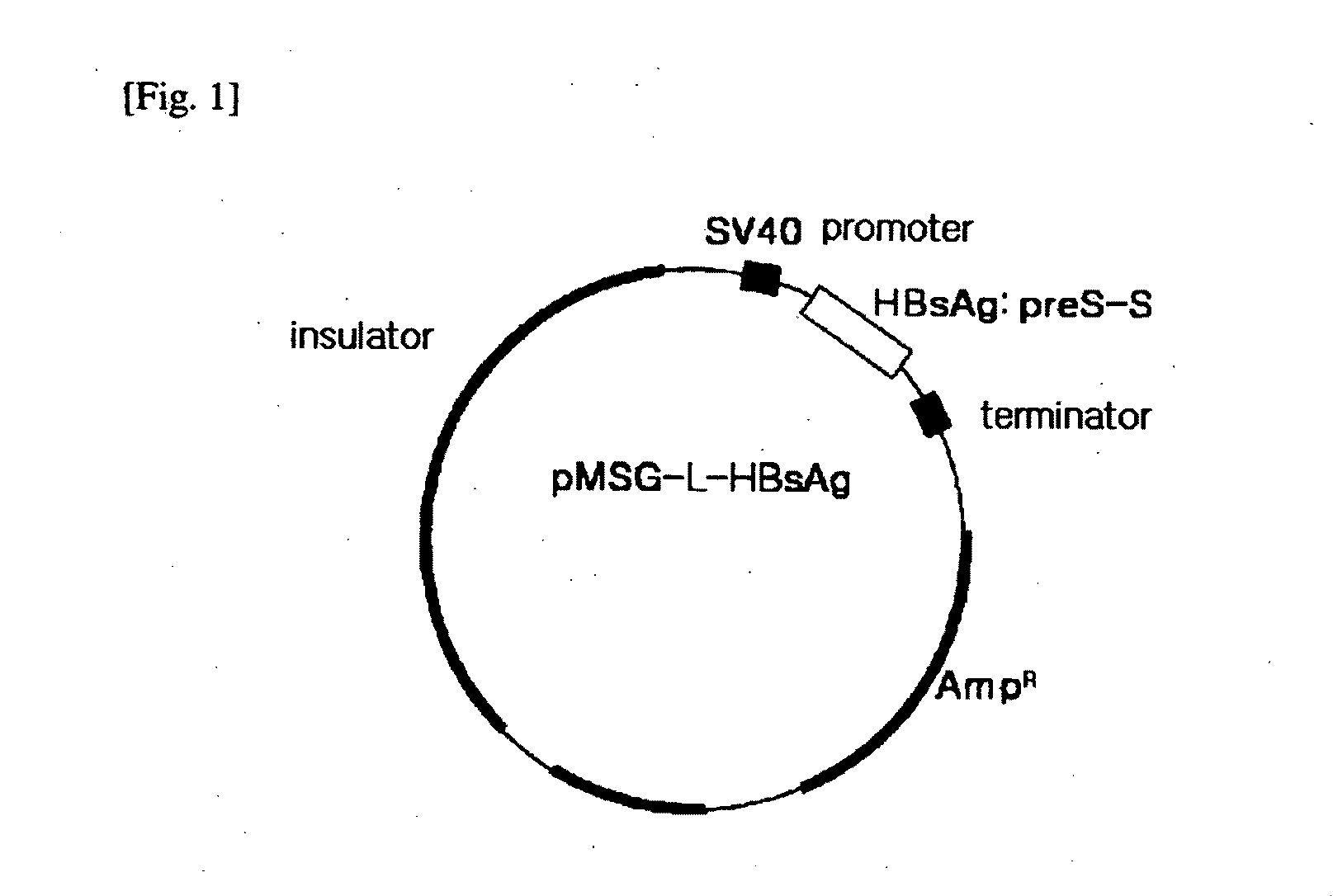
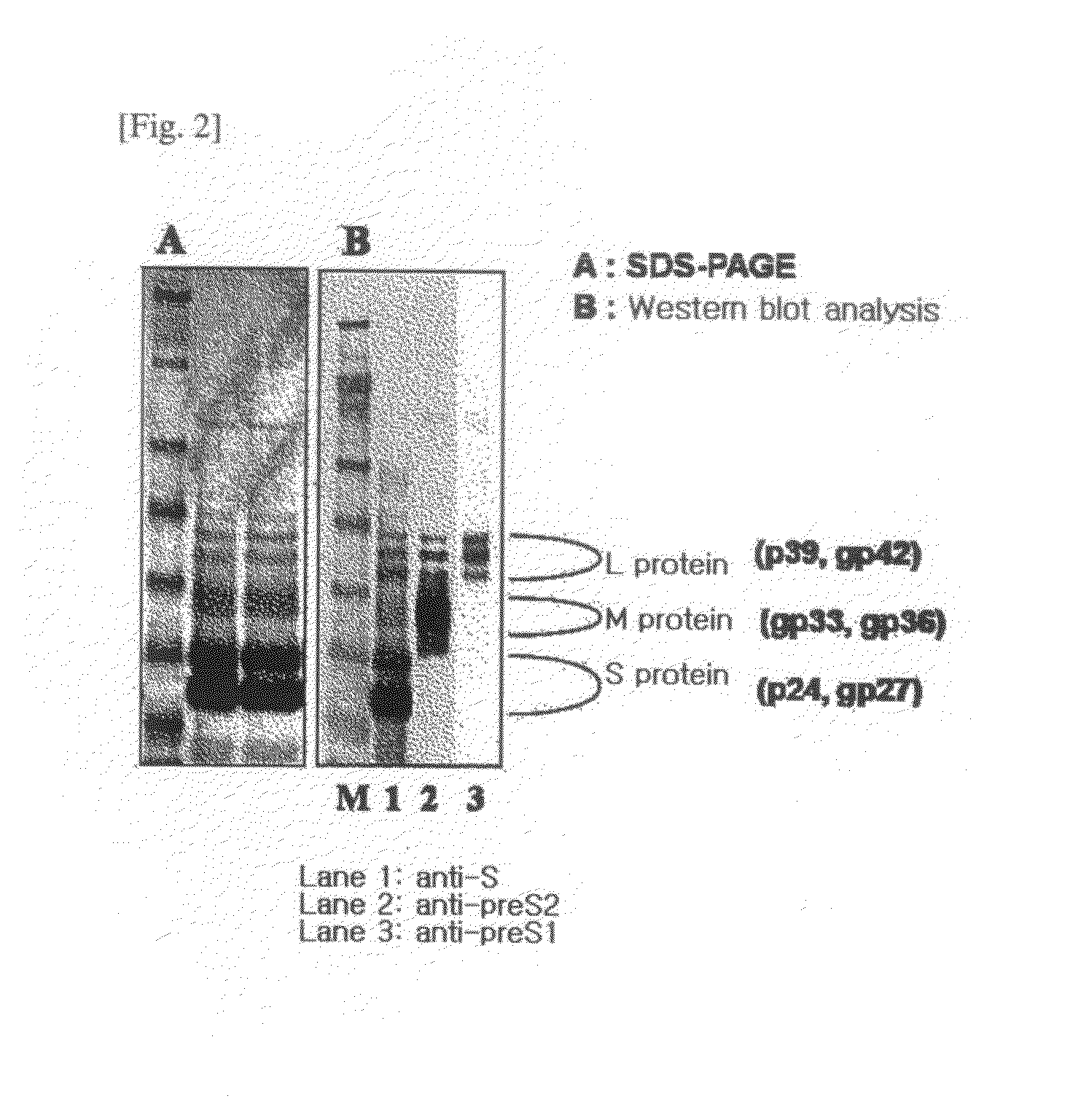
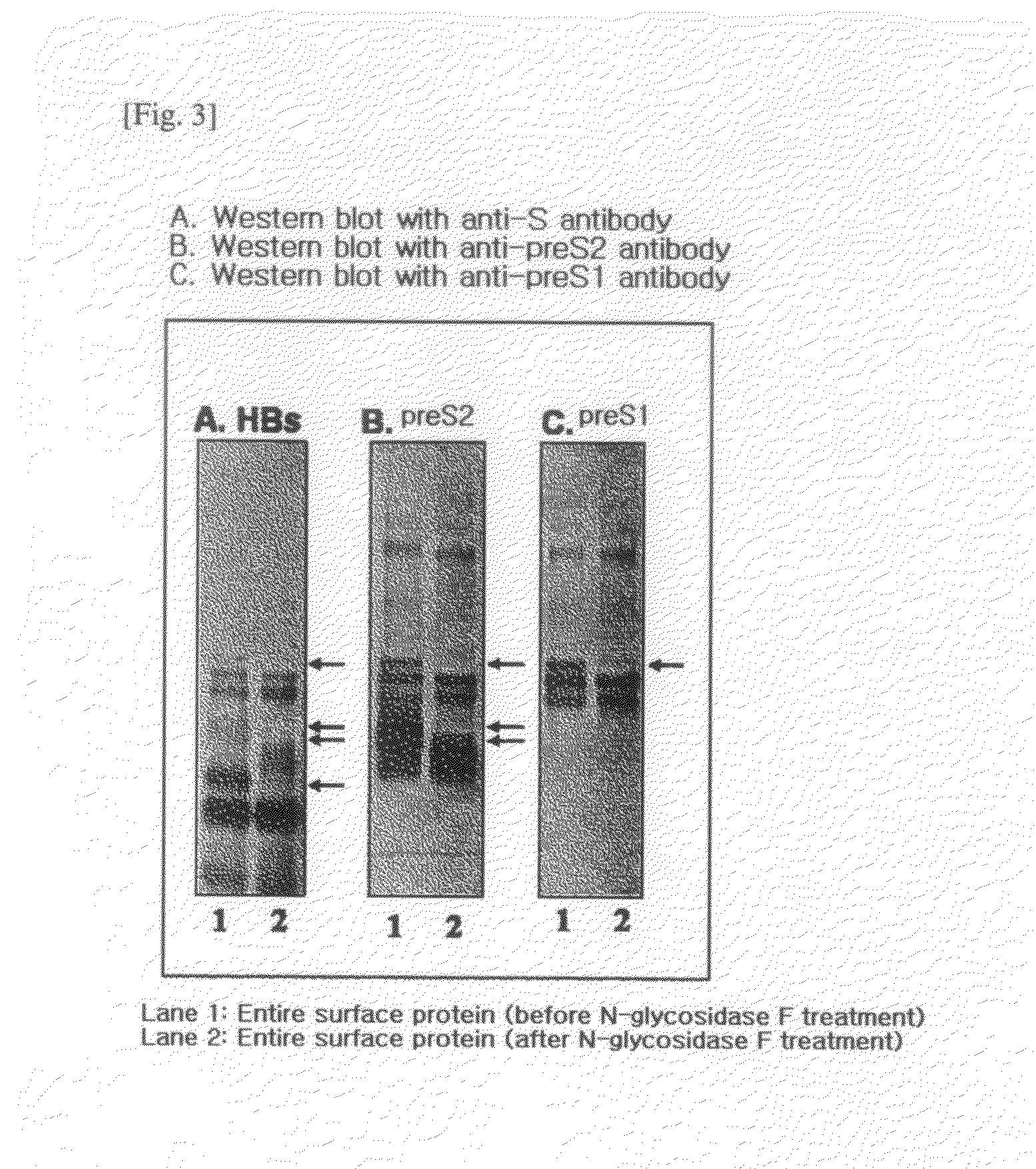
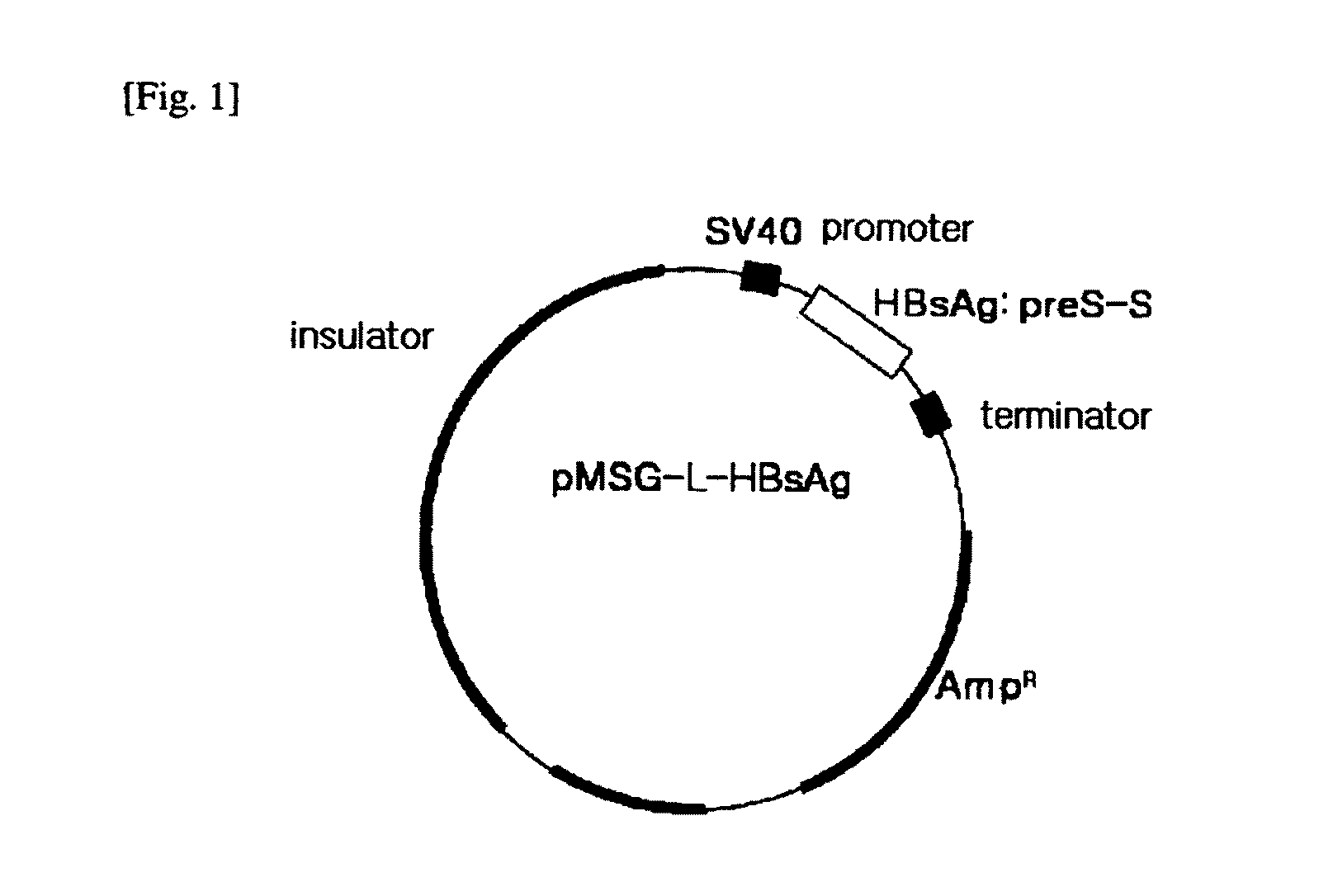
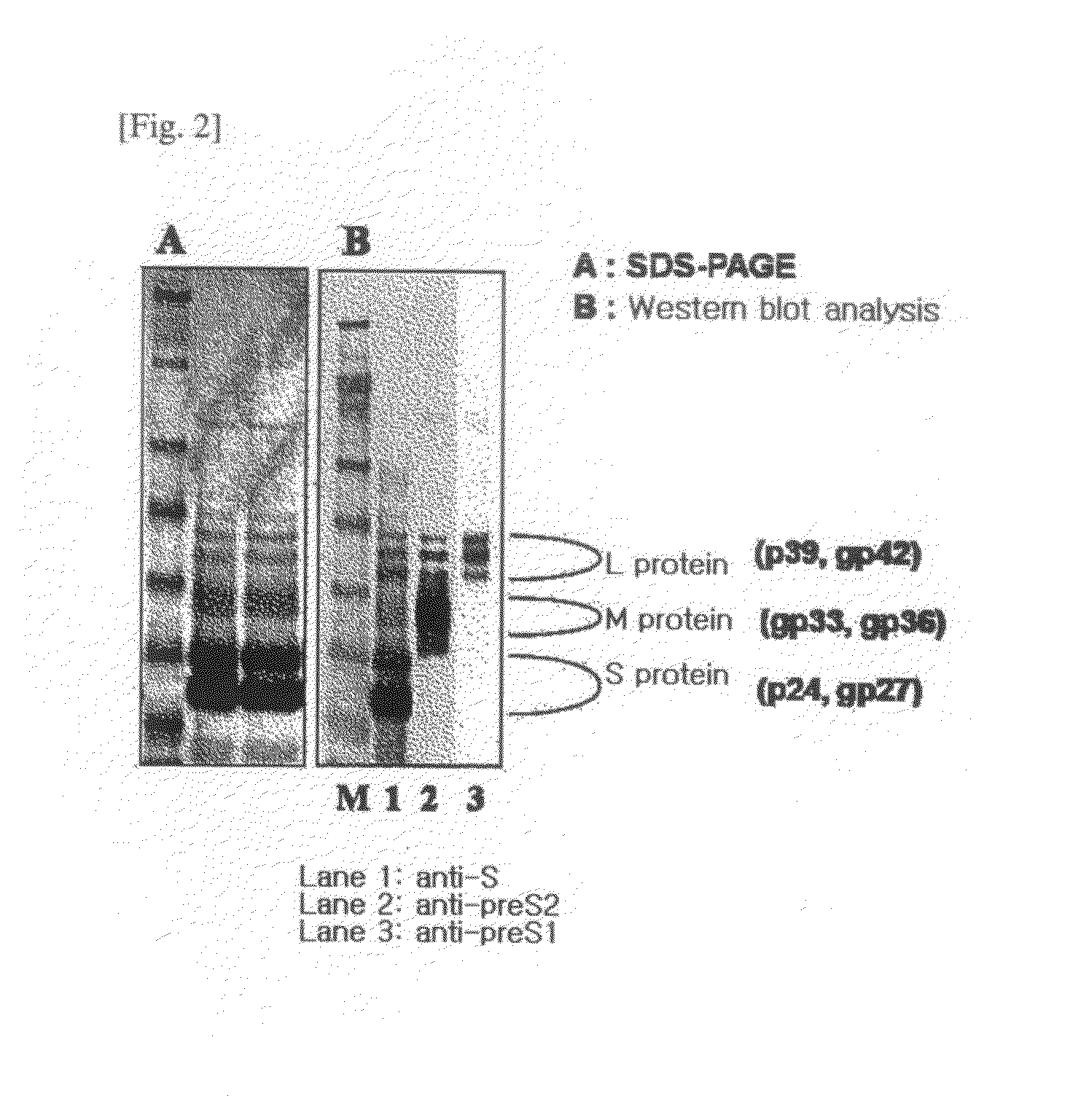
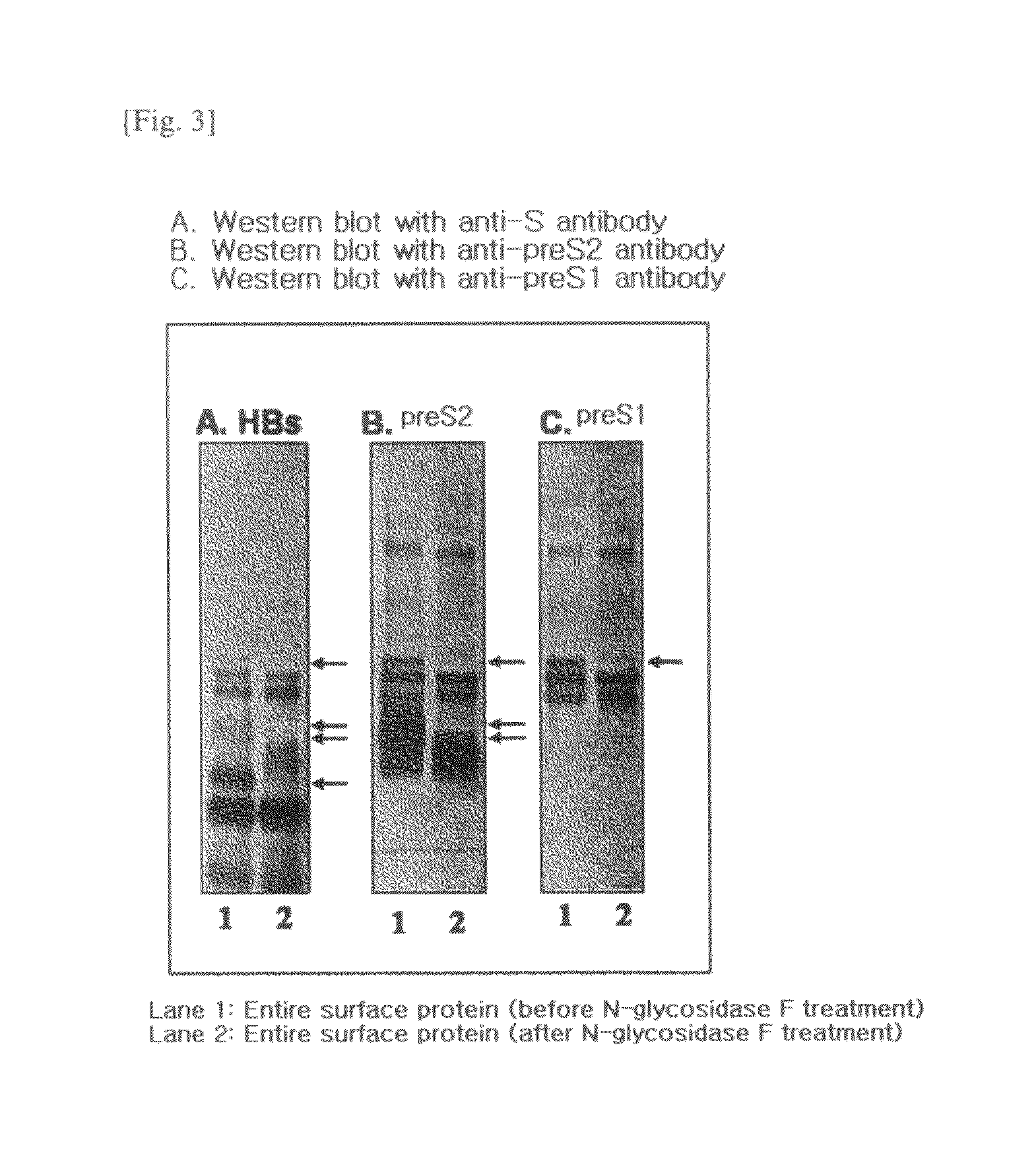
HBV Vaccine and a Process of Preparing the Same
The present invention relates to an HBV vaccine comprising an entire hepatitis B surface antigen of L protein, M protein and S protein, in which the produced antigens form virus-like particles, and a multi-antigen vaccine further comprising an HBV core antigen in addition to the entire surface antigen, and a method for preparing the same. The vaccines provide various epitopes and have excellent immunogenicity to induce a strong humoral immune response as well as a cell-mediated immune response.
Owner:CHA VACCINE RES INST CO LTD
Methods for amyloid removal using anti-amyloid antibodies
InactiveUS8105594B2Nervous disorderSugar derivativesCell mediated immunityCell-mediated immune response
Owner:UNIV OF TENNESSEE RES FOUND
