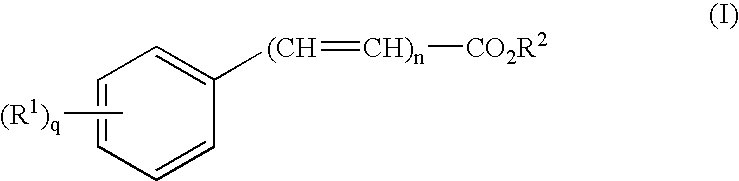Patents
Literature
151 results about "Topical drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Topical drug. Drug or medication applied to a specific area of the skin and affecting only the area to which it is applied.
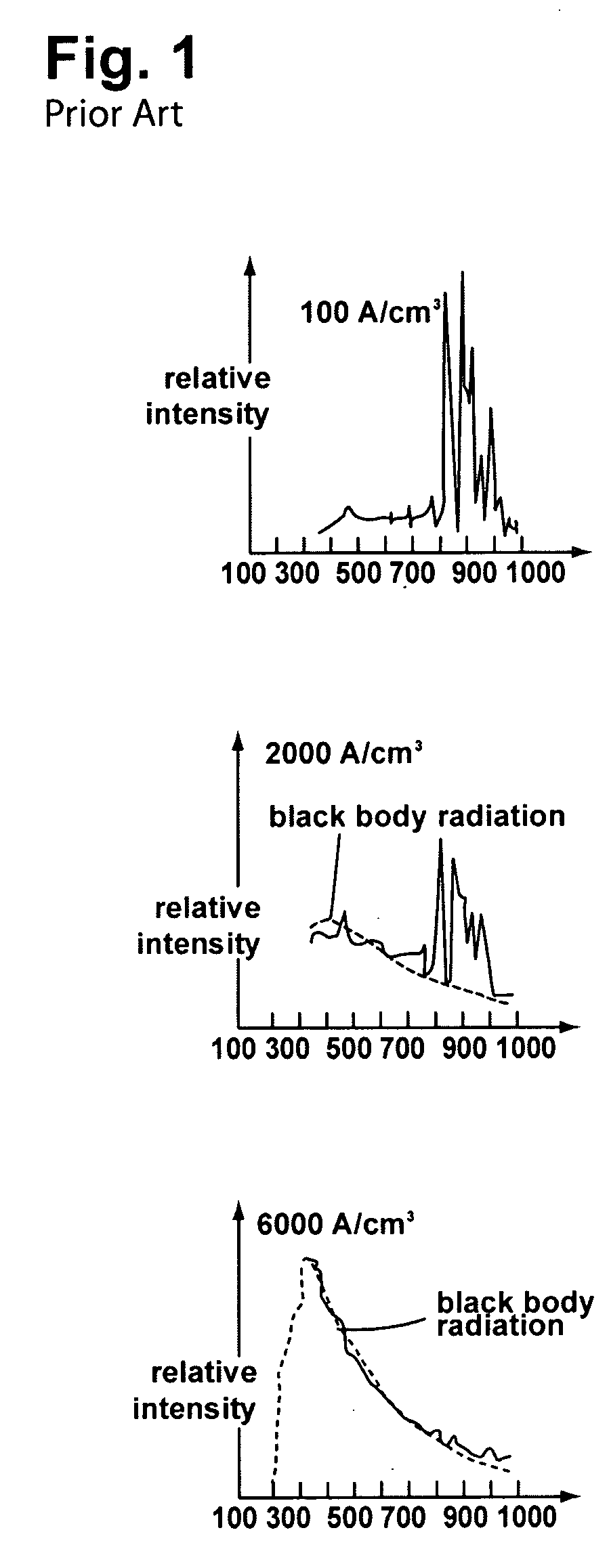
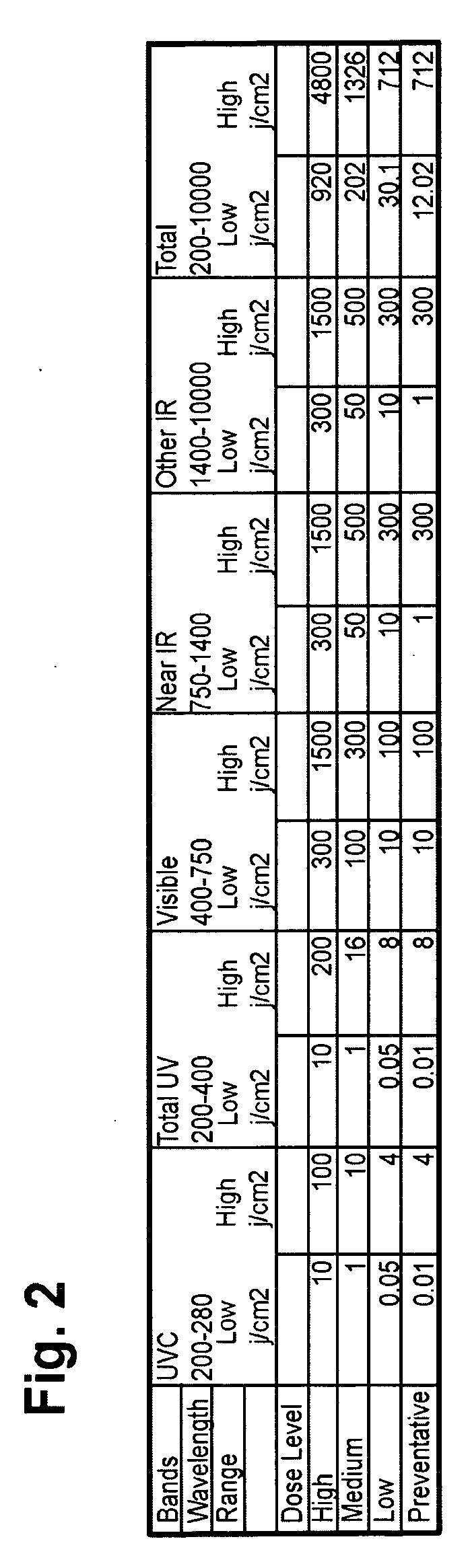
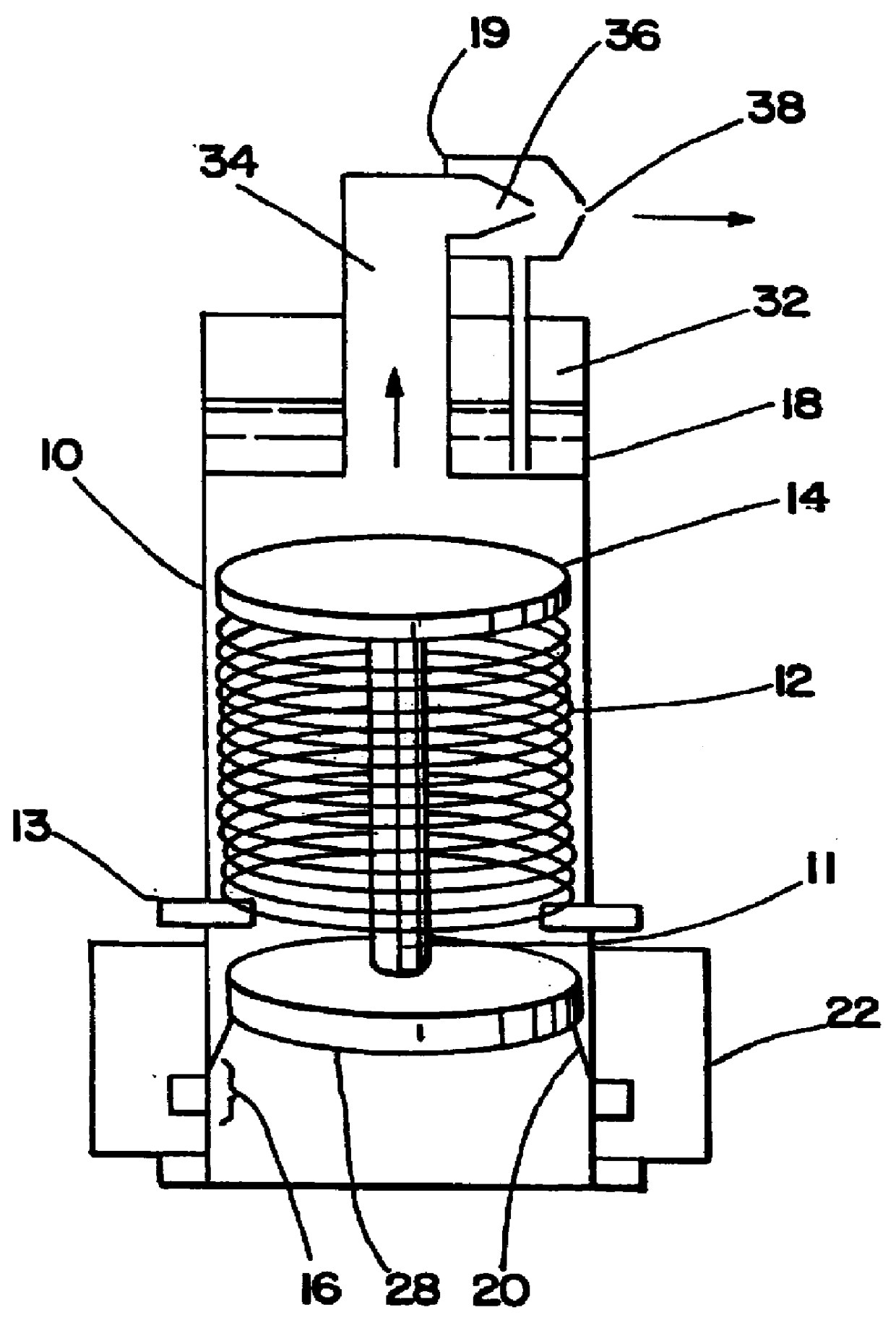
Phototherapy Treatment and Device for Infections, Diseases, and Disorders
A device to treat infections, diseases, and disorders. The device can be used to treat part of a larger organism such as using the device to kill cancerous cells in humans or animals or to kill parasites. The invention can also may be used to treat fungal and bacterial nail infections of the hands and feet which are difficult to treat with oral and topical drugs.
Owner:CUMBIE WILLIAM E +1
Methods of delivering stable topical drug compositions
InactiveUS20060127469A1Easy and administration routeNot to wasteOrganic active ingredientsPeptide/protein ingredientsRoom temperatureTopical drug
A method of delivering a drug composition comprises providing a carrier having a phosphatidylcholine component and a drug entrapped therein, and applying the composition to the skin for transdermal delivery of the drug, wherein the composition is stable at room temperature.
Owner:TRANSDERMAL BIOTECHNOLOGY INC
Topical drug delivery using phosphatidylcholine
InactiveUS20060105955A1Topical deliveryEasier and pleasanterOrganic active ingredientsPeptide/protein ingredientsTopical drugTransdermal medication
The present invention relates to compositions and methods for transdermal drug delivery comprising formulating a phosphatidylcholine carrier composition containing the drug and applying the composition to the skin.
Owner:TRANSDERMAL BIOTECHNOLOGY INC
Apparatus and method for delivery of micro and submicro quantities of materials
A process and apparatus (invention) permitting the distribution of micro or submicro liter or gram (exceptionally small) quantities of liquids and powders, (hereafter materials) in patterns and quantities that are especially well-suited for ophthalmologic patient self-application of drugs to his own eye(s) with substantially no overdose. The invention is thus also highly beneficial in those many topical drug applications where overdoses should be avoided. This is particularly applicable for the eye, where overdoses can have many deleterious medical consequences. Use of the invention permits exceptionally small doses of ophthalmologic drugs. Moreover, it also permits the repeatability of the quantity size of the materials chosen for delivery. In the case of drugs, particularly opthomologic drugs, this means that the invention permits the repeated multiple distribution of the same chosen drug / carrier quantity resulting in a uniform repeatable dosage. Variation of dose from one application to another is very unacceptable. The preferred apparatus of the invention employs a venturi configuration inventively modified which uses gas under pressure to pump exceptionally small quantities of material from a reservoir for delivery. In especially preferred embodiments of the invention, said reservoir is pressurized by at least a quantity of gas at somewhat above atmospheric. prior to the removal of any material by pumping.
