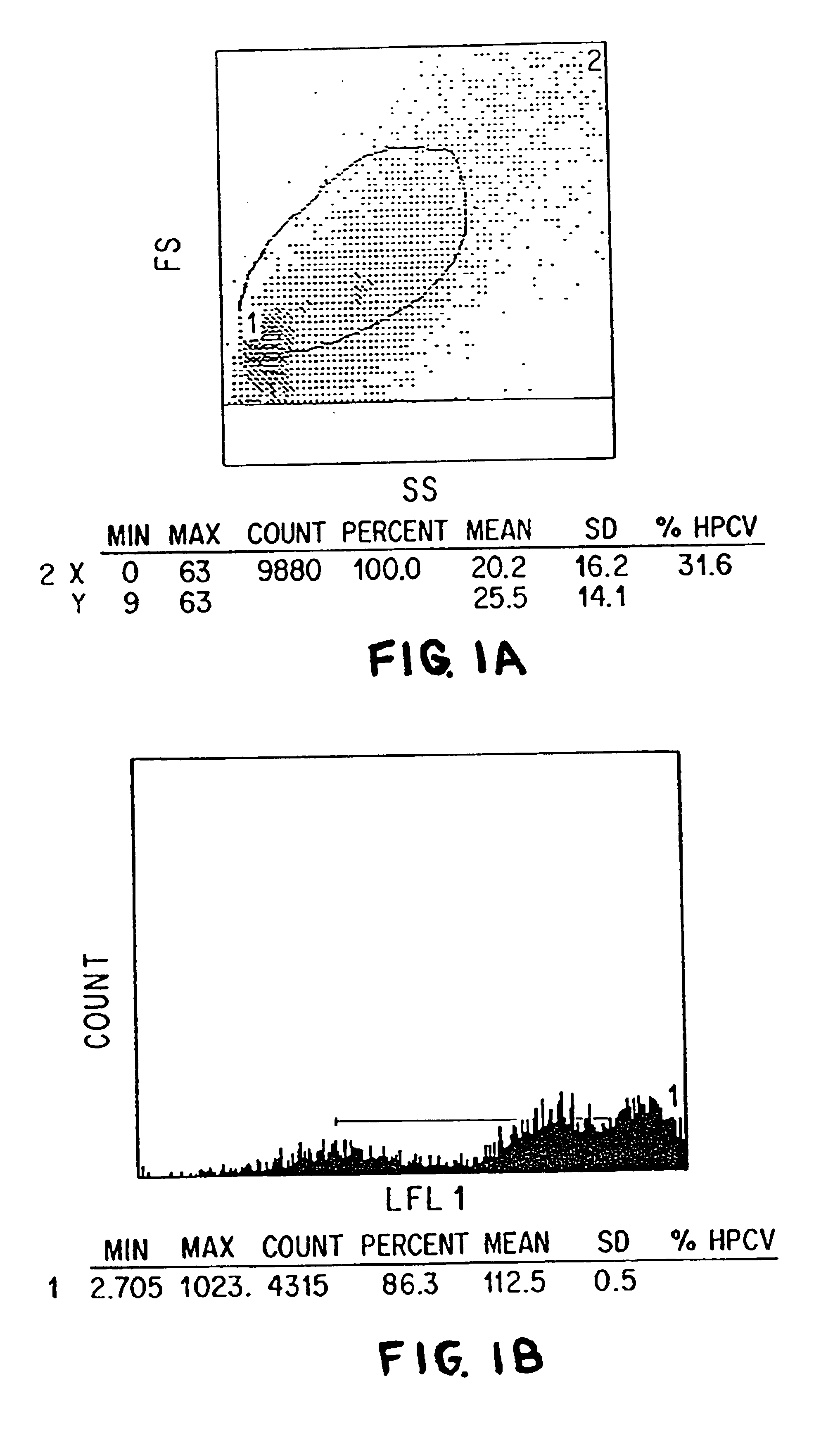