T-cell vaccination with viral vectors via mechanical epidermal disruption
ActiveUS20110274649A1Strong immune responseReduce doseAntibacterial agentsAntimycoticsAdjuvantCuticle
Attenuated, replication-deficient viruses such as vaccinia viruses are used to deliver an exogenous viral, bacterial, parasitic or tumor antigen to an epidermal tissue such as the skin, lungs or gastrointestinal tract, which has been mechanically disrupted, in an amount effective to elicit or stimulate a cell mediated immune response. The epidermal tissue may be mechanically disrupted by a device such as a scarification needle or an abrader device. The epidermis may be mechanically disrupted prior to, at the same time, or immediately after the administration of the vaccine. The vaccine can be used to induce immunity against a pathogen, such as a virus, bacteria, or parasite, or against a cancer in a subject that has or is at risk of developing cancer. In some embodiments a co-stimulatory molecule, a growth factor, an adjuvant and / or a cytokine is administered before, with or after the viral vaccine. In some embodiments, the co-stimulatory molecule is co-expressed with the antigen by the virus.
Owner:TREMRX
Novel compositions and uses therefor
ActiveUS20040241177A1Increase ratingsMore responsiveViral antigen ingredientsGenetic material ingredientsLong latencyProtection sex
The invention is directed to the use of (i) a first antigen corresponding to a target antigen of interest, together with (ii) a second antigen, corresponding to a modified form of the target antigen, whose rate of intracellular proteolytic degradation is increased, enhanced or otherwise elevated relative to the first antigen, in compositions and methods for inducing both humoral and cellular immunity in an individual. The ability to provide compositions, which are capable of inducing both host-protective antibody and cell-mediated immune responses, facilitates the generation of immunogenic compositions capable of combating, inter alia, conditions that have long latency periods and, therefore, benefit from the dual approach of prophylaxis and therapy in one delivery.
