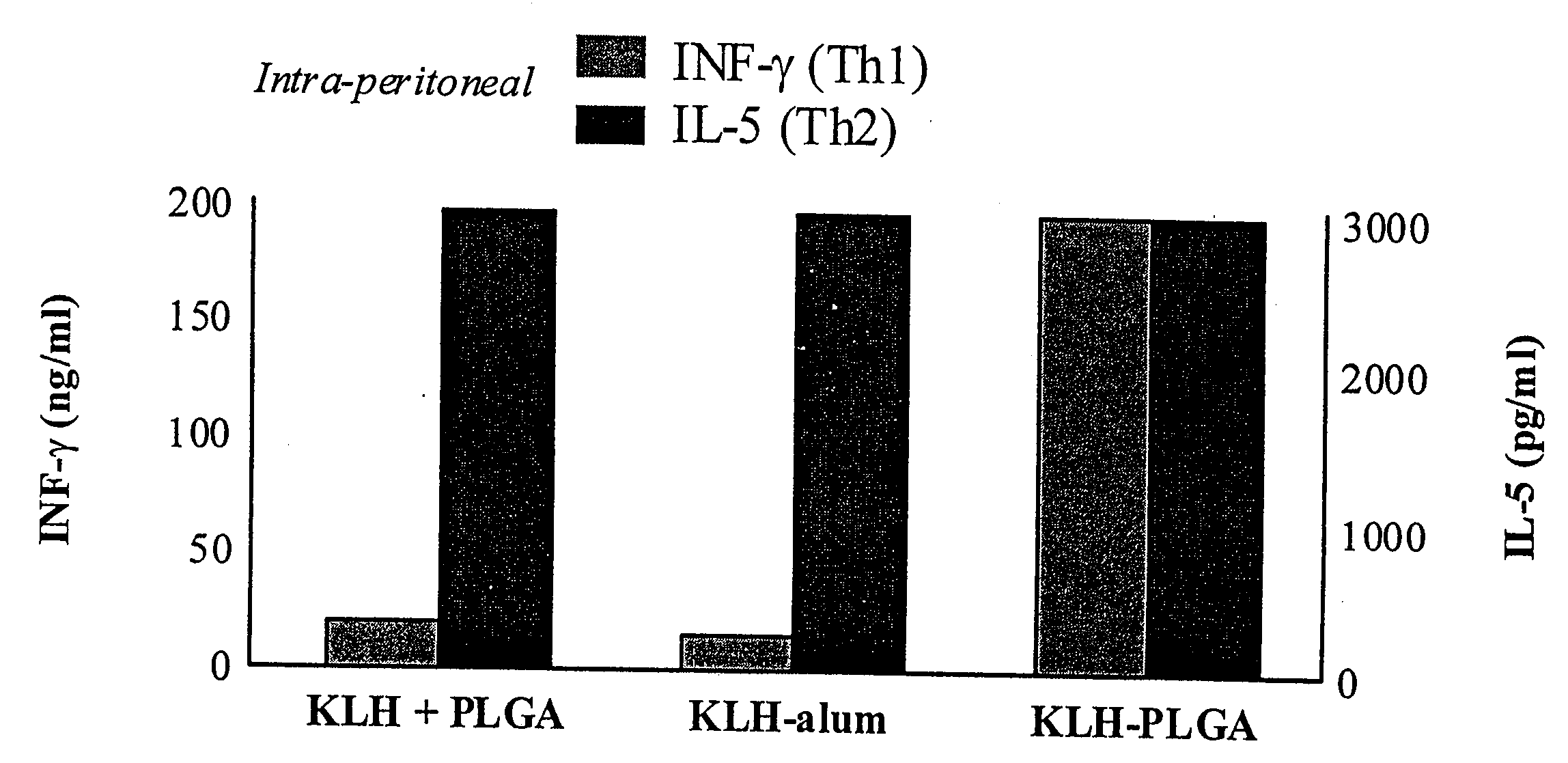
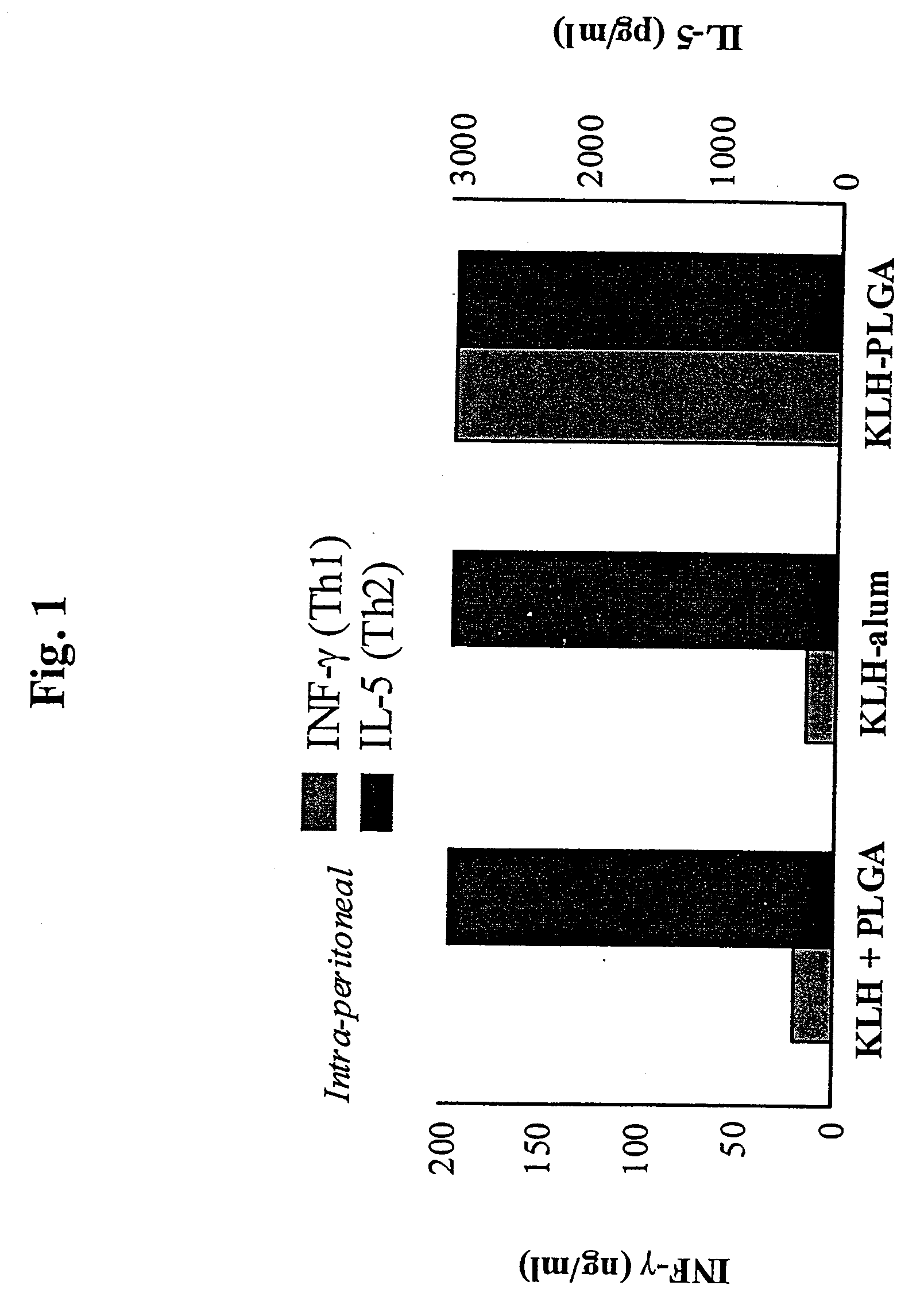
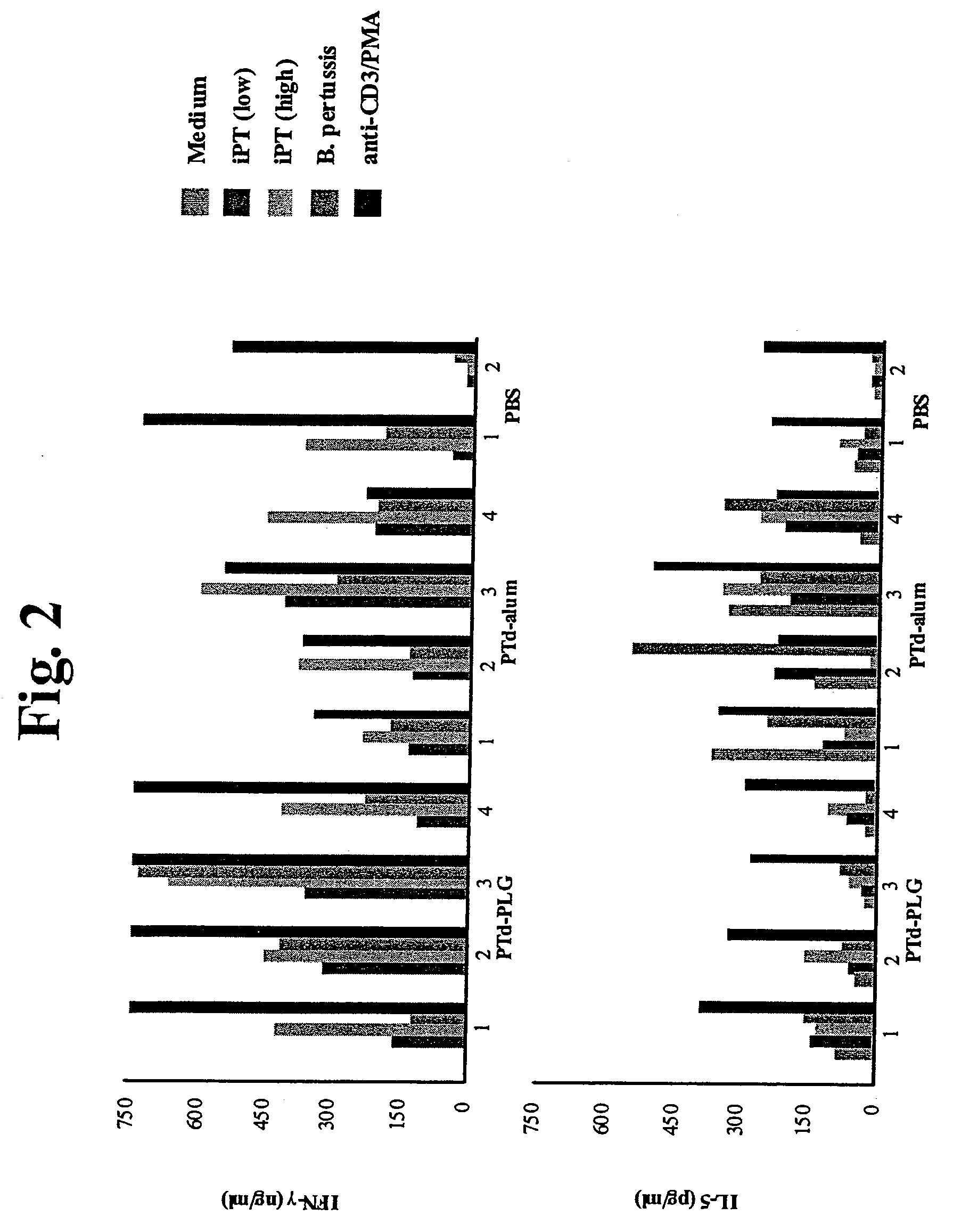
Method for Inducing a Cell-Mediated Immune Response and Improved Parenteral Vaccine Formulations Thereof
a cell-mediated immune response and cell-mediated technology, applied in the field of vaccine formulations, can solve the problem of no general method for predicting or anticipating the nature of the immune response, and achieve the effect of protecting immunity against the infectious agen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of KLH−PLGA Microparticles Using a Solvent Evaporation Method
[0044] A polymer solution of PLGA [poly (D,L-lactide-co-glycolide), 50:50; i.v.=0.94 dl / g; supplied by Boehringer Ingelheim] in dichloromethane (10% PLGA in 10 ml DCM) was prepared two hours prior to use and subsequently chilled 30 minutes prior to use. The antigen, KLH (supplied by Calbiochem as a powder), was prepared as an aqueous solution (5.1 mg KLH in 1 ml water) containing 2% PVA. A first water-in-oil emulsion was prepared by adding the antigen solution to the polymer solution and homogenising for 1 min. at 24,000 rpm on ice. This first emulsion was poured slowly into an aqueous solution of PVA (40 ml, 3% PVA) forming a second water-oil-water emulsion and homogenisation was continued for 2 min. with a 15 sec. break [1 min.; 15 sec. break; 1 min.]