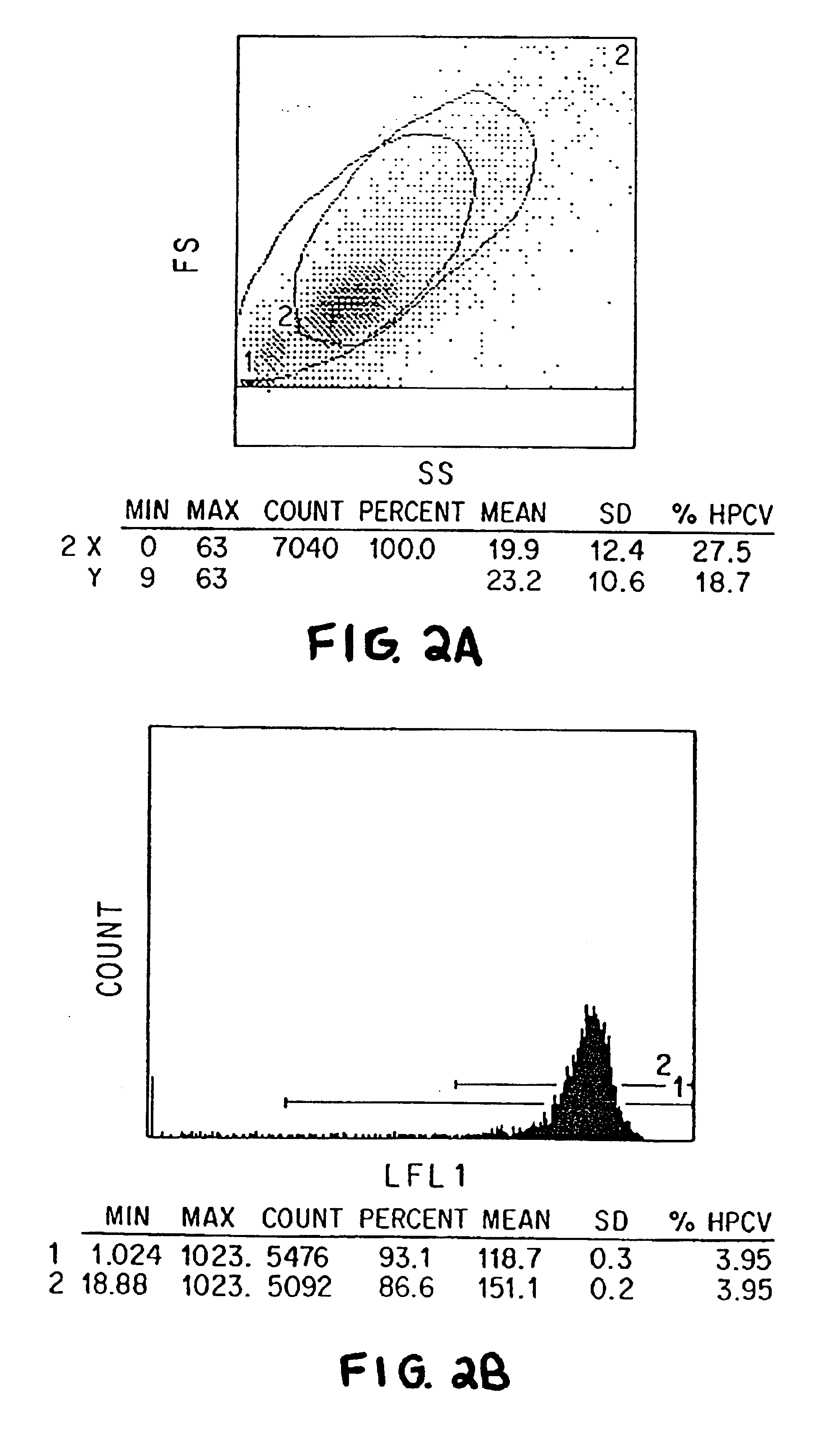