Owner:JINGANG MEDICINE AUSTRALIA PTY LTD
Modulators of p-selectin glycoprotein ligand 1
InactiveUS20100080819A1Induce depletionInduce of inductionCompound screeningApoptosis detectionDiseaseNatural Killer Cell Inhibitory Receptors
Multimeric compounds that bind to P-Selectin Glycoprotein 1 (PSGL-1) on the surface of T cells or natural killer (NK) cells can be used to induce T cell or NK cell depletion and / or to induce T cell or NK cell apoptosis. The multimeric compounds and methods of the invention can be used to control unwanted T cell- or NK cell-mediated immune responses in conditions such as inflammatory diseases, autoimmune diseases, transplant rejection, and allergic diseases.
Owner:ABGENOMICS COOPERATIEF U A
Diagnostic assay for measuring a cell mediated immune response
ActiveUS7608392B2Reduce exposureAmenable to analysisMicrobiological testing/measurementBiological testingImmune effectsWild life
The present invention relates generally to a diagnostic assay and, more particularly, an assay for measuring cell-mediated immune reactivity. Even more particularly, the present invention provides an assay and a kit for measuring a cell-mediated response to an antigen using whole blood or other suitable biological sample. The assay may be conducted using ligands to immune effector molecules or at the nucleic acid level, screening for expression of genes encoding the immune effector molecules. The assay is useful in therapeutic and diagnostic protocols for human, livestock and veterinary and wild life applications.
Owner:CELLESTIS
Hivcon: an HIV immunogen and uses thereof
InactiveUS20080089901A1Elicit immune responsePeptide/protein ingredientsAntibody mimetics/scaffoldsHIV ProteinsA-DNA
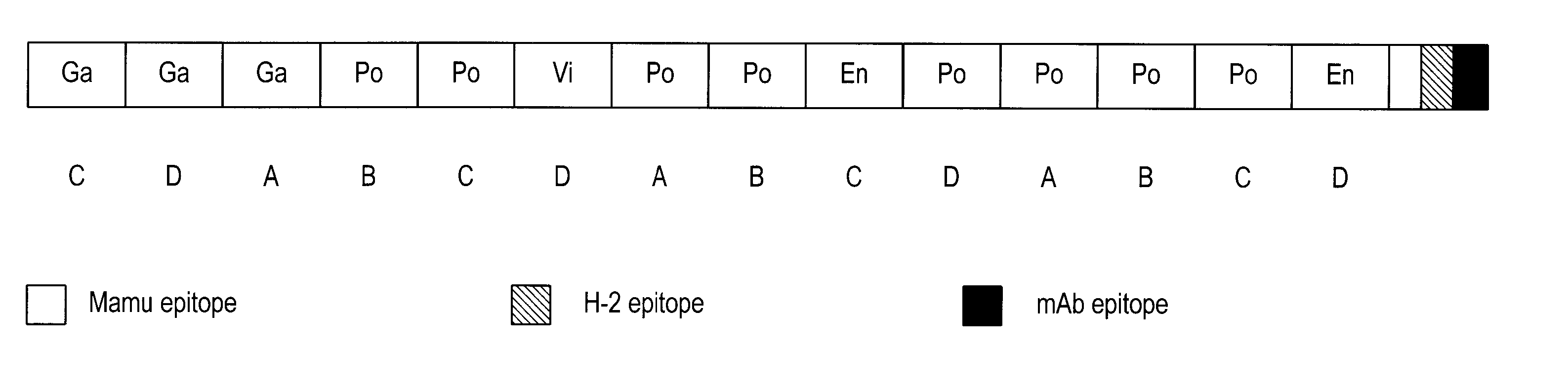
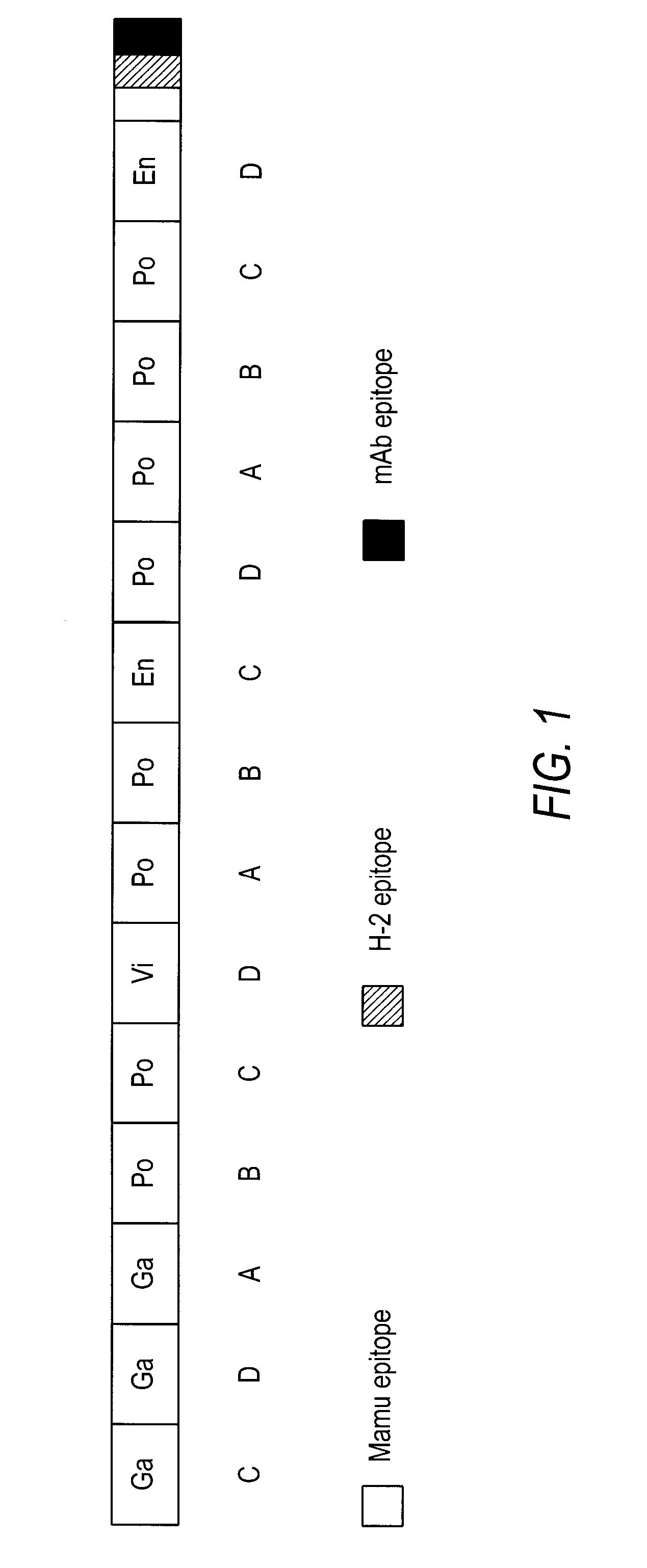
The present invention provides artificial fusion proteins (AFPs) designed to elicit an anti-HIV immune response, as well as nucleic acid molecules and expression vectors encoding those proteins. The AFPs of the invention may comprise domains from various HIV proteins, such as Gag, Pol, Vif, and Env proteins, which are partial sequences. HIVCON is an AFP in which the HIV domains are from several HIV clade consensus sequences and which optionally contains additional domains which may be useful, for example, in monitoring expression levels or laboratory animal immune responses. Other aspects of the invention may include compositions and methods for inducing an anti-HIV immune response in a subject, preferably with a DNA prime-MVA boost strategy, and to induce a cell-mediated immune response.
Owner:MEDICAL RESEARCH COUNCIL
HBV vaccine and a process of preparing the same
The present invention relates to an HBV vaccine comprising an entire hepatitis B surface antigen of L protein, M protein and S protein, in which the produced antigens form virus-like particles, and a multi-antigen vaccine further comprising an HBV core antigen in addition to the entire surface antigen, and a method for preparing the same. The vaccines provide various epitopes and have excellent immunogenicity to induce a strong humoral immune response as well as a cell-mediated immune response.