Owner:ROGERS ROBERT L +1
NSAID formulations, based on highly adaptable aggregates, for improved transport through barriers and topical drug delivery
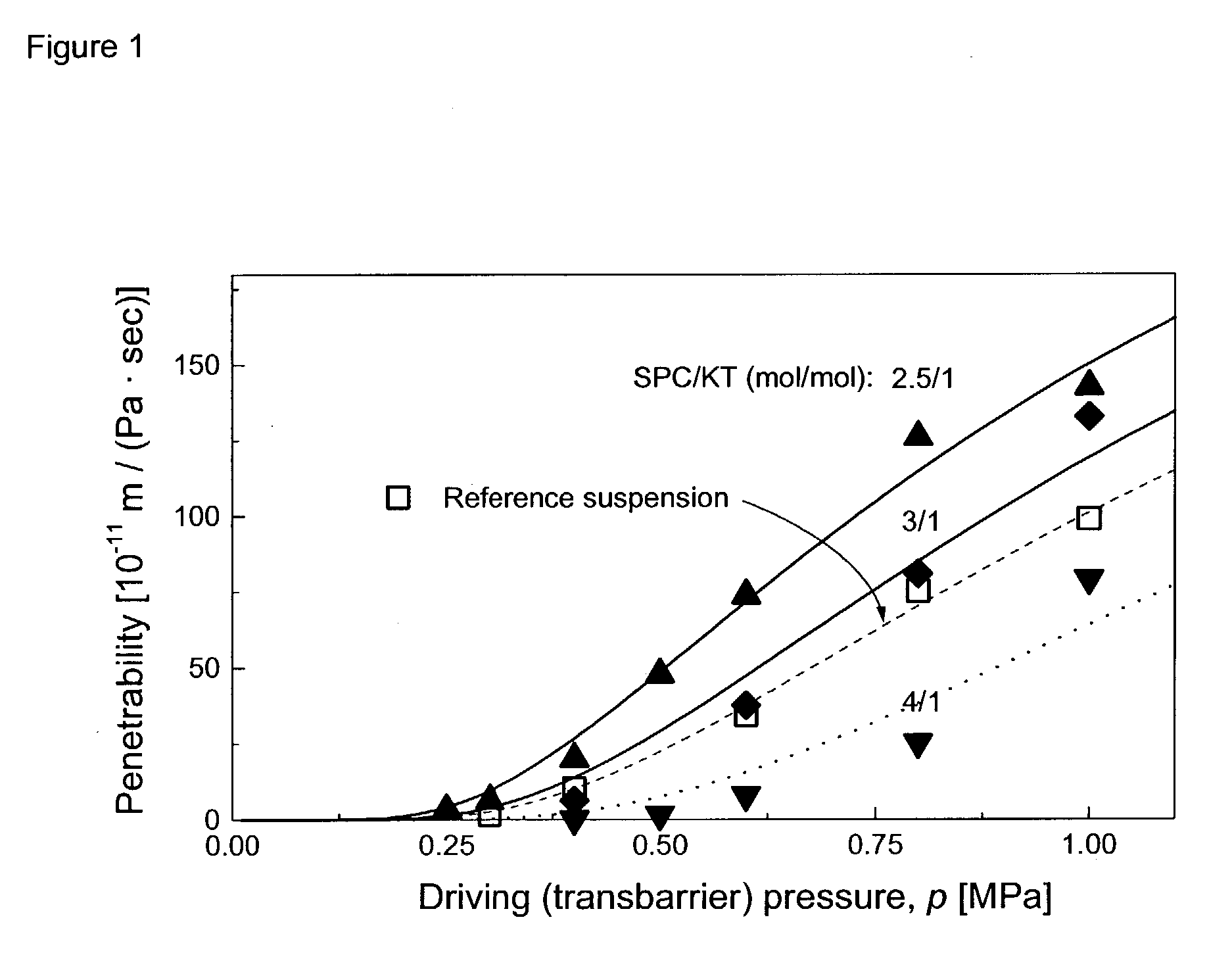
The invention describes novel formulations of nonsteroidal anti-inflammatory drugs (NSAIDs) based on complex aggregates with at least three amphipatic components suspended in a suitable, e.g. pharmaceutically acceptable, polar liquid medium. A suitably ionised NSAID is one of the two, amongst said three, components that tends to destabilise lipid membranes, the other system component with such activity being typically a surfactant. In contrast, the remaining amongst said at least three amphipatic components typically forms a stable lipid membrane on it's own. An essential characteristics of the resulting, relatively large, aggregates is an improved ability to penetrate pores, in a semi-permeable barrier, at least 30%, and often much smaller than the average diameter of the complex aggregate. This enables said aggregates to mediate NSAID transport through semi-permeable barriers including mammalian skin. As a result of the skin penetration by NSAID loaded large aggregates, the drug delivered transcutaneously with such carriers gets deeper into the tissue than the corresponding NSAID from a solution on the skin surface. This is believed to be due to the special ability of suitable large carriers to bypass the local sink of blood capillaries at the epidermal-dermal junction in the skin. The carrier-mediated delivery of locally applied NSAIDs thus allows therapy of deep tissues under the drug administration site, which is medically highly desirable.
Owner:IDEA AG
Tricot-like pouch for the delivery of topical drugs and cosmetics
InactiveUS6156323ASoft and cost-effective and convenientEasy to useCosmetic preparationsBiocideTopical drugDermatology
This invention relates to pouch or pledget delivery system having an apertured film or fabric covering which assists in evenly and safely distributing topical medicaments.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Topical drug delivery system with dual carriers
InactiveUS20120082632A1Enhancing primary effectOptimization mechanismBiocideCosmetic preparationsMammalian tissuePharmaceutical drug