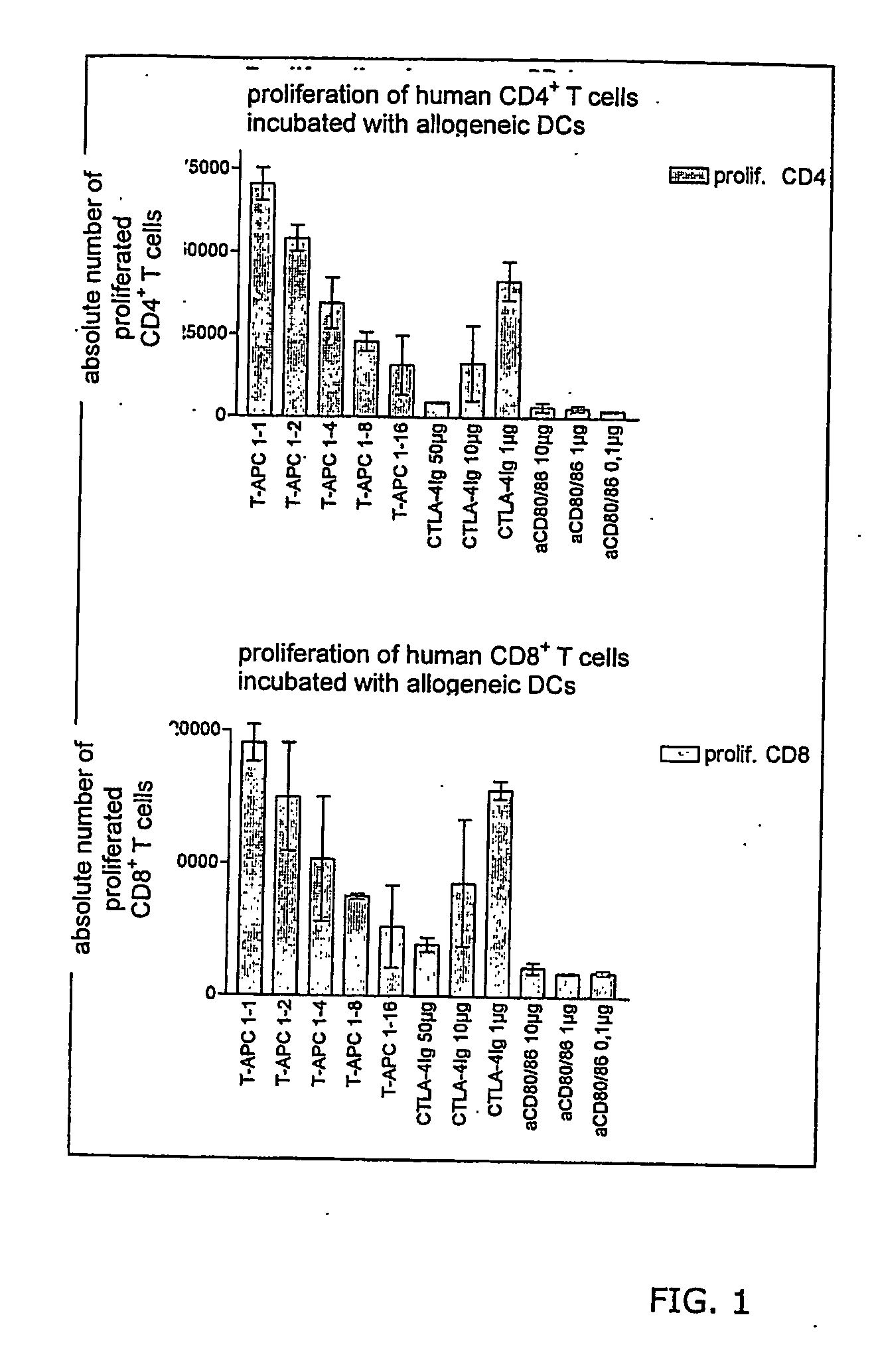