Owner:CHA VACCINE RES INST CO LTD
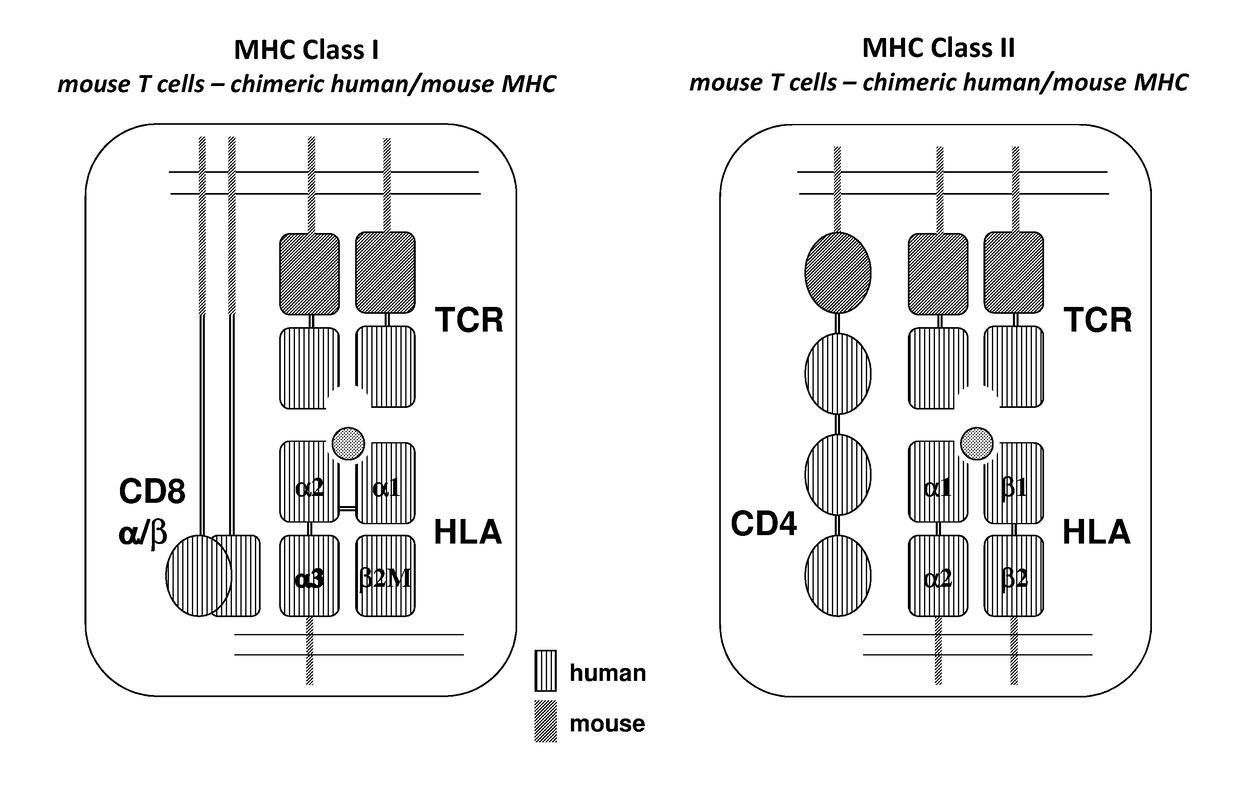
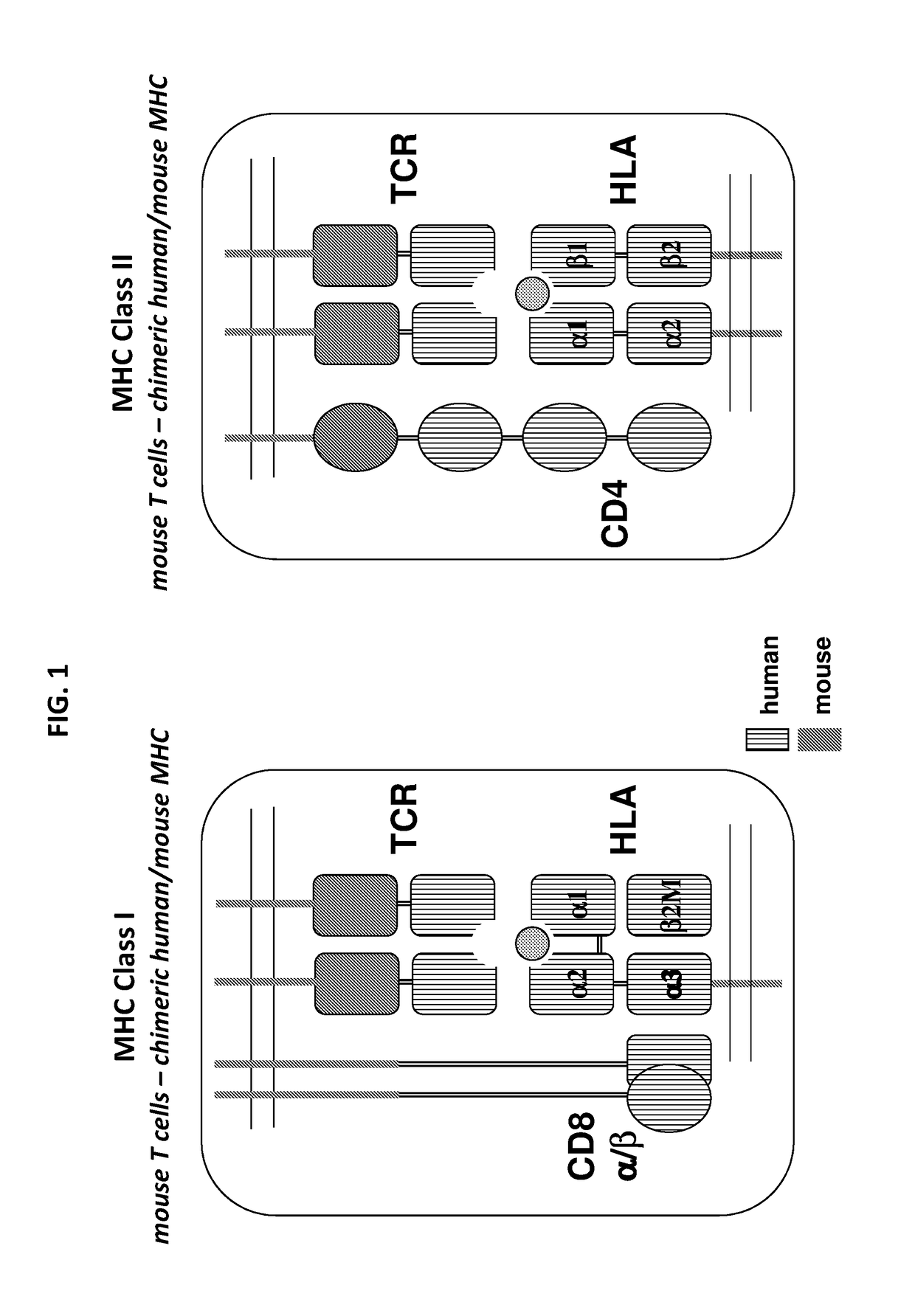
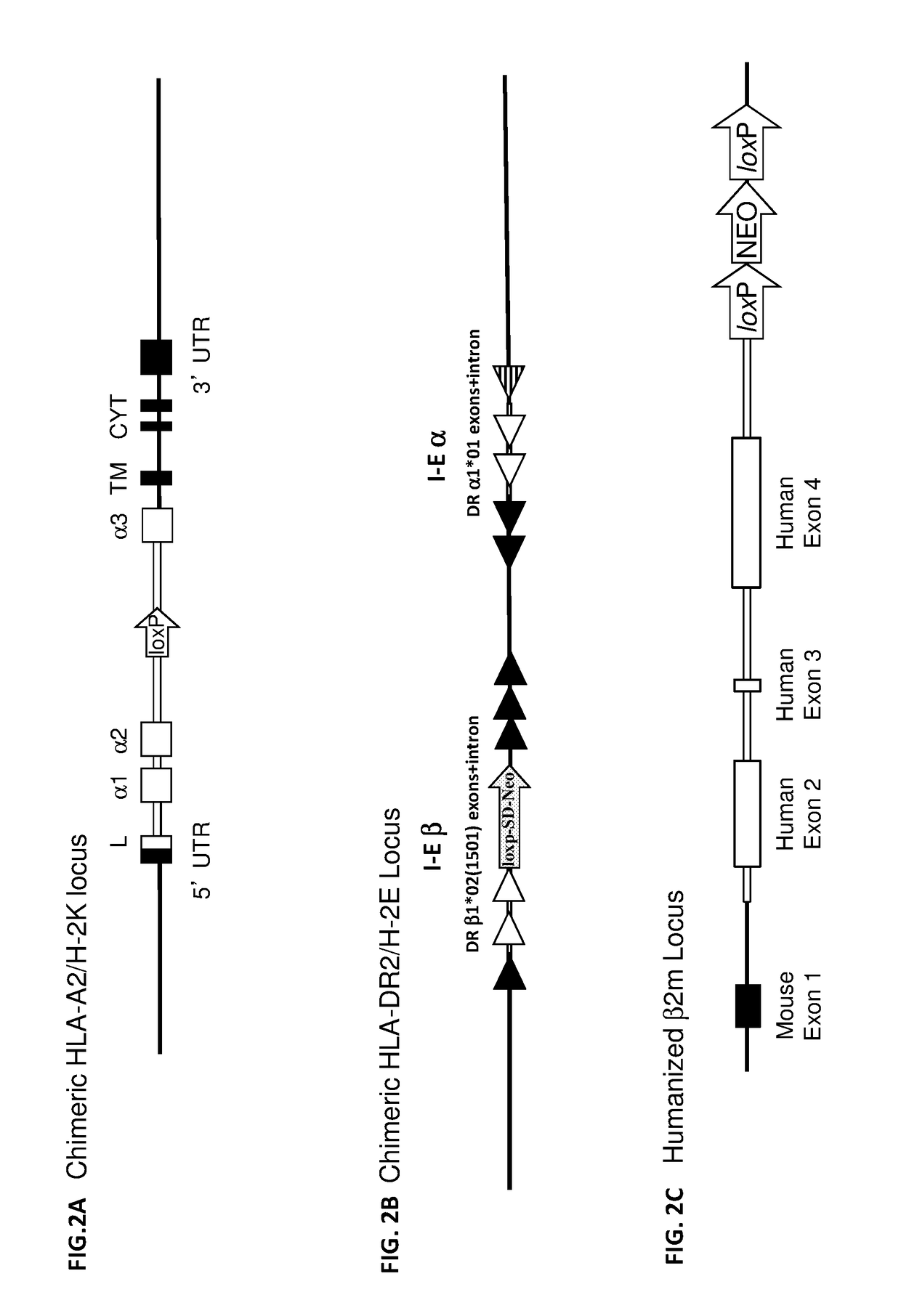
Humanized t cell mediated immune responses in non-human animals
ActiveUS20180139940A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyGenetically Engineered AnimalsCD8
Disclosed herein are non-human animals (e.g., rodents, e.g., mice or rats) genetically engineered to express a humanized T cell co-receptor (e.g., humanized CD4 and / or CD8 (e.g., CD8α and / or CD8β)), a human or humanized T cell receptor (TCR) comprising a variable domain encoded by at least one human TCR variable region gene segment and / or a human or humanized major histocompatibility complex that binds the humanized T cell co-receptor (e.g., human or humanized MHC II (e.g., MHC II α and / or MHC II β chains) and / or MHC I (e.g., MHC I α) respectively, and optionally human or humanized β 2 microglobulin). Also provided are embryos, tissues, and cells expressing the same. Methods for making a genetically engineered animal that expresses at least one humanized T cell co-receptor (e.g., humanized CD4 and / or CD8), at least one humanized MHC that associates with the humanized T cell co-receptor (e.g., humanized MHC II and / or MHC I, respectively) and / or the humanized TCR are also provided. Methods for using the genetically engineered animals that mount a substantially humanized T cell immune response for developing human therapeutics are also provided.
Owner:REGENERON PHARM INC
Mesoporous alum nanoparticles as a universal platform for antigen adsorption, presentation, and delivery