A drug delivery system, formed as a tissue penetrating solution, comprising:a solvent suitable for solubilizing a non-liquid active ingredient into a solution; a diluent for diluting the solvent to optimize the solution for mammalian tissue compatibility; and a stabilizer for maintaining the solution chemically stable and substantially free from oxidation during storage for a pre-determined shelf life period.
Owner:PHILLIPS D HOWARD +1
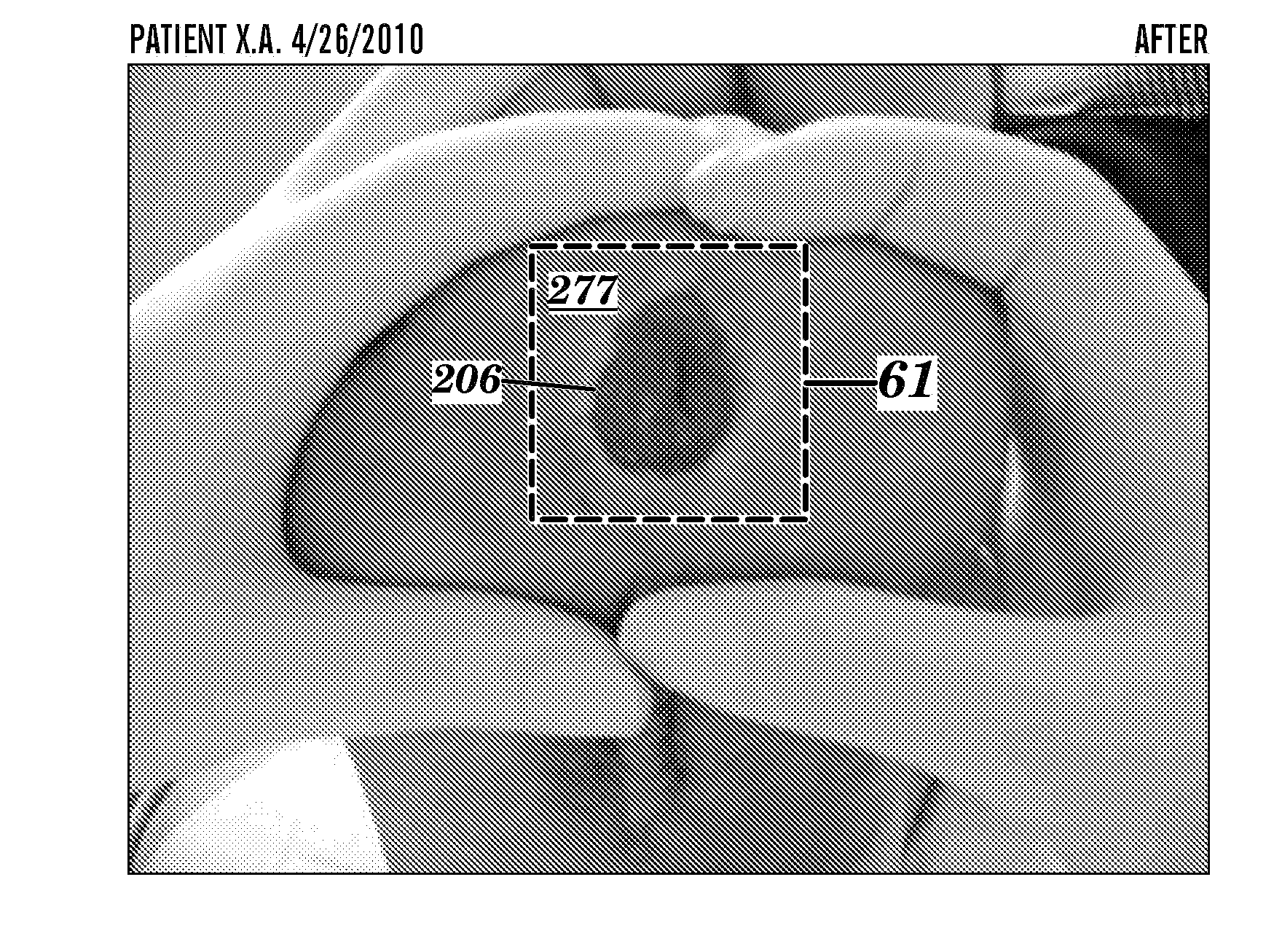
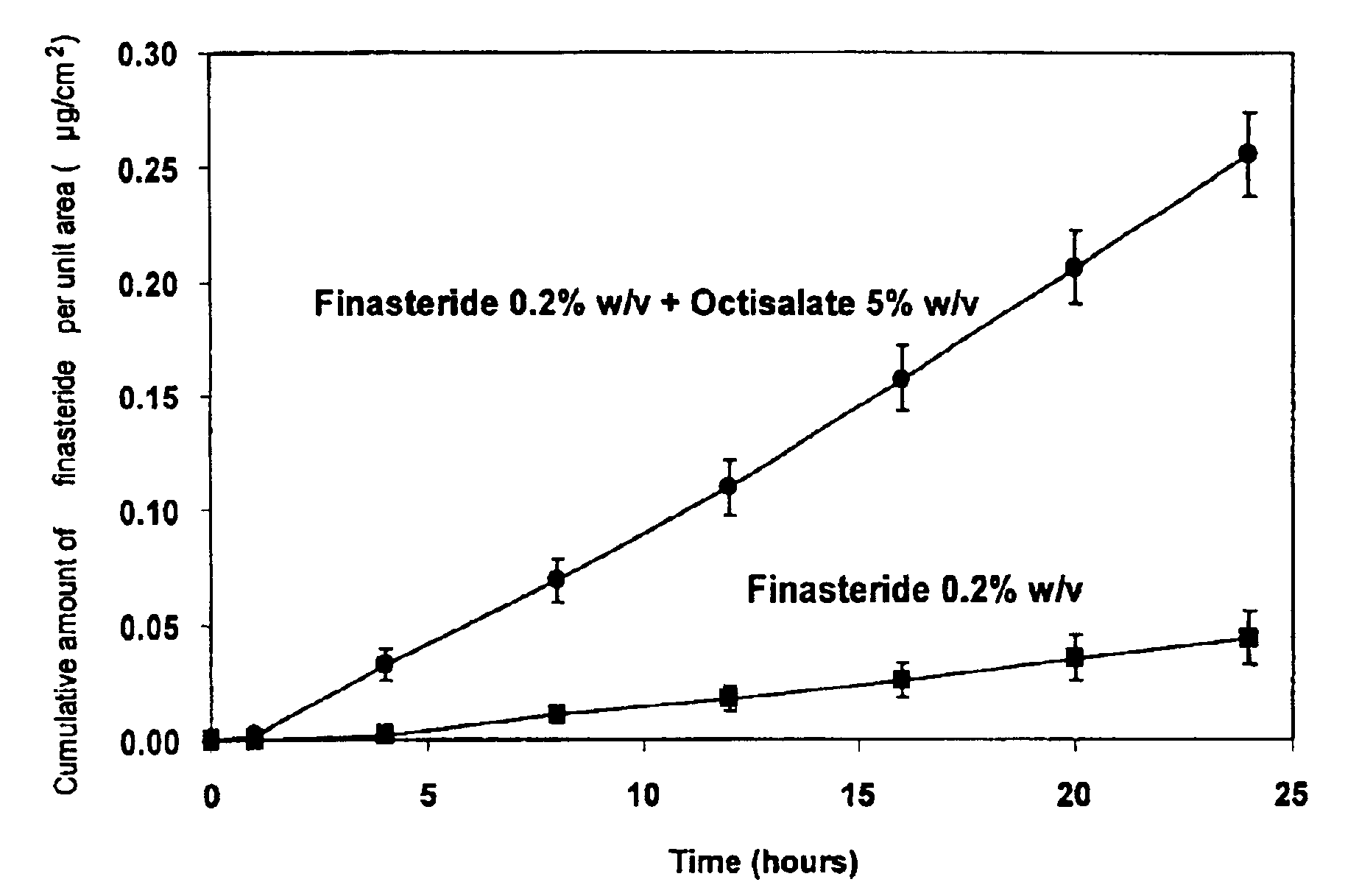
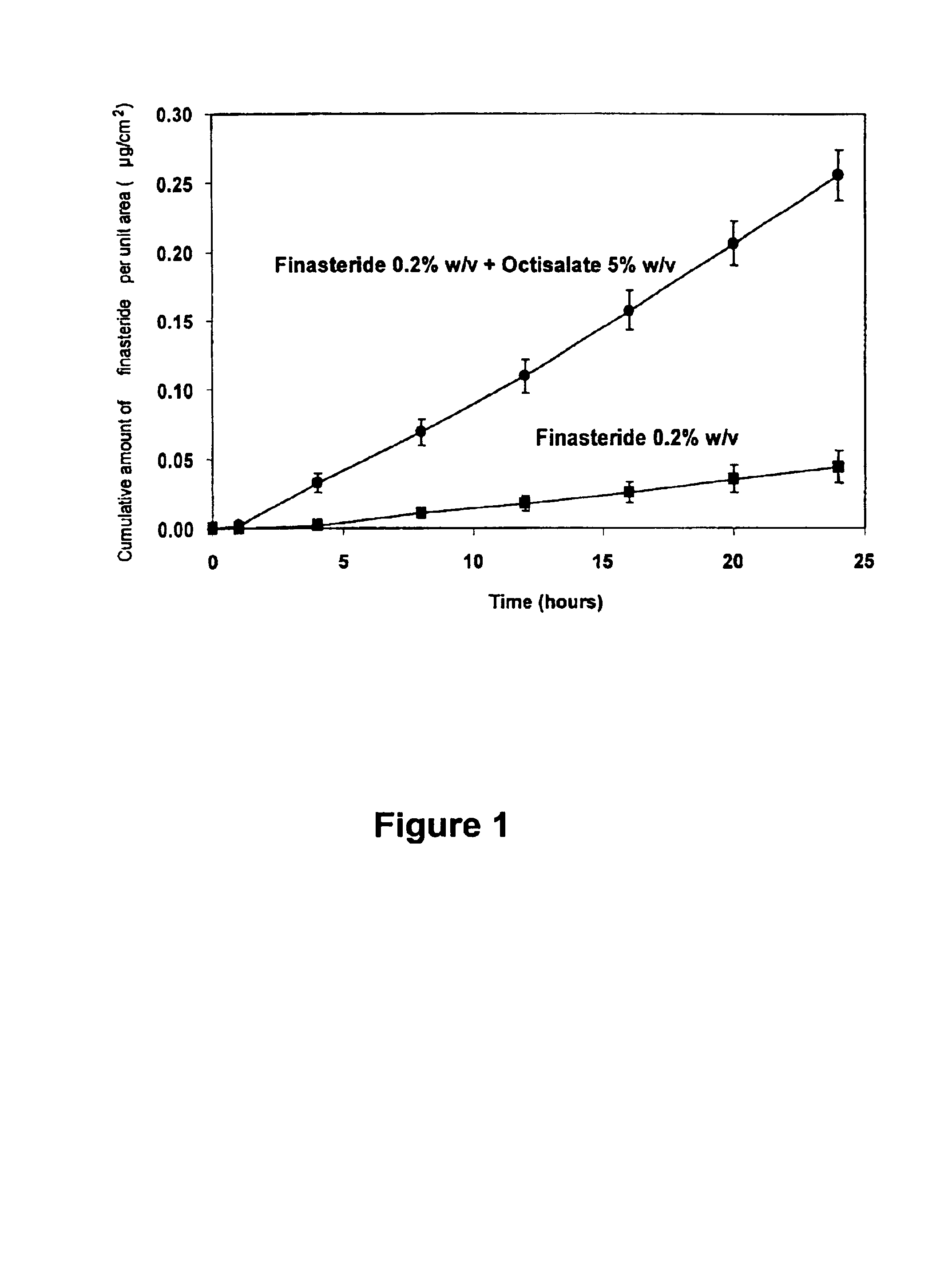
Topical delivery of anti-alopecia agents
InactiveUS6998138B2Improve absorption efficiencyLess irritatingCosmetic preparationsOrganic active ingredientsMedicineWhole body
The present invention provides a topical drug delivery system which comprises: a therapeutically effective amount of an anti-alopecia agent; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and a volatile liquid. The invention also provides a method for administering at least one systemic acting anti-alopecia agent to an animal which comprises applying an effective amount of the anti-alopecia agent in the form of the drug delivery system of the present invention.
Owner:ACRUX DDS +1
Gelled immunomodulating topical compositions and a method of treating warts and other human papilloma virus skin infections
Topical drug compositions of this invention contain delayed type contact sensitizing haptens in a unique non-flowable, non-toxic, non-volatile, anhydrous gel composition to achieve retained site application on warts and other human papilloma virus (HPV) skin infections. The preferred gelled compositions contain, but are not limited to, the sensitizing haptens, squaric acid dibutylester and diphenylcyclopropenone in optimized blends of Polysorbate 80, Isopropyl myristate uniquely gelled with Polyoxyl 40 stearate to form a penetrant of keratinized epitheliiuim of warts for direct application wherein virucidal pharlacologic action is induced by Th-1 cell mediated immune responses with resultant releases of CD4 helper T cells, CD8 killer T cells and cytokines to attack the human papilloma viruses. The commonly used vehicles with these contact sensitizers are acetone, petrolatum, or water containing emulsion creams which do not have the capacity to penetrate the keratinized wart surfaces and are therefore minimally effective in treating warts.
Owner:HAPTEN PHARMA
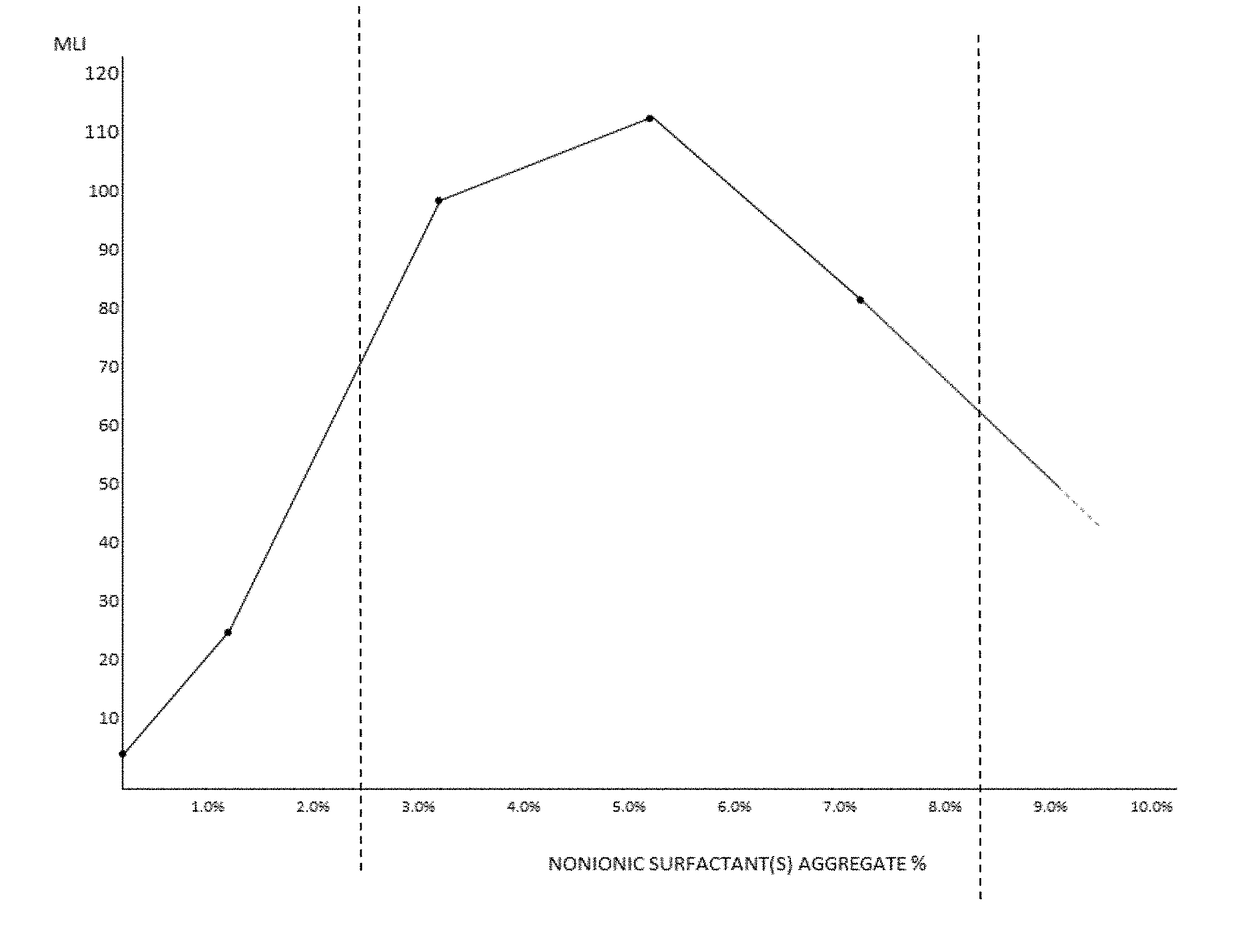
Artificial tear, contact lens and drug vehicle compositions and methods of use thereof
PendingUS20180098937A1Enhancing contact lens wear timeReduce decreaseOrganic active ingredientsSenses disorderPolyolMedicine
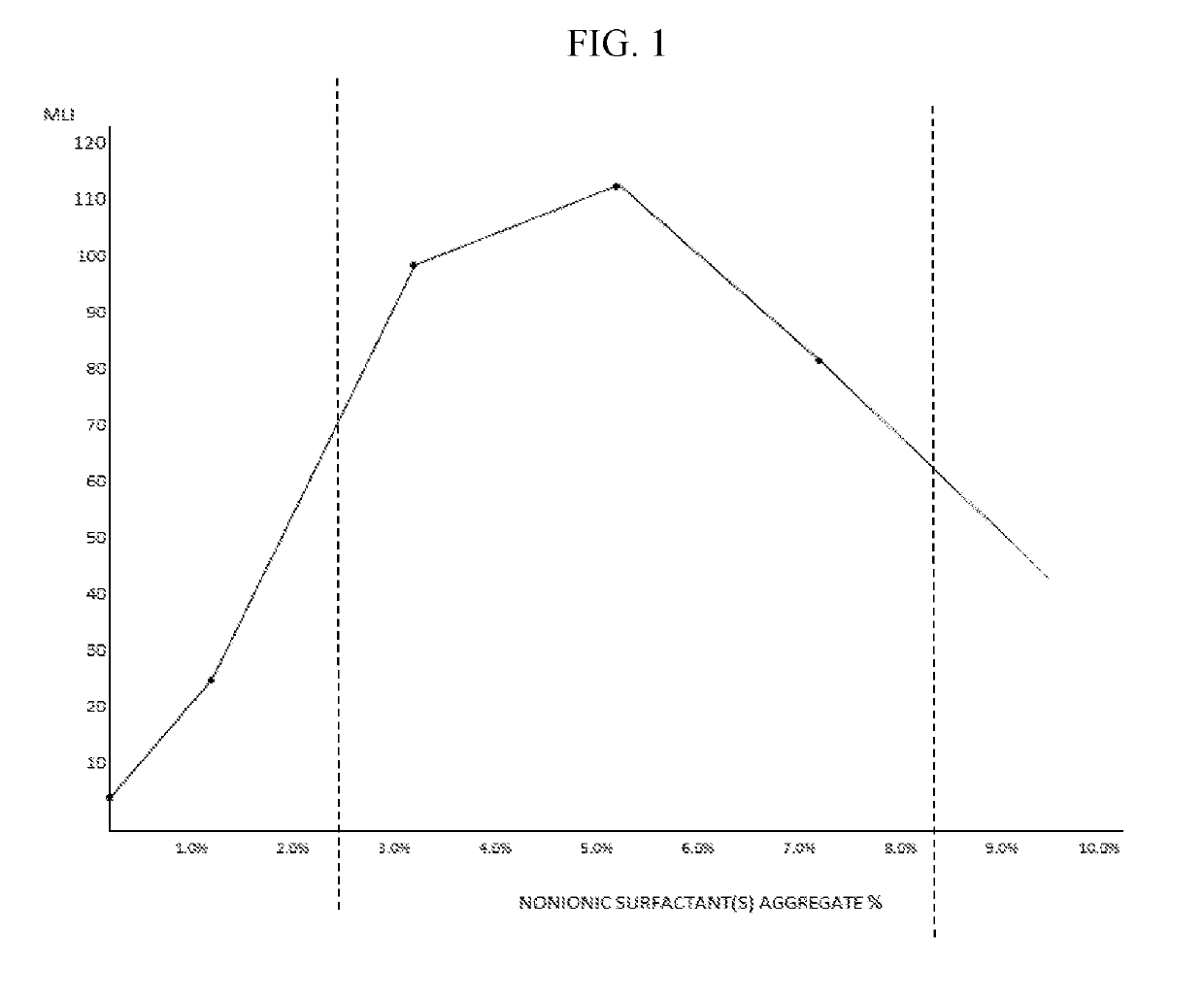
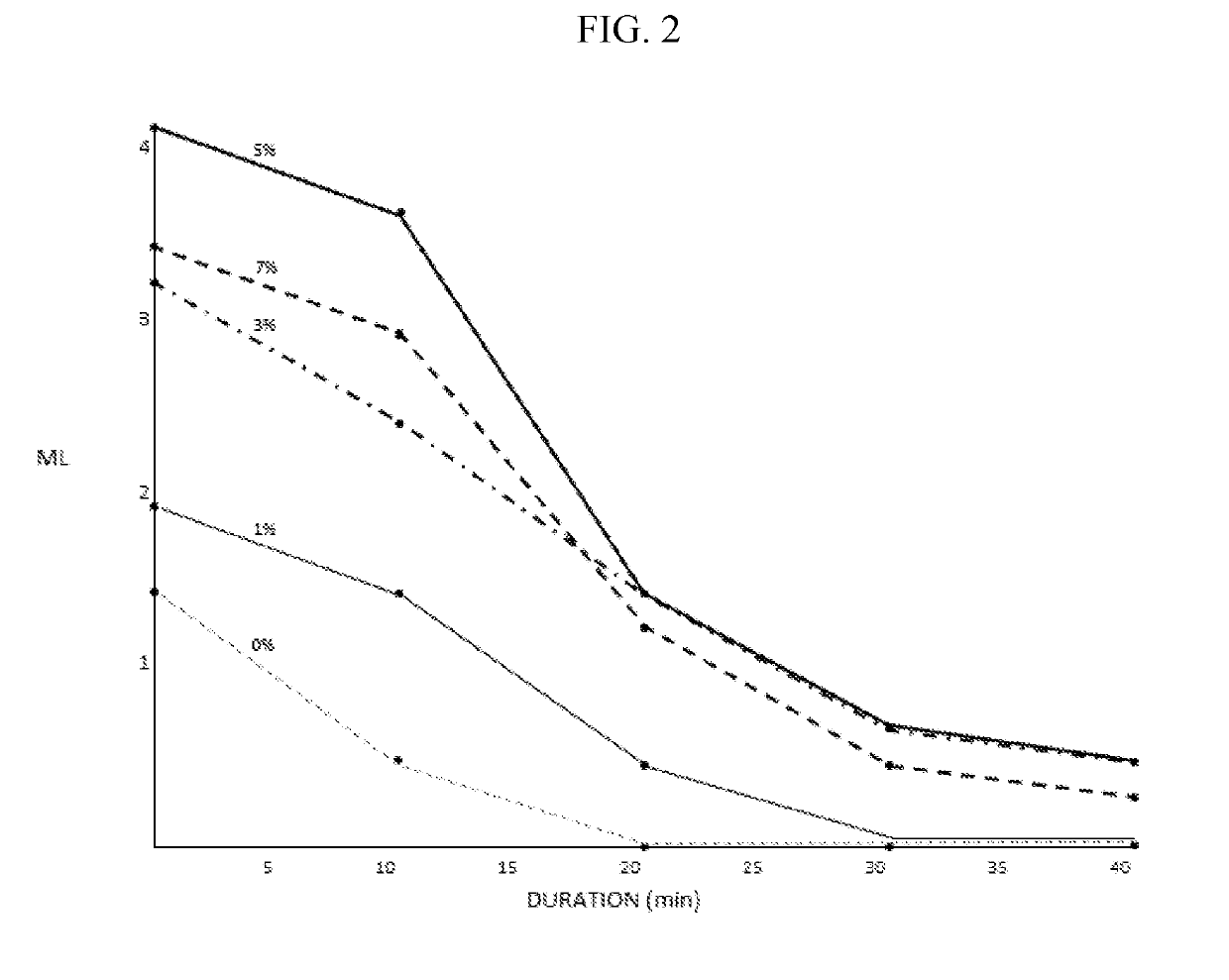
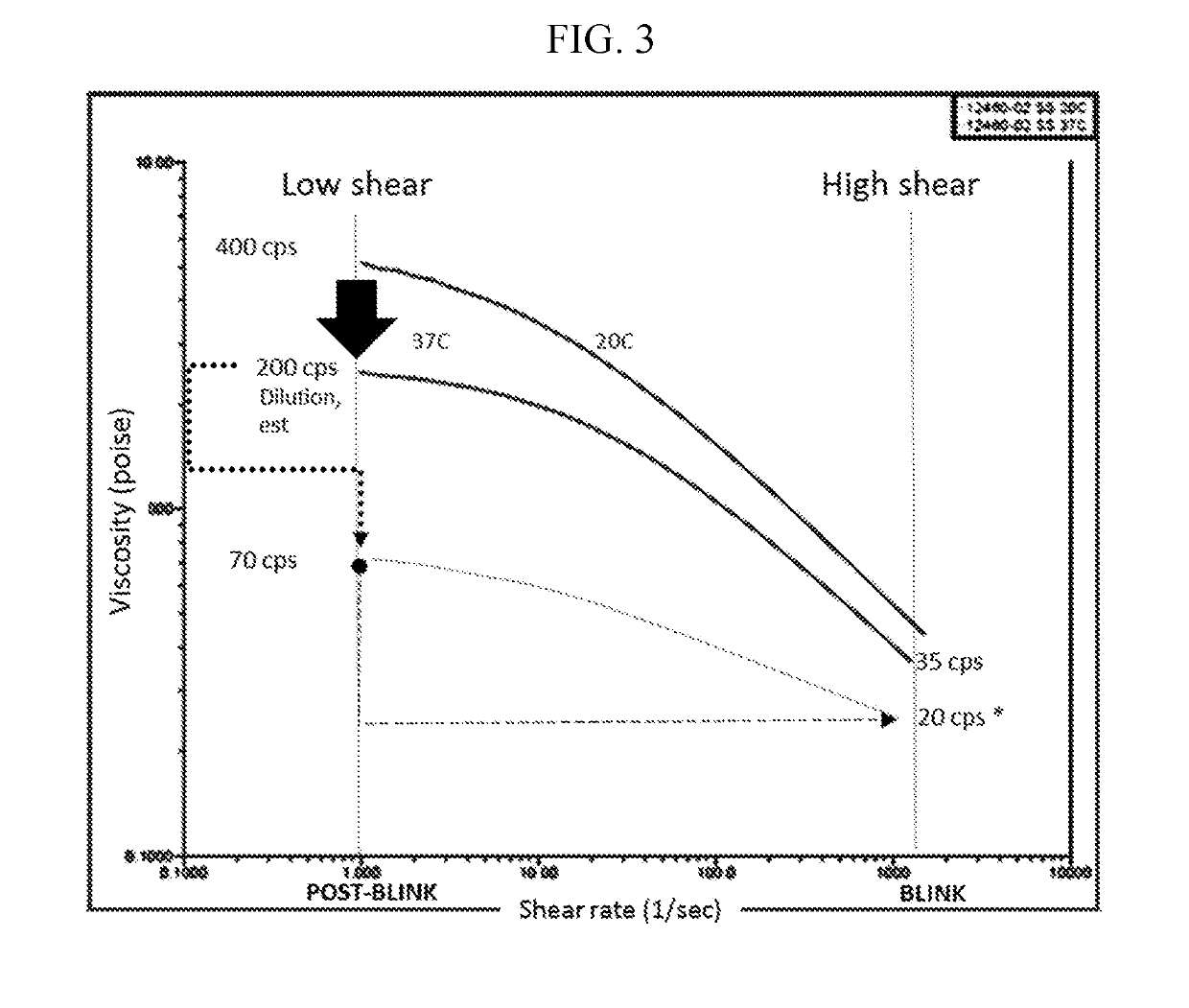
The invention provides artificial tear compositions, artificial tear-gel compositions, contact lens storage compositions, contact lens treatment compositions, ophthalmological drug vehicle compositions and topical drug vehicle compositions comprising one or more nonionic surfactants with one or more non-Newtonian viscosity enhancing excipients and one or more of a polyol and or an electrolyte and methods of their use.
Owner:PS THERAPIES LTD +1
Prophylactic and therapeutic treatment of topical and transdermal drug-induced skin reactions
Botanically derived anti-irritants for prophylactic and therapeutic treatment of adverse skin reactions from application of transdermal or topical drug delivery system, permits the effective administration of a drug from a delivery system in which the drug, of a component of the delivery system comprises a skin irritant; and the delivery systems formed thereby.
Owner:MORNINGSIDE VENTURE INVESTMENTS
Transdermal drug delivery formulations with optimal amounts of vasodilators therein
InactiveUS20060013866A1Maximize efficiencyEasy to transportSalicyclic acid active ingredientsBiocideVascular dilatationDelivery vehicle
Topical drug delivery formulations with optical amounts of vasodilator. Vasodilator chemicals applied topically dilate the blood vessels in the skin tissue, which have been shown to facilitate or inhibit systemic or skin tissue deposition of drug substances. The level of stimulation and / or inhibition has been found to be dependent on the concentration and the identity of the specific vasodilator chemical(s) used as well as the drug molecule(s) to be delivered. This work teaches the need to consider specific formulation requirements when dealing with vasodilator chemicals for the creation of successful delivery vehicles in the transdermal drug delivery system. These requirements for very low concentrations of vasodilators were an unexpected and a surprise finding, in contrast to the concentrations of the vasodilators typically used to elicit an increase in skin blood flow.
Owner:BIOCHEMICS
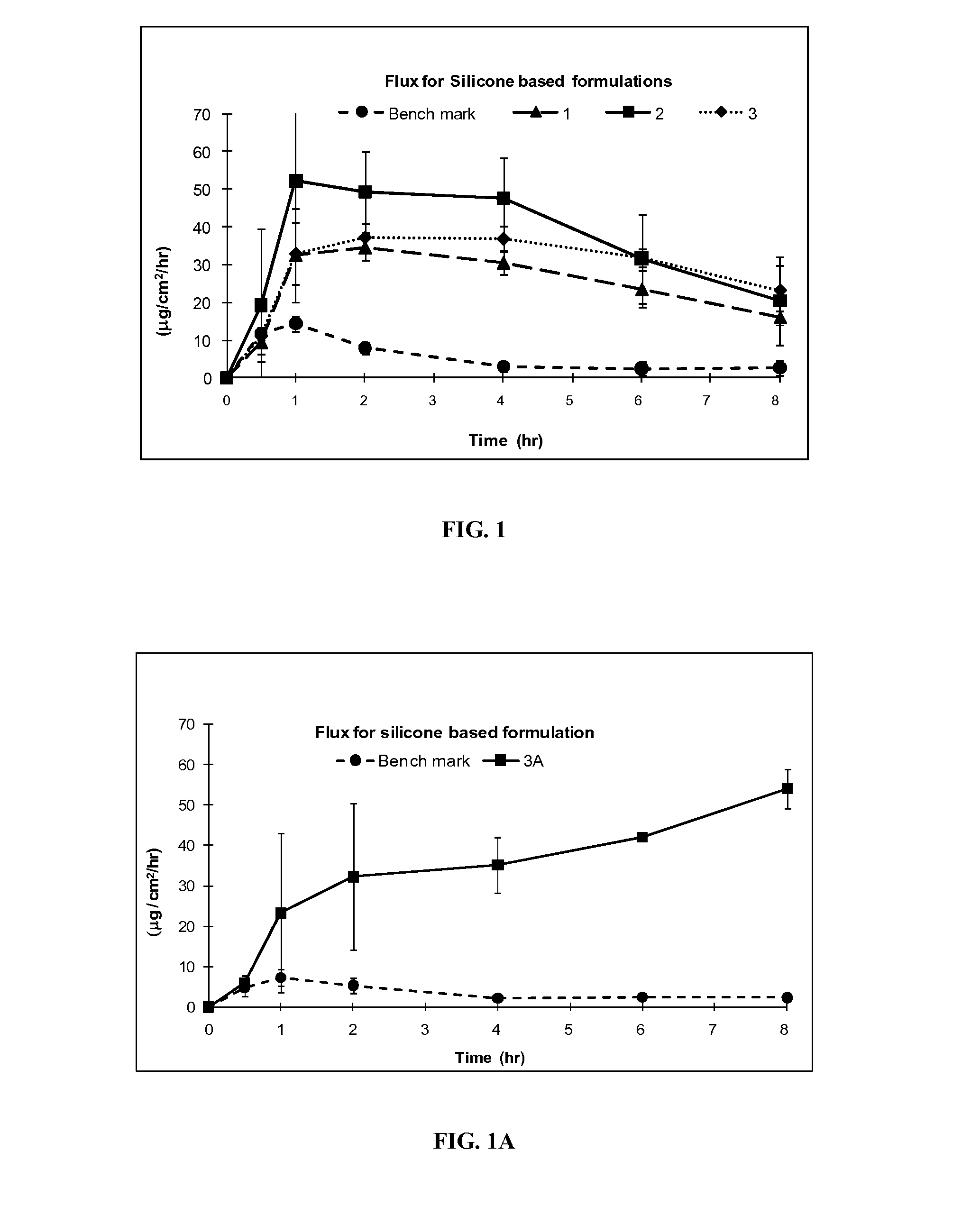
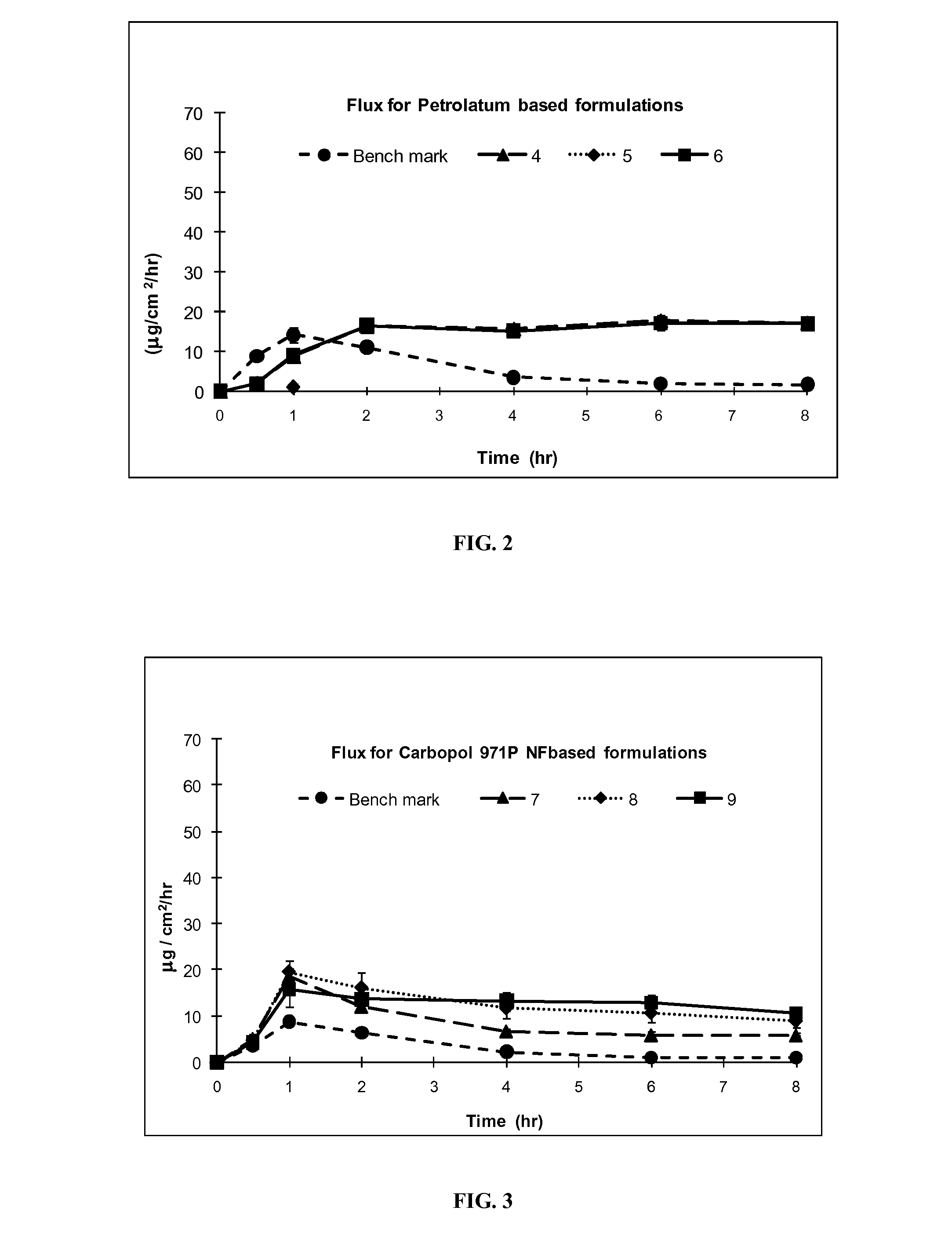
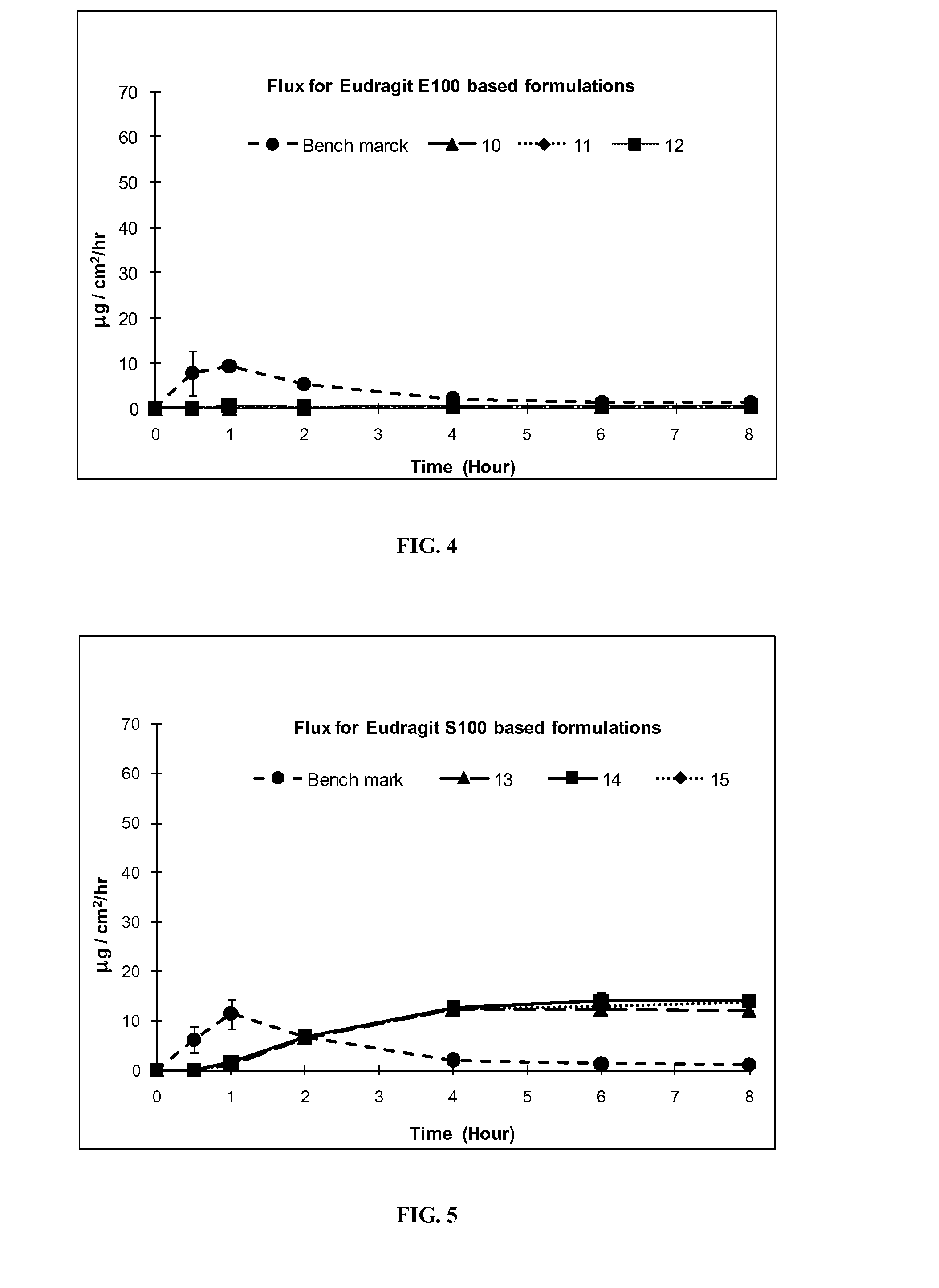
Silicone based emulsions for topical drug delivery
A water-in-oil emulsion is provided in which the lipophilic phase of the emulsion contains a silicone fluid and an emulsifier, a hydrophilic phase, and a pharmaceutically active compound. The active pharmaceutical ingredient is dissolved or dispersed in the emulsion and is partitioned in the emulsion so that all or a portion of the amount of the chemical compound dissolved or dispersed in the emulsion is dissolved or dispersed in the aqueous phase of the emulsion. The emulsion of the invention provides increased penetration into skin of the chemical compound dissolved or dispersed in the aqueous phase.