. The resulting emulsion was stirred for 2 hours to evaporate the dichloromethane. The antigen-loaded particles (75% yield) were collected by centrifugation (10...
example 2
Preparation of PTd−PLGA Microparticles Using a Solvent Evaporation Method
[0051] Using a method substantially the same as that described in Example 1 above, PTd (supplied by Katetsuken) loaded PLGA particles were prepared. The polymer solution was 6.7% PLGA in 15 ml DCM and the antigen solution was 744 μg PTd in 2 ml water containing 0.9% PVA. The first water-in-oil emulsion was poured into 80 ml aqueous PVA (3% PVA) to form the water-oil-water emulsion. The emulsion was left over night to evaporate the DCM. After collection (88% yield), the microparticles were washed with chilled autoclaved water (30 ml).
[0052] Characterisation of these particles, identified as PTd-1 in Table 1 below, showed that the microparticles formed were smooth and spherical in appearance with at least 50% of the particles less than 5 microns in diameter. Laser light diffractometry showed that the particles had a D50% of 2.5 μm. The microparticles were loaded with antigen at 0.12 μg / mg, representing an entra...
example 3
Preparation of FHA−PLGA Microparticles Using a Solvent Evaporation Method
[0055] A procedure substantially similar to that used in Example 2 was employed for the preparation of FHA-loaded PLGA microparticles. Two batches of FHA−PLGA microparticles were prepared. For these two batches (FHA-1 and FHA-2 in Table 2 below) the polymer solution was 4% PLGA in 20 ml DCM and the antigen solution was 0.87 μg FHA in 2 ml water containing no PVA. The first water-in-oil emulsion was poured into 80 ml aqueous PVA (3% PVA) to form the water-oil-water emulsion. The characteristics of these two batches are given in Table 2 below. FHA-1 and FHA-2 were pooled (the pooled microparticles are labelled FHA-3 in Table 2) for antigen release determination and i.p. protection studies (see Example 7 below). SEM analysis showed the FHA-1 and FHA-2 microparticles to be smooth and spherical in nature with at least 50% of the particles less than 5 microns in diameter.
TABLE 2ExampleLoadingD50%No.(mg / mg)% EE(μm)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| sizes | aaaaa | aaaaa |
| sizes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com