InactiveUS20160151482A1Promotes effective uptakeEasy to packPowder deliveryOrganic active ingredientsNanoparticleAlum
The present invention relates to mesoporous alum nanoparticles which can be used as a universal platform for antigen adsorption, presentation and delivery to provide immune compositions, including vaccines and to generate an immune response (preferably, both humoral and cell mediated immune response), preferably a heightened immune response to the presentation of one or more antigens to a patient or subject.
Owner:STC UNM +1
Method for Inducing a Cell-Mediated Immune Response and Improved Parenteral Vaccine Formulations Thereof
A method of inducing either a TH1 polarised immune response, a TH2 polarised immune response or a combined TH1 and TH2 response to an antigen and associated vaccine formulations are disclosed. A method is provided for inducing a polarised TH1 response by parenteral administration of microparticles sized such that at least 50% of the microparticles are less than 5 μm, the microparticles containing antigen entrapped or encapsulated by a biodegradable polymer. Additionally, a method is provided for inducing a polarised TH2 response by parenteral administration of nanoparticles sized such that at least 50% of the nanoparticles are less than 600 nm, the nanoparticles containing antigen entrapped or encapsulated by a biodegradable polymer. Vaccine formulations containing the B. pertussis antigens PTd, FHA or a combination of PTd and FHA are provided.
Owner:MERRION RES III LTD
Methods for tailoring the immune response to an antigen or immunogen
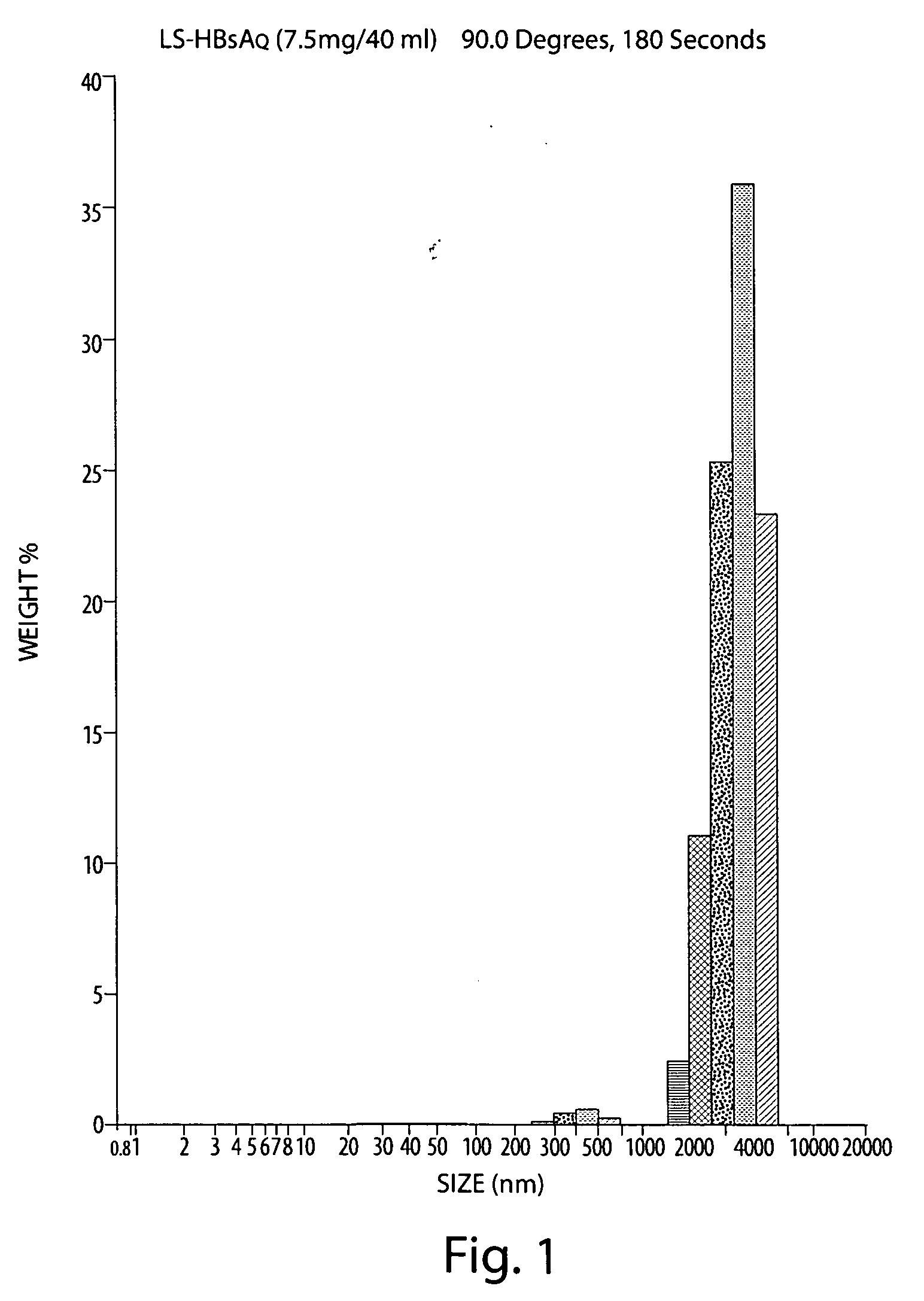
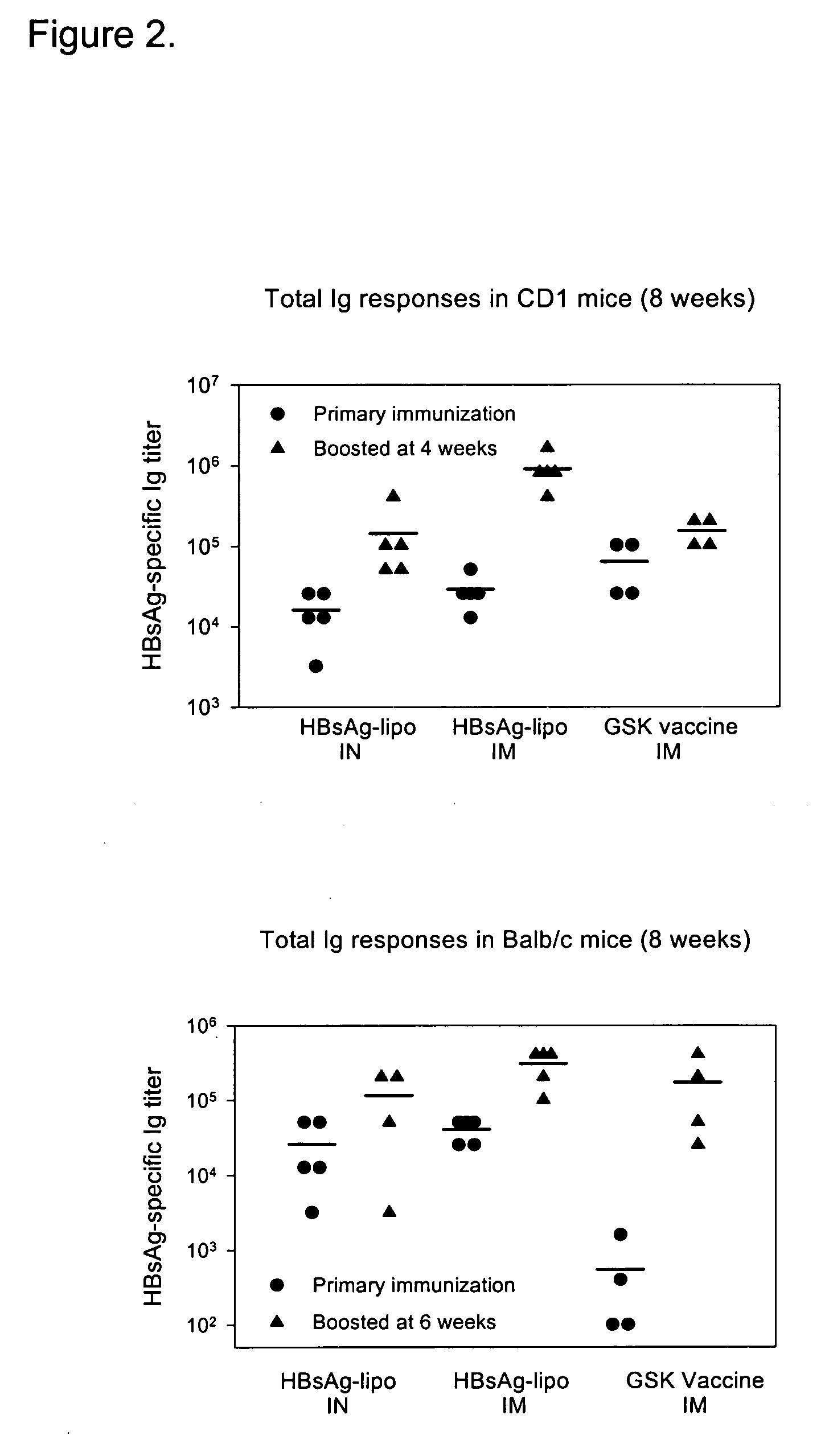