Owner:DOW PHARMA SCI INC
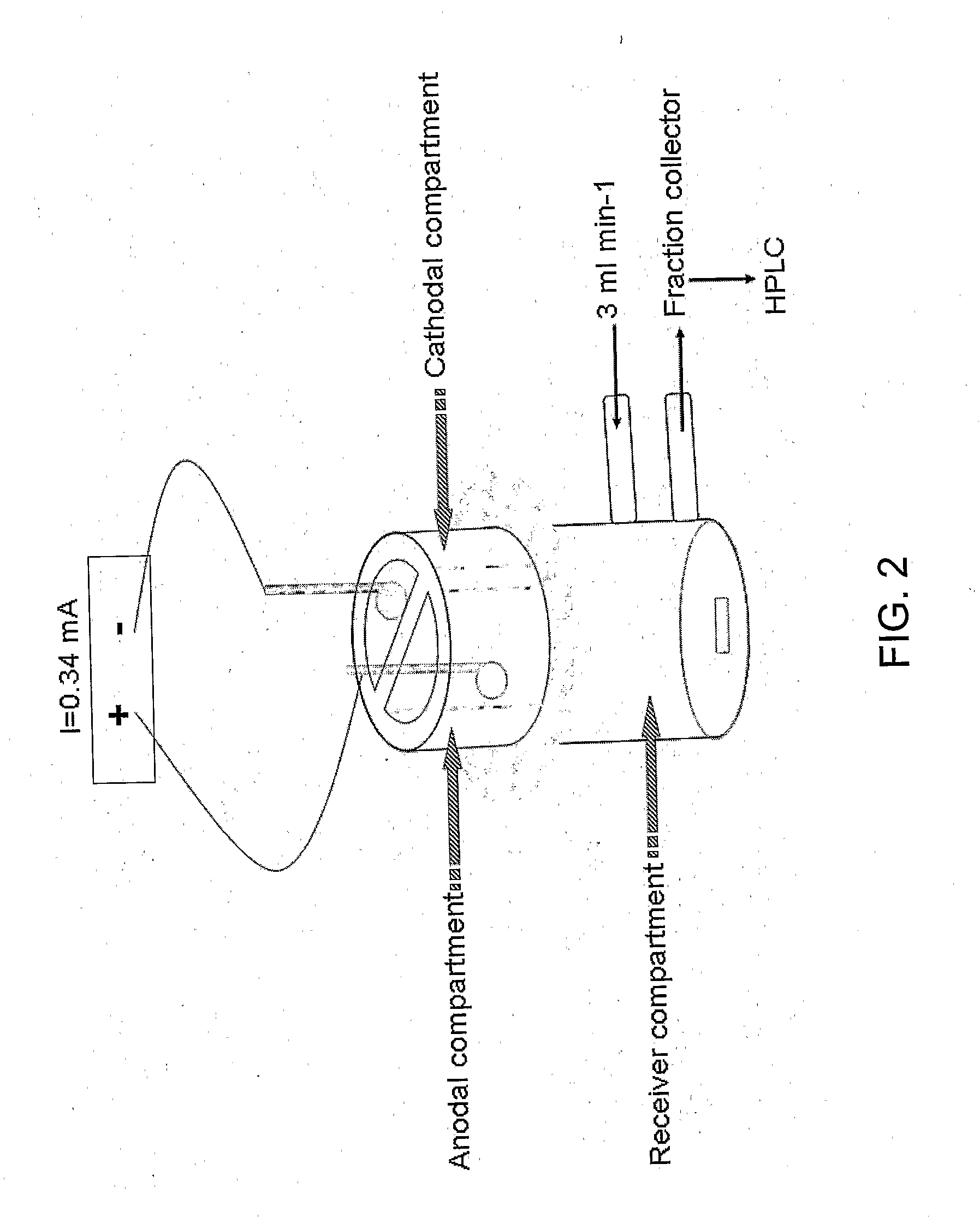
Topical drug delivery by iontophoresis
The invention generally concerns methods of topical drag delivery. Delivery according to the invention may be via electrotransport of compounds through the skin, for example by iontophoresis. In certain embodiments improved methods for the delivery of compounds, such as antimicrobial agents are described.
Owner:UNIVERSITY OF GENEVA
Freeze-dried excipient and matched solvent packaging double-cavity bag
InactiveCN104443822AProtection stabilityImprove stabilityContainers with multiple articlesFreeze-dryingMedicine
The invention relates to a freeze-dried excipient and a matched solvent packaging double-cavity bag, in particular to a double-cavity bag which enables a freeze-dried excipient applied to foods or health foods or oral drugs or topical drugs or cosmetics and a solvent to be independently stored and used in the same package and prepared to be readily available. The packaging double-cavity bag for the freeze-dried excipient has the advantages of protecting the stability of active ingredients, achieving single-dose packaging, being convenient to carry and use, avoiding contamination, lowering the cost and the like.
Owner:李和伟
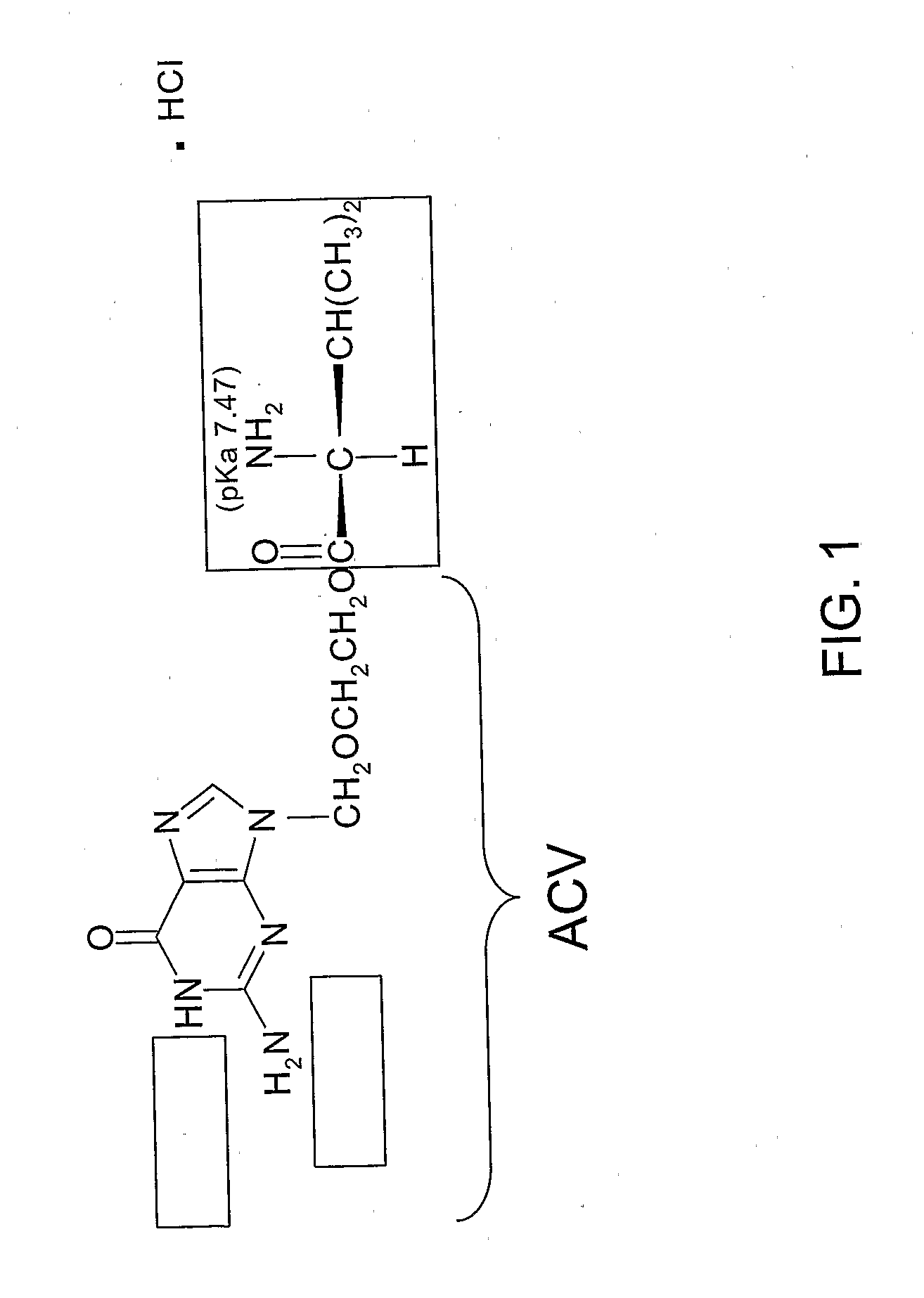
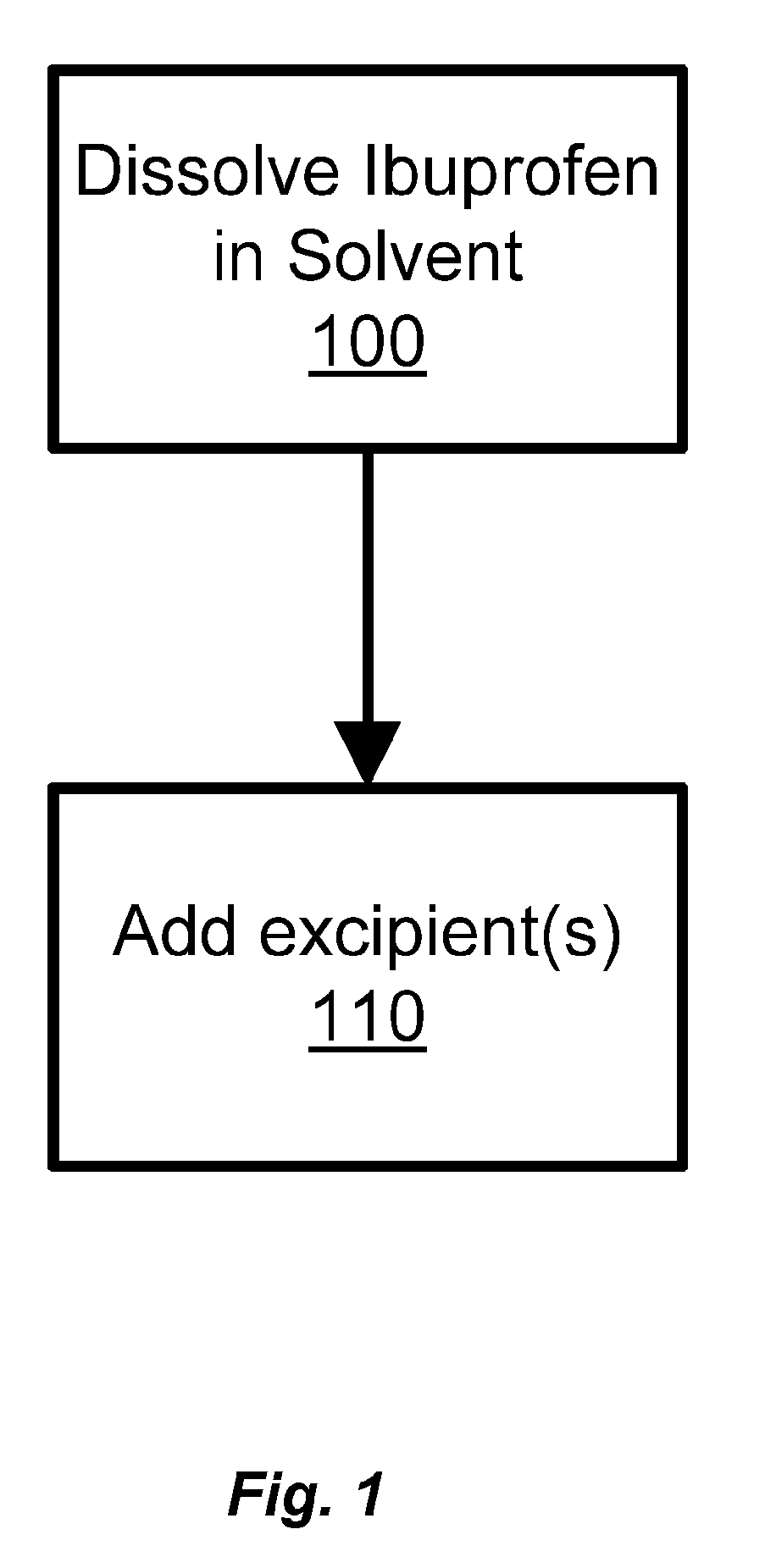
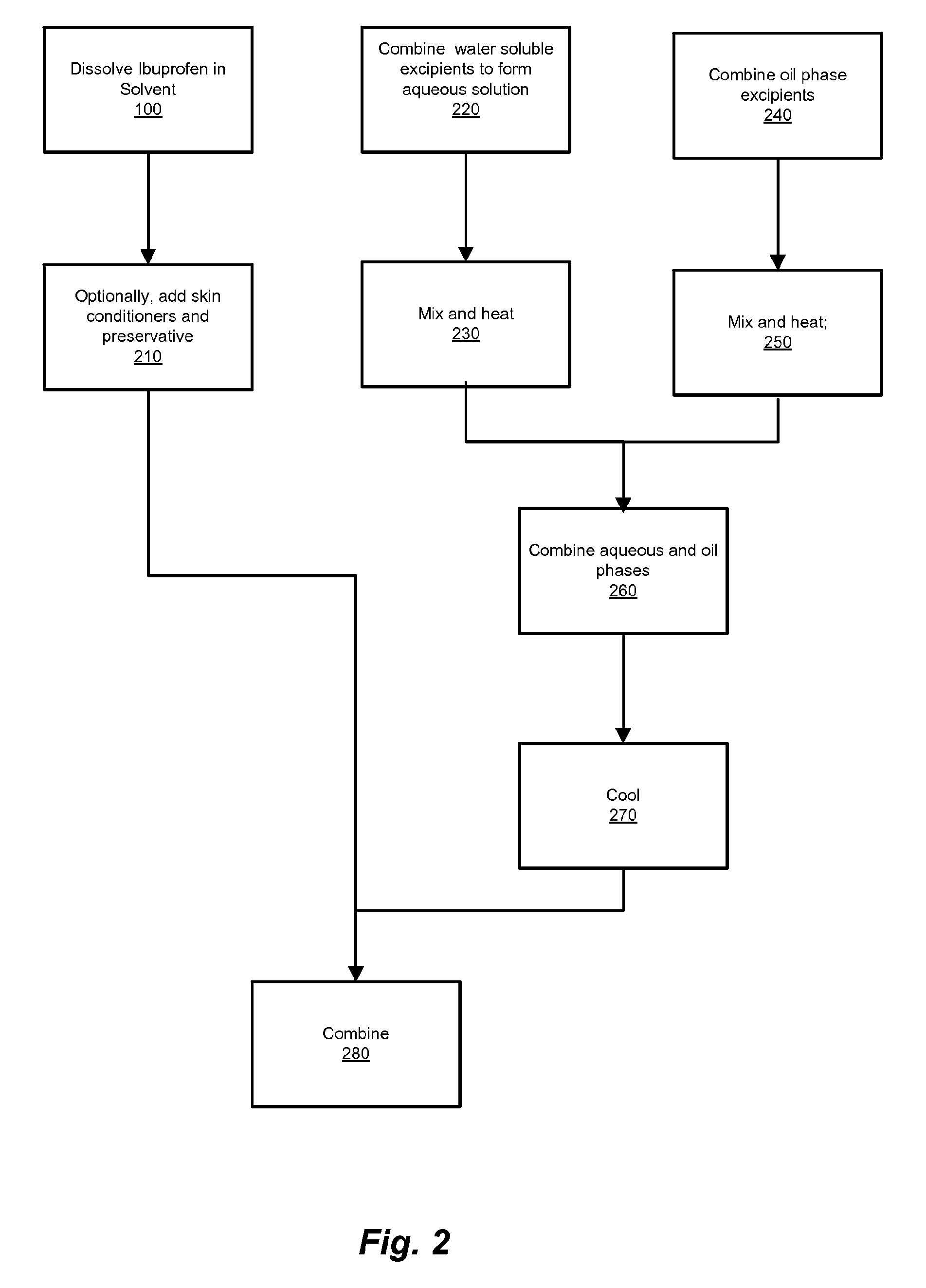
Ibuprofen for Topical Administration
InactiveUS20100137443A1Prevent degradationAdverse side effect commonlyBiocideAntipyreticPropanoic acidTopical drug
Set forth herein is a preparation of ibuprofen (2-(4-isobutylphenyl)propionic acid) in the free acid form that is suitable for topical administration. The topical ibuprofen formulation is prepared by dissolving the free acid form of ibuprofen, or preparing a homogeneous suspension of the free acid form of ibuprofen, in the presence of a pharmaceutically acceptable solvent so as to produce a topical drug formulation compatible with the penetration of 2-(4-isobutylphenyl) propionic acid through the skin tissue. Topical formulations of ibuprofen can be based on a pharmaceutically acceptable solvent such as, e.g., a pyrrolidone solvent or dimethylacetamide.
Owner:BIOCHEMICS
Drug vehicle compositions and methods of use thereof
ActiveUS20190224120A1Good effectHigh viscosityOrganic active ingredientsSenses disorderOcular surface diseaseDrug carrier
