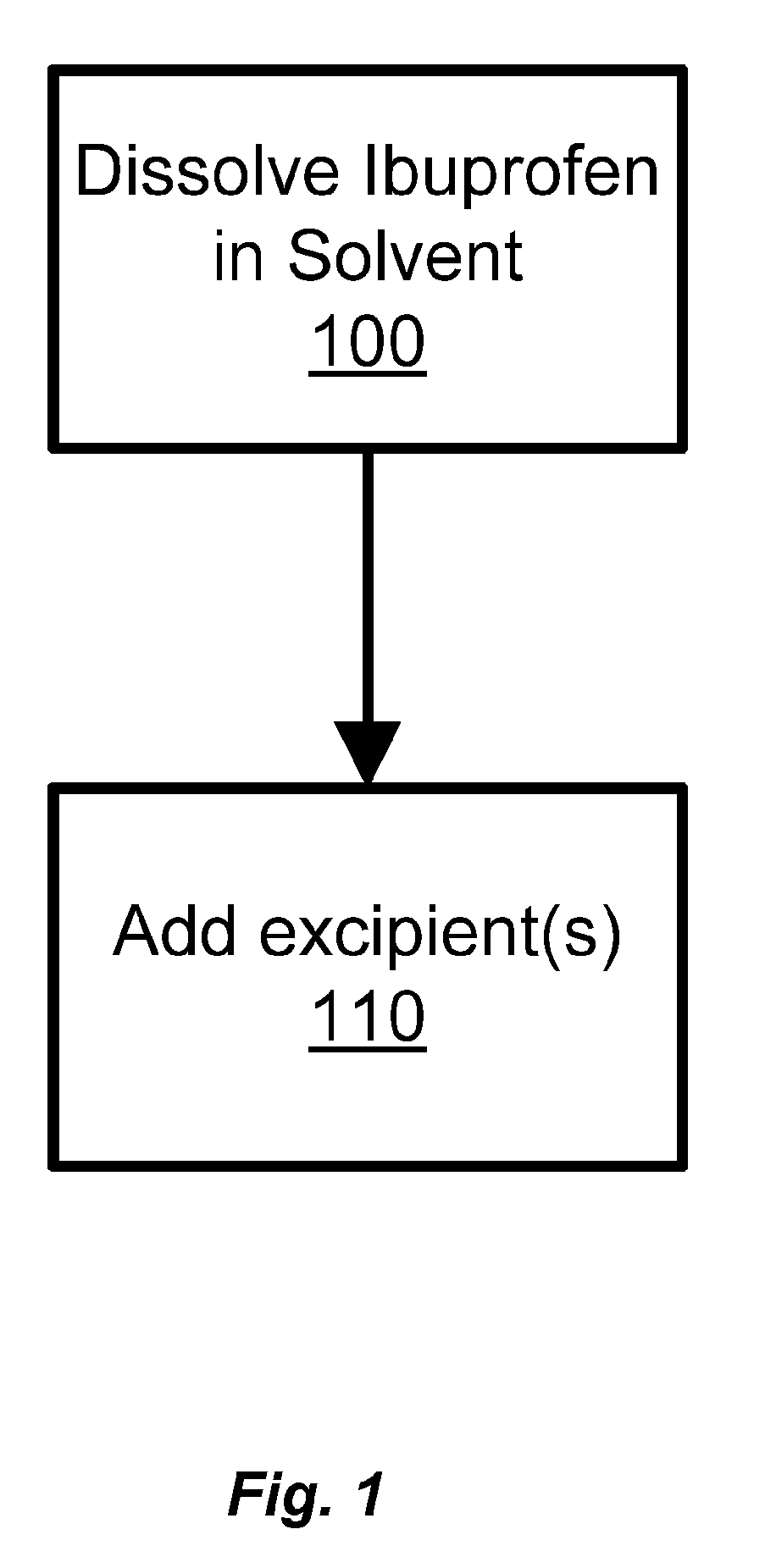
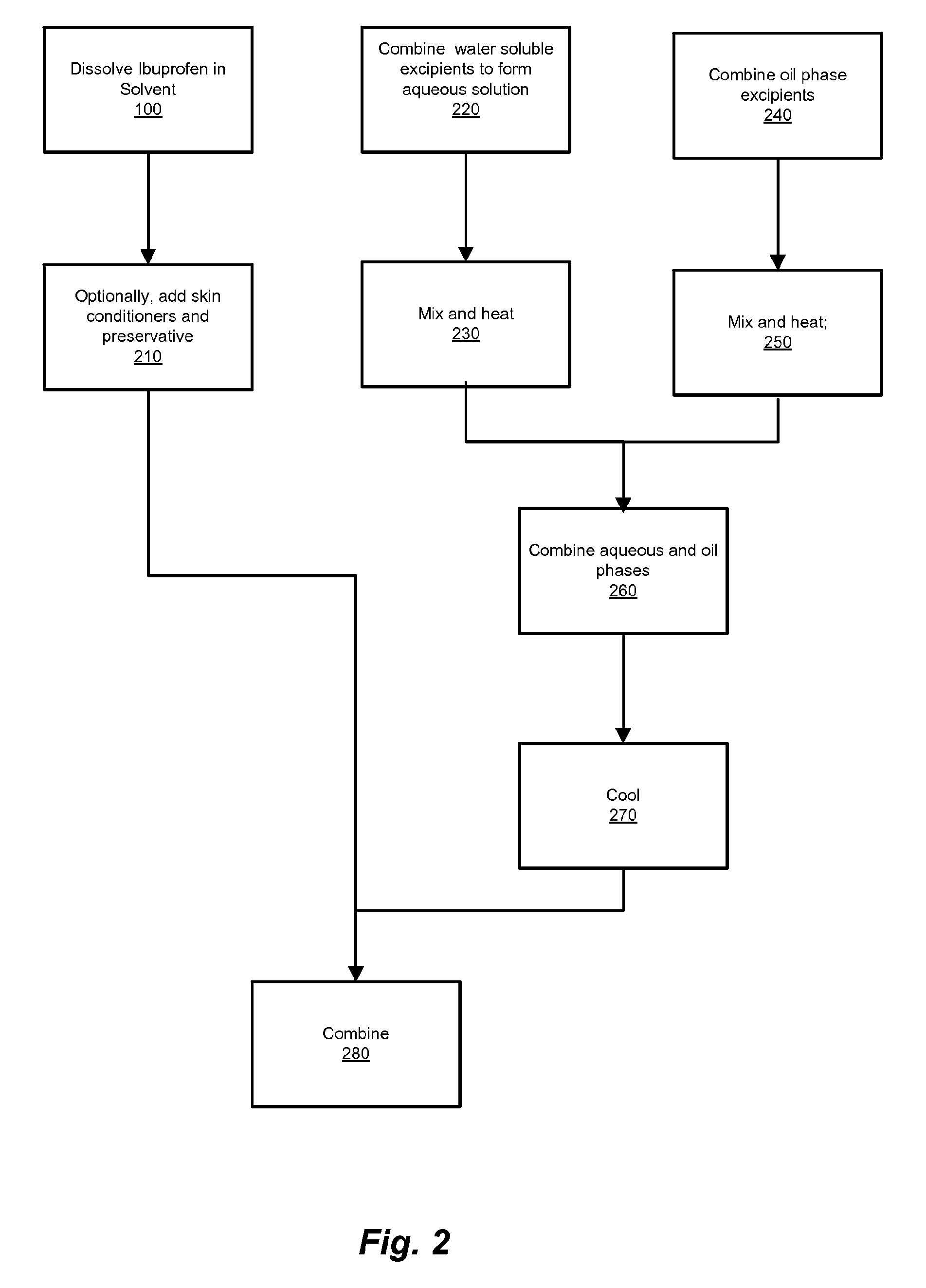
Ibuprofen for Topical Administration
a technology of ibuprofen and topical administration, which is applied in the field of topical compositions of ibuprofen, can solve the problems of inability to tolerate oral intake of ibuprofen, ineffective dosing, and limited use of ibuprofen in its free acid form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0046]
TABLE 1Wt % (ofexcipient)Phase AL-Arginine Base0.2Methylparaben0.2Water5Phase BIBU (USP grade)3.5Menthol2Eucalyptus Oil2N-methyl-2-pyrrolidone1Phenoxyethanol0.7Phase CCetyl Alcohol5Soybean Oil17.5Glyceryl Stearate6Beeswax22Petrolatum10Ethyl Oleate12.8Vitamin E TPGS2Capric Glyceride10Propylparaben0.1TOTAL:100
[0047]The formulation of Example 1 is prepared as follows. In Tank 1, dissolve (±)-2-(4-isobutylphenyl)propionic acid into N-methyl-2-pyrrolidone until completely solubilized. Add the remaining ingredients of Phase B and mix until completely dissolved. In the Main tank, add the ingredients of Phase C, mix while heating to 70° C. In tank 2, add the ingredients of Phase A, mix