The invention relates to methods and reagents for immunizing animals to elicit specific cellular and humoral immune-responses against specific antigens, such as viral antigens, including HBsAg antigen. The invention provides methods of using specifically prepared immunogen in fresh or lyophilized liposome, proper routes of administration of the immunogen, proper doses of the immunogen, and specific combinations of heterologous immunization including DNA priming in one administration route followed by liposome-mediated protein antigen boost in a different route to tailor the immune responses in respects of enhancing cell mediated immune response, cytokine secretion, humoral immune response, immune protection and selective skewing of T helper responses to be Th1, Th2, or a mixed or balanced Th response.
Owner:MUCOSAL VACCINE TECH LLC
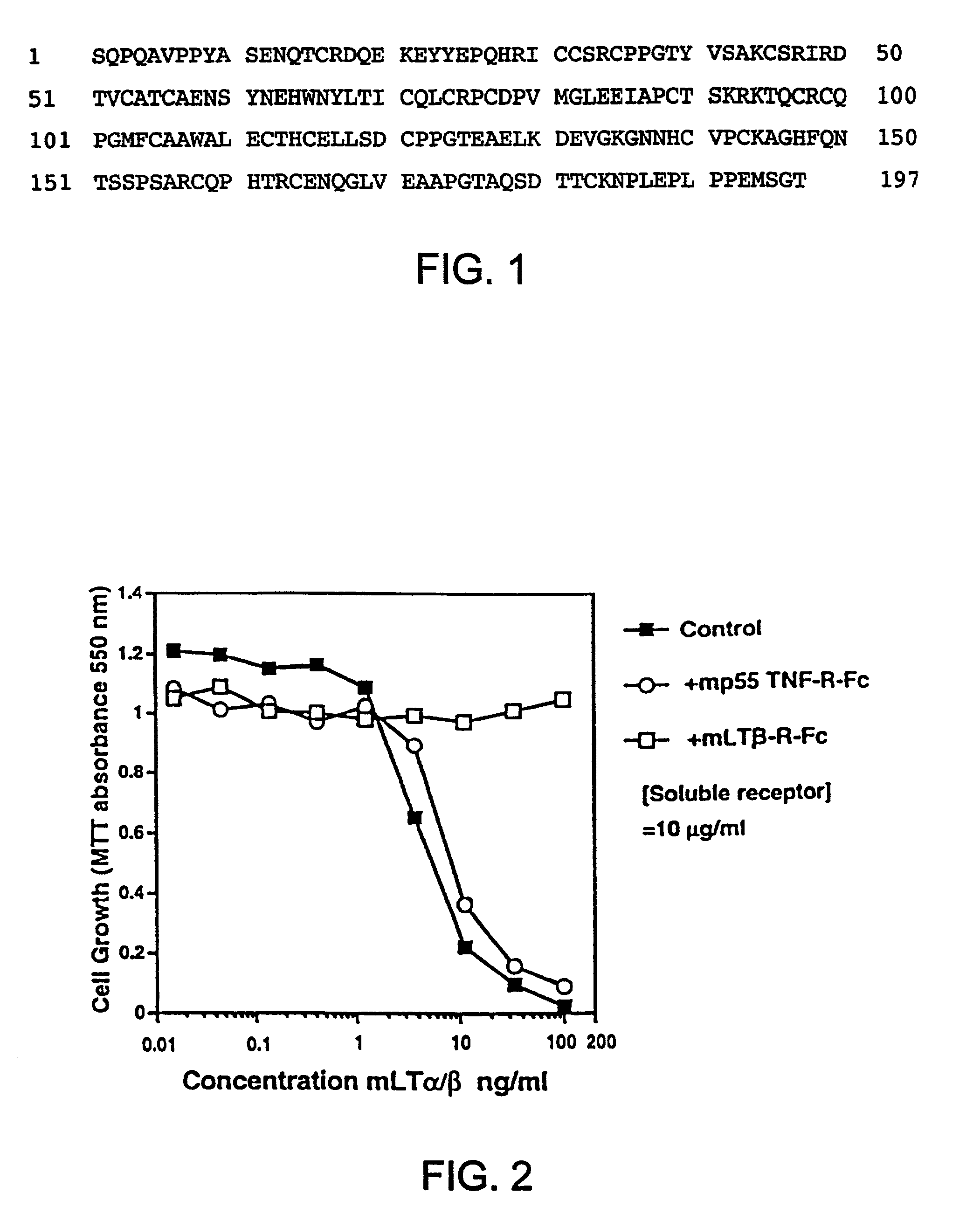
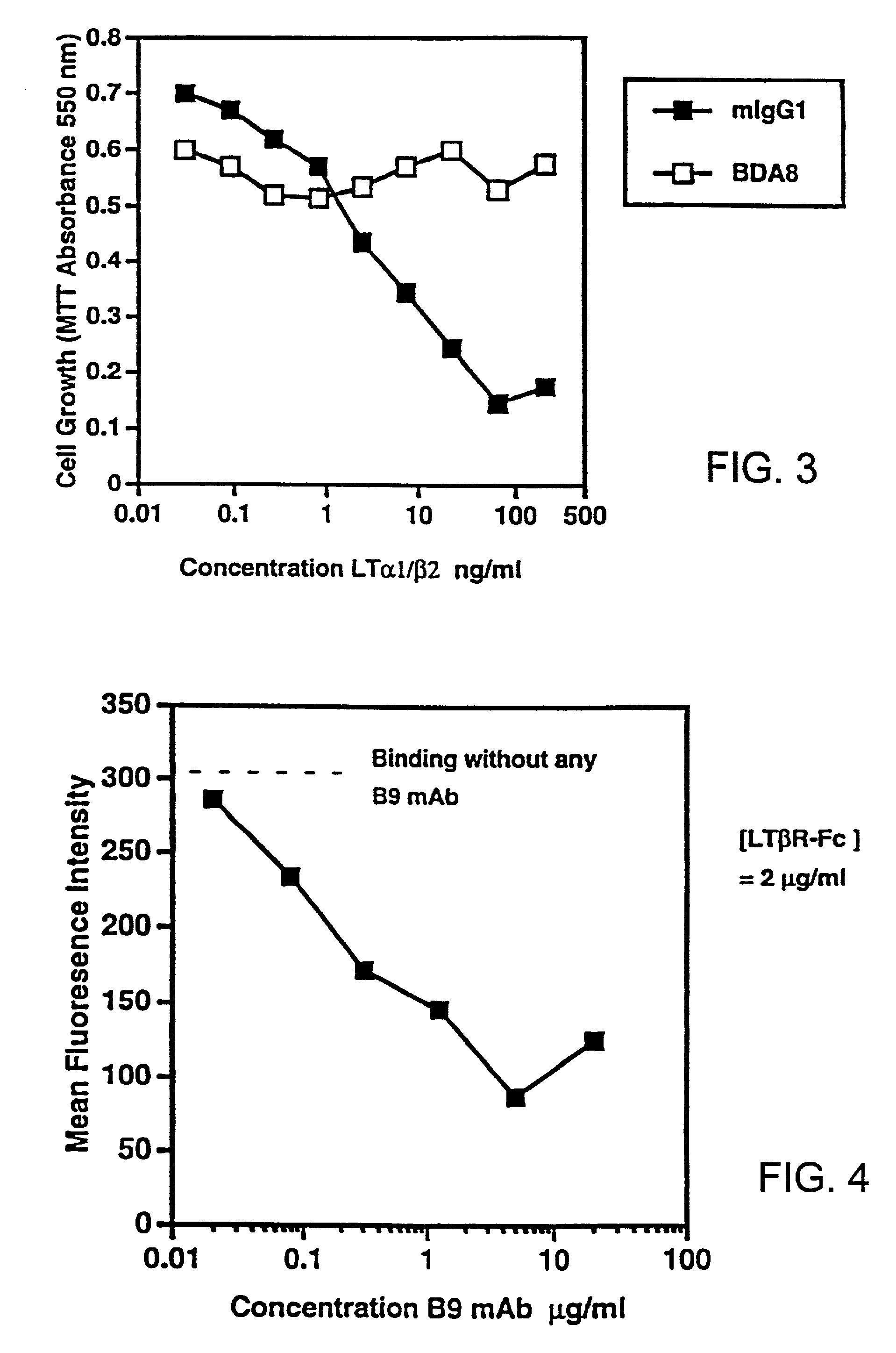
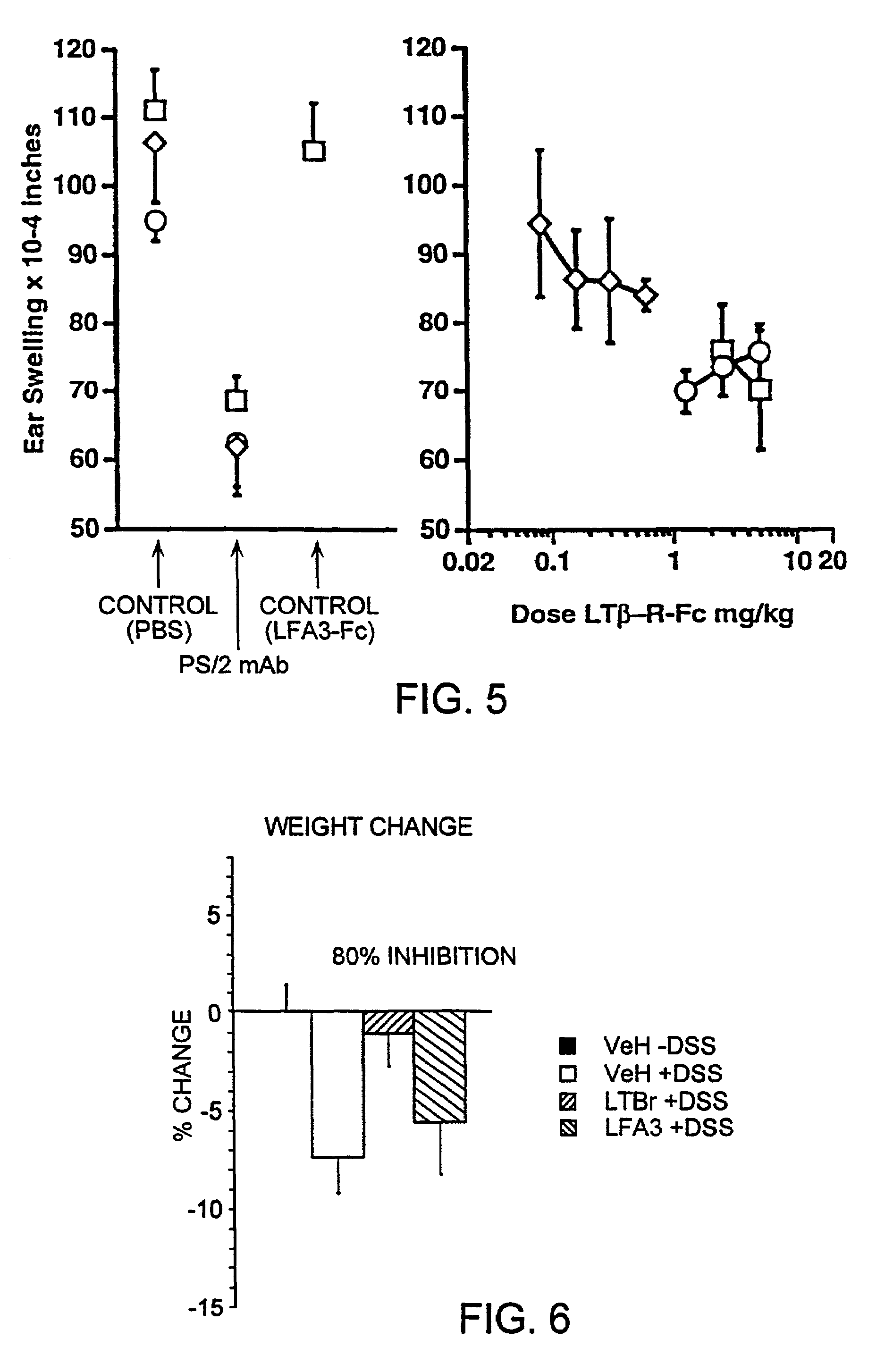
Methods for inhibiting lymphotoxin β receptor signalling
This invention relates to compositions and methods comprising “lymphotoxin-β receptor blocking agents”, which block lymphotoxin-β receptor signalling. Lymphotoxin-β receptor blocking agents are useful for treating lymphocyte-mediated immunological diseases, and more particularly, for inhibiting Th1 cell-mediated immune responses. This invention relates to soluble forms of the lymphotoxin-β receptor extracellular domain that act as lymphotoxin-β receptor blocking agents. This invention also relates to the use of antibodies directed against either the lymphotoxin-β receptor or its ligand, surface lymphotoxin, that act as lymphotoxin-β receptor blocking agents. A novel screening method for selecting soluble receptors, antibodies and other agents that block LT-β receptor signalling is provided.
Owner:BIOGEN MA INC
Adjuvant combinations of liposomes and mycobacteriaial lipids for immunization compositions and vaccines
InactiveUS20060286128A1High protective immune responseIncreased protective immune responseAntibacterial agentsBacterial antigen ingredientsLipid formationActive agent
The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldioctadecylammonium-bromide / chloride (DDA) and a lipid extract from The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from <The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis. The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis<The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis BCG The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis BCG <The present invention provides a vaccin adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis BCG. The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from <i>Mycobacterium bovis BCG<i>. <The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from <i>Mycobacterium bovis BCG<i>. The total lipid extract contains both apolar lipids, polar lipids, and lipids of intermediate polarity of which the apolar lipids were found to induce the most powerful immune responses. The total lipids may be extracted with chloroform / methanol and re-dissolved in water before the addition of surfactant. This preparation may be used to induce prominent cell-mediated immune responses in a mammal in order to combat pathogens, or as a treatment for cancer.
Owner:AGGER ELSE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com