The invention is directed to topical drug vehicle platform compositions for ophthalmological and dermatological use. These compositions comprise a means to sequester tears and an ophthalmological drug. The invention is further directed to methods of treating a spectrum of ocular surface disease epitheliopathies including but not limited to dry eye in a human or mammal. The invention is further directed to contact lenses, punctum plugs, pellets or any other device used to deliver drugs to the surface of the eye, coated or infused with compositions of the invention.
Owner:PS THERAPIES LTD +1
Cyclic ether side group-containing amphiphilic polymer lyophilized powder and composition thereof, and applications of composition
The present invention relates to cyclic ether side group-containing amphiphilic polymer lyophilized powder and a composition thereof, and applications of the composition, wherein the lyophilized powder is the lyophilized powder of a cyclic ether side group-containing amphiphilic polymer or the drug-loaded lyophilized powder adopting a cyclic ether side group-containing amphiphilic polymer as an auxiliary material, the amphiphilic polymer is selected from one or a plurality of ABA type and BAB type amphiphilic block copolymers comprising a degradable polyester A containing a 1,4,8-trioxaspiro[4.6]-9-undecanone (TOSUO) unit and a polyethylene glycol block B, and the polyester A is a copolymer of caprolactone, lactide, glycolide and TOSUO. According to the present invention, the lyophilized powder can be rapidly dispersed into an aqueous medium at a temperature of 4-25 DEG C to form a flowable liquid and can be rapidly gelated under the body temperature environment; and the pyrogen-free powder injection after the sterilization treatment can be used for the clinical topical drug administration application, is sued for the topical drug administration on the intratumoral position, the peritumoral position, the thoracic cavity, the peritoneal cavity, the Intravesical position, the position in the liver, the intranasal position, the intraocular position, the intravaginal position and other positions, and the drug can be subjected to long-acting slow-release.
Owner:TIANJIN UNIV
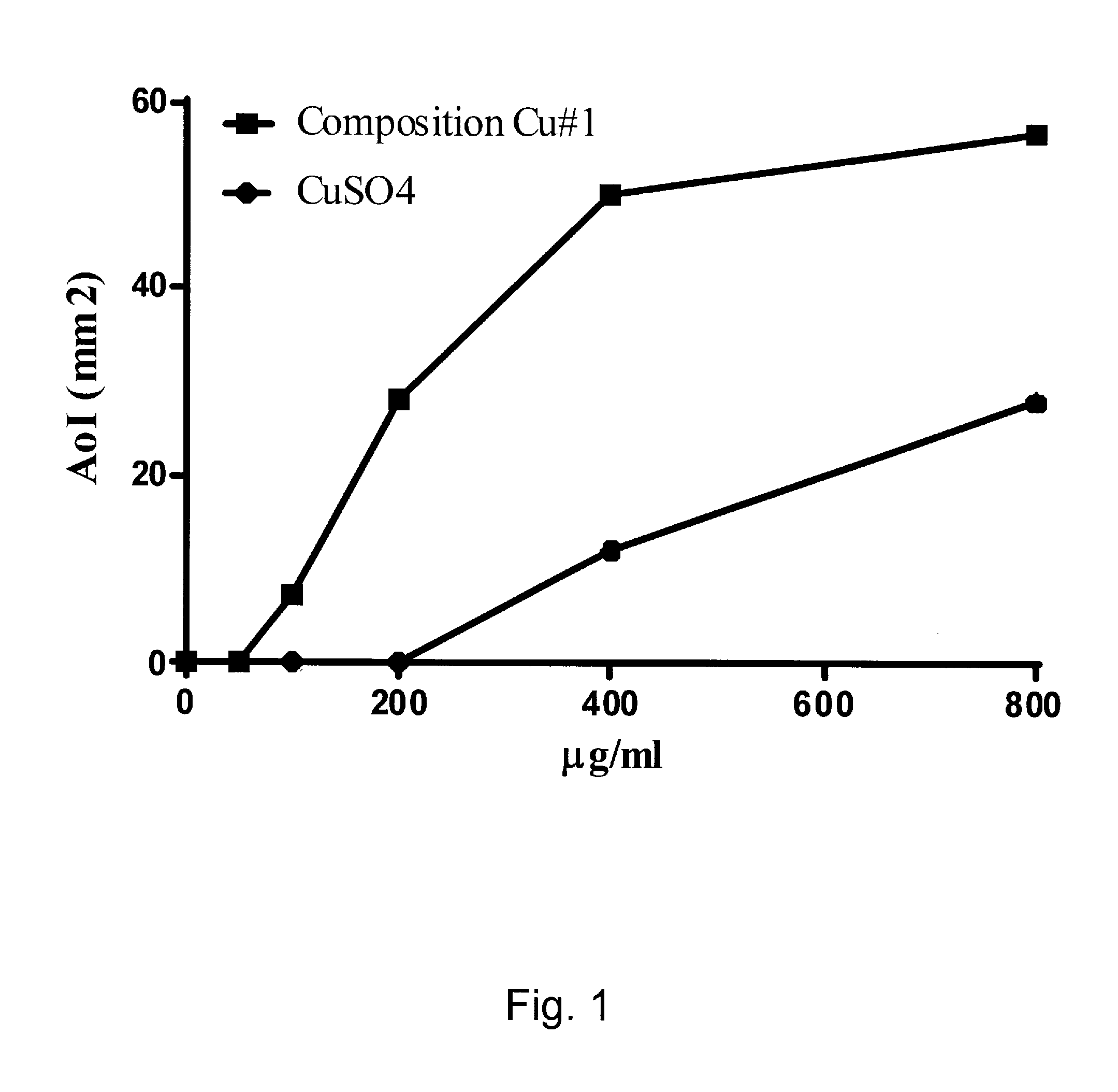
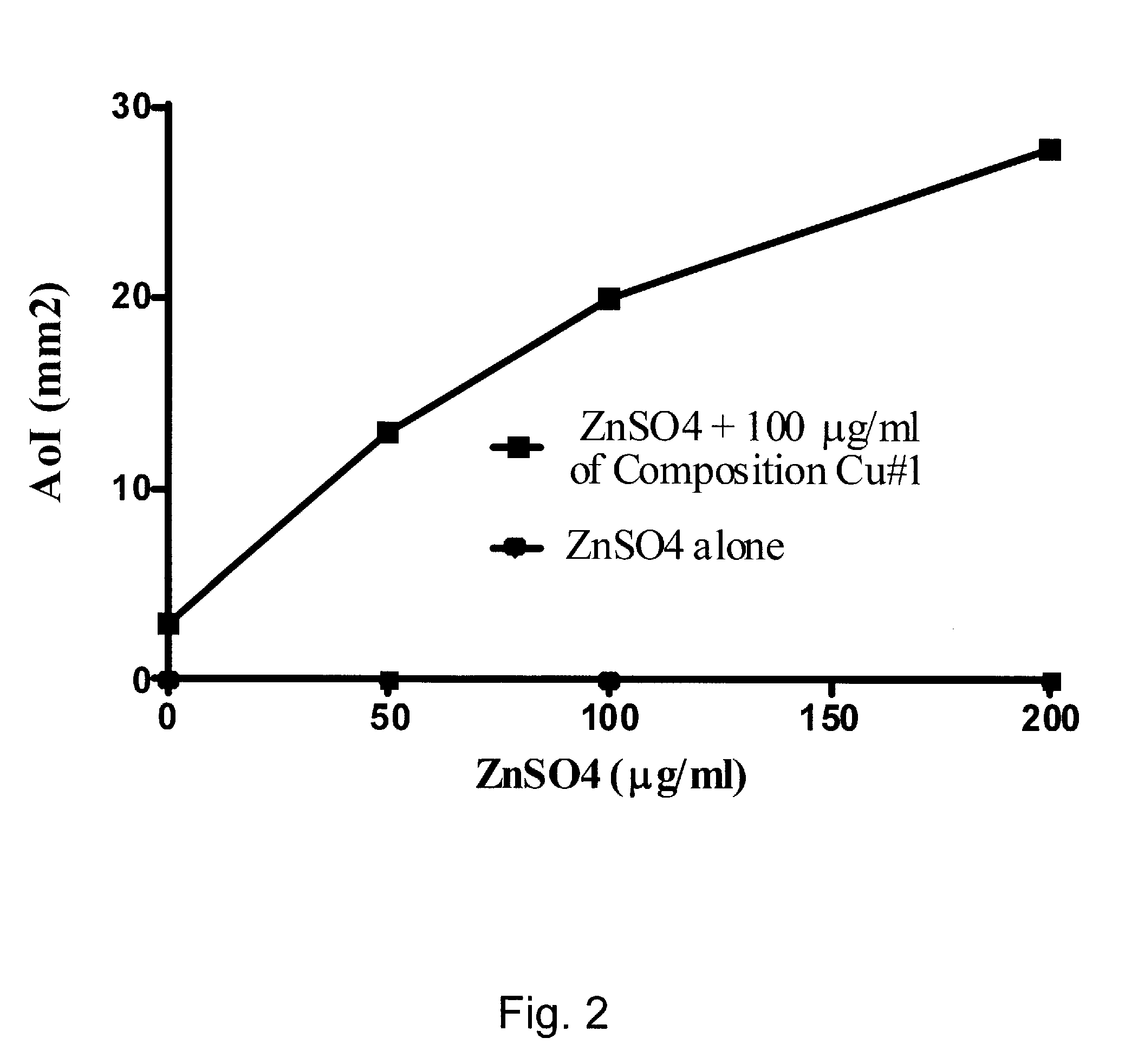
Acid-Solubilized Copper-Ammonium Complexes and Copper-Zinc-Ammonium Complexes, Compositions, Preparations, Methods, and Uses
An antimicrobial composition is disclosed that contains an acid-solubilized copper ammonium or copper-zinc ammonium complex that is effective against microorganisms such as nosocomial or environmental bacteria, fungi, viruses, and the like. The antimicrobial composition can be used in the preparation of a medicament for treating microbes or a microbial infection, and may contain a carrier to create a cream, soap, wash, spray, dressing, cleanser, cosmetic product, topical drug product, or other antimicrobial product.
Owner:VM AGRITECH LTD
External-use sertaconazole nitrate suppositorium
InactiveCN101375835ASimple preparation processLow toxicityOrganic active ingredientsSuppositories deliverySertaconazole NitrateTreatment effect
The invention provides an external nitric acid Sertaconazole suppository, which is a suppository of different specifications made by mixing the powder of ground nitric acid Sertaconazole and melt suppository base, glycerol and Polysorbate 80. The suppository has the beneficial effects that the preparation process of the suppository is simple, the eutherapeutic and low-toxic nitric acid Sertaconazole are used as the main medicaments, a greater cure effect can be achieved by using a small amount of medicament for local application, mycotic infection is inhibited, the cure effect is good, the use is convenient, etc.
Owner:HAINAN HAISHEN TONGZHOU PHARM CO LTD
Topical Formulation Compositions Containing Silicone Based Excipients To Deliver Actives To A Substrate
The present disclosure relates to a semi-solid topical drug delivery formulation including a silicone-based excipient, at least one volatile solvent, at least one active configured to be topically delivered through a patient's skin for an intended therapeutic application, and at least one enhancer. The formulation may additionally optionally include at least one agent that provides occlusivity when the formulation is applied onto a patient's skin. The at least one active may be a healthcare and / or pharmaceutical active.
Owner:DOW CORNING CORP
Topically applied sustained-release antibiotic preparation
InactiveCN1883706AIncrease concentrationReduce concentrationAntisepticsSaccharide peptide ingredientsTreatment effectWhole body
Disclosed is a topical applicated antibiotics slow release agent as a slow release injection or a slow release implantation agent, comprising slow release microspheres and menstruum. Said release microsphere comprises slow release assist materials and antibiotics, and said menstruum is a special menstruum comprising suspending agents of sodium carboxymethylcellulose, etc, with a viscosity of 100cp-3000cp(at 20-30 DEG C). Said slow release assist materials are selected from the group of EVAc, polifeprosan, PLA, PLGA, sebacic acid copolymer, albumen glue, gelatin, and etc. Said slow release implantation agent is prepared with slow release microspheres or by melt method, etc. The disclosed slow release agent can release drugs for 10 days by topical applicated or injected at a focus, decreasing whole body toxicity obviously while obtaining and sustaining effective topical drug concentration at the focus.
Owner:JINAN KANGQUAN PHARMA TECH
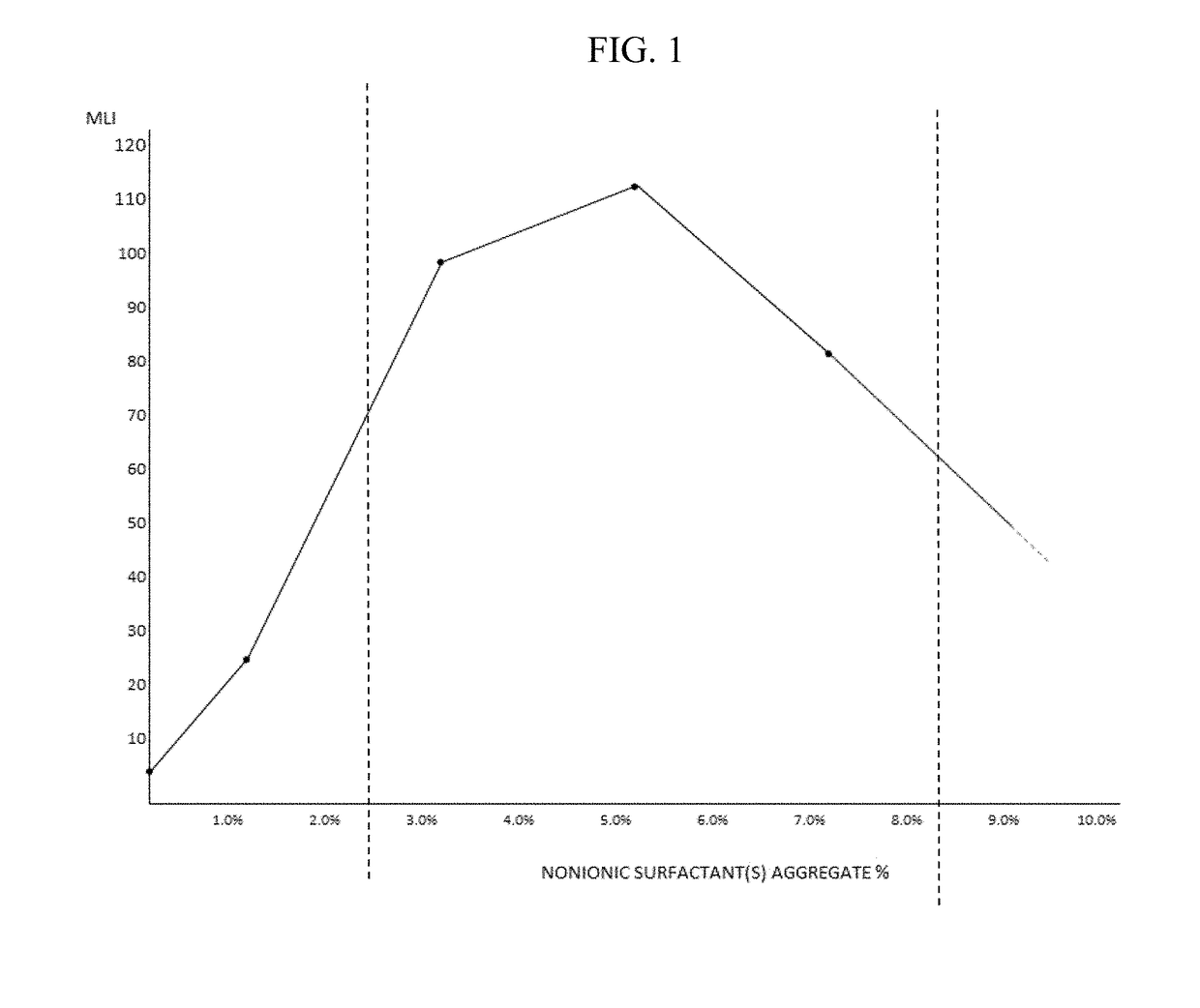
Artificial tear, contact lens and drug vehicle compositions and methods of use thereof
ActiveCN110114119AAvoid breakingAvoid dischargeOrganic active ingredientsSenses disorderActive agentTopical drug
The invention provides artificial tear compositions, artificial tear-gel compositions, contact lens storage compositions, contact lens treatment compositions, ophthalmological drug vehicle compositions and topical drug vehicle compositions comprising one or more nonionic surfactants with one or more non-Newtonian viscosity enhancing excipients and one or more of a polyol and or an electrolyte andmethods of their use.
Owner:ピーエスセラピーリミテッド
Topical drug delivery system
InactiveUS20060127483A1Increase the number ofImprove drug stabilityPowder deliveryOrganic active ingredientsWater basedIrritation
The present invention relates to topical drug compositions and methods for topical drug delivery which promote stability of a drug component and facilitate the penetration of the drug component into the skin of the host. The invention also relates to topical drug compositions containing a suitable vasoactive agent, such as a prostaglandin, and methods for effectively delivering said active ingredient to the host. These compositions and methods are useful for the treatment of sexual dysfunction, in both men and women. The invention also relates to methods for formulating or preparing the composition of the present invention. To minimize irritation, the composition is water based.
Owner:GLYCOBIOSCI +1
Compositions and Methods of Topical Drug Delivery for the Treatment of Carpal Tunnel Syndrome
InactiveUS20110008413A1Good skin permeabilityRelieve symptomsBiocideOrganic active ingredientsCTS - Carpal tunnel syndromeTopical drug
The present invention generally relates to transdermal drug delivery systems. More particularly, the present invention provides compositions and transdermal drug delivery systems for the treatment and / or relief of symptoms associated with carpal tunnel syndrome or tendonitis.
Owner:MSK PHARMA
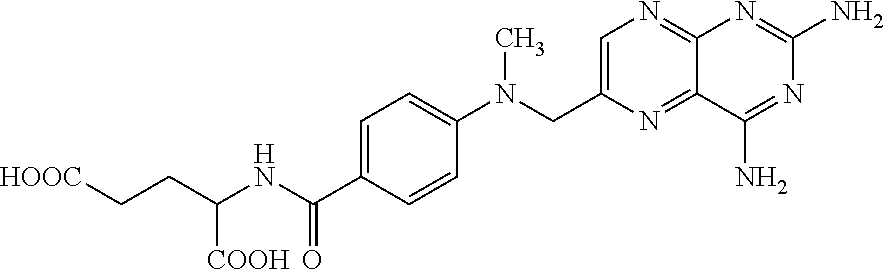
Topical Pharmaceutical Composition, Method for Producing the Topical Pharmaceutical Composition, Use of the Topical Pharmaceutical Composition and Method for the Topical Treatment of Psoriasis, Atopic Dermatitis or Chronic Eczema
This invention relates to a topical pharmaceutical composition comprising a combination of methotrexate, alpha bisabolol and allantoin; a process for producing the same and the use of the composition in the treatment of plaque psoriasis (psoriasis vulgaris), atopic dermatitis and chronic eczema. The composition of this invention can be used alone or in combination with other topical or systemic therapies. The present invention further discloses a process for producing the pharmaceutical composition.
Owner:BIOLAB SANUS FARMACEUTICA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com