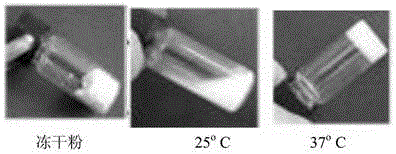
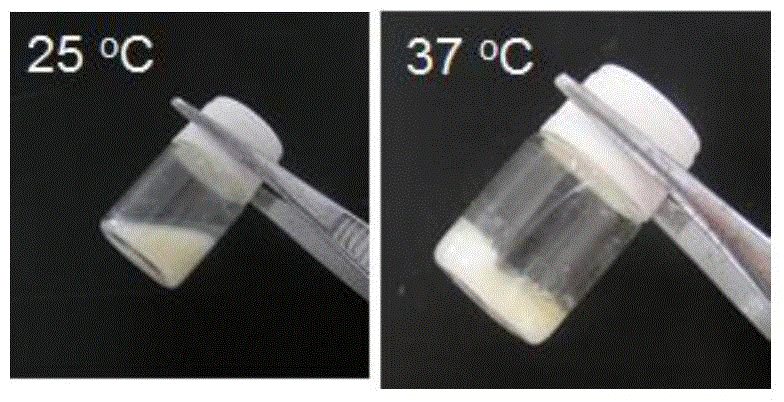
Cyclic ether side group-containing amphiphilic polymer lyophilized powder and composition thereof, and applications of composition
A technology based on amphiphilic polymers and cyclic ether side groups, which can be used in drug combinations, freeze-dried delivery, and medical preparations containing active ingredients, etc., and can solve problems such as non-degradability, difficult large-scale preparation, and difficult injections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add polyethylene glycol (1.50g) with a relative molecular mass of 1000 into the reactor, heat it to melt the polyethylene glycol, vacuum to remove water and cool it naturally; add caprolactone (2.79g), TOSUO (0.21g) and Stannous octoate (14mg), after adding each reactant, vacuum, fill with nitrogen, finally vacuum seal, react at 120°C for 24 hours under stirring; add dichloromethane to the reactor to dissolve the synthesized polymer, then Pour into cold n-hexane for precipitation, filter and collect the precipitate. This operation is repeated three times. The precipitate is separated by suction filtration and dried in vacuum to obtain ABA type amphiphilic polymer P-1. The heavy metal content is less than 0.001% (mass), and the residual monomer and solvent content is less than 0.05% (mass).
Embodiment 2
[0024] Polyethylene glycol monomethyl ether (7.2g) with a molecular weight of 700 was added to the reactor, heated to melt, vacuumed to remove water and then cooled naturally; add caprolactone (18.36g), TOSUO (3.24g) and caprylic acid in sequence Stannous (108mg), after adding each reactant, vacuumize, fill with nitrogen, finally vacuum seal, react at 160℃ for 10 hours; add dichloromethane to the reactor to dissolve the synthesized polymer, then pour into cold n-hexane The precipitate was collected by filtration. This operation was repeated three times. The precipitate was separated by suction filtration and dried under vacuum to obtain a diblock copolymer containing pendant cyclic ether groups; the above product (22.4g) was dissolved in anhydrous toluene (100ml ), heat and vacuum, remove the water absorbed by the polymer to the remaining 50ml of toluene, then add hexamethylene diisocyanate (432mg), stir and react at 60℃ for 8-24 hours, pour the reaction liquid into cold n-hexan...
Embodiment 3
[0026] The device and operation are the same as those in Example 1 and Example 2, except that the relative molecular weight of polyethylene glycol and the ratio between monomers are changed to the data in Table 1. According to the raw material composition shown in Table 1, Table 1 is prepared. In other block copolymers. Two or more precipitation purifications are required until the content of heavy metals and their compounds in the polymer (mainly stannous octoate) is less than 0.001%, and the residual monomer and solvent content is less than 0.05%.
[0027] The content of heavy metals is detected in accordance with the national FDA Pharmacopoeia standard method, and the monomer and organic solvent residues are determined by gas chromatography. The composition of the amphiphilic polymer is shown in Table 1
[0028] Table 1 Composition of biodegradable amphiphilic block copolymer containing cyclic ether side groups
[0029]
[0030] M B Represents the relative molecular mass of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com