while heating to 70° C. Transfer the contents in Tank 2 into the main tank, mix for 10 minutes, and cool to 40° C. (degrees Celsius) or less. Transfer contents in Tank 1 into Main Tank at 40° C. (degrees Celsius) or less. Mix until blended (˜20 minutes).
example 2
[0048]
TABLE 2Wt % (ofexcipient)Phase AL-Arginine Base0.2Methylparaben0.2Water5Phase BIBU (USP grade)20Menthol2Soybean Oil14.8Eucalyptus oil2Phenoxyethanol0.7Dimethylacetamide2Phase CBeeswax18Petrolatum10Glyceryl Stearate5Cetyl Alcohol5Vitamin E TPGS2Capric Glyceride10Ethyl Oleate3Propylparaben0.1TOTAL:100
[0049]The formulation of Example 2 is prepared as follows. In Tank 1, dissolve menthol into eucalyptus oil until completely solubilized; add remaining ingredients of Phase B and pass through nano-equipment to reduce the particle size. In the main tank, add the ingredients of Phase C, mix while heating to 70° C. In tank 2, add the ingredients of Phase A, mix while heating to 70° C. Transfer the contents from Tank 2 into Main Tank, mix for 10 minutes; cool to 40° C. (degrees Celsius) or less. Transfer the contents of Tank 1 into the Main Tank at 40° C. (degrees Celsius) or less. Mix until blended (˜20 minutes).
example 3
[0050]
TABLE 3Preparation of Batch No. 176ZX03(% w / w)Ibuprofen in free acid form10KOH2L-Arginine Base0.5Carbopol ® 980NF (2.5%)4Veegum ® HV (10%)35Methylparaben0.2Syloid 244 FP4Phenoxyethanol0.7Water14Menthol5Eucalyptol5N,N-dimethylacetamide3Olive Oil5Lemon Oil0.5Vitamin E TPGS2Propylparaben0.1Glyceryl Monostearate7DC Elastomer 102TOTAL100
[0051]One hundred grams (100 g) of a 2.5% Carbopol® 980NF solution is prepared as follows. While heating 97.5 g water to 70° C., add 2.5 g Carbopol® 980NF powder with strong mixing (i.e.,. such that a vortex should turn). Mixing is continued until the solution is hydrated and free of clumps at 70° C. The solution is removed from heat and left at room temperature overnight, and then mixed again before use.
[0052]One hundred grams (100 g) of a 10% Veegum® HV solution is prepared as follows. While heating 90 g of water to 70° C., 10 g Veegum® HV is added with strong mixing (i.e., a vortex should turn). Mixing is continued for 30 minutes at 70° C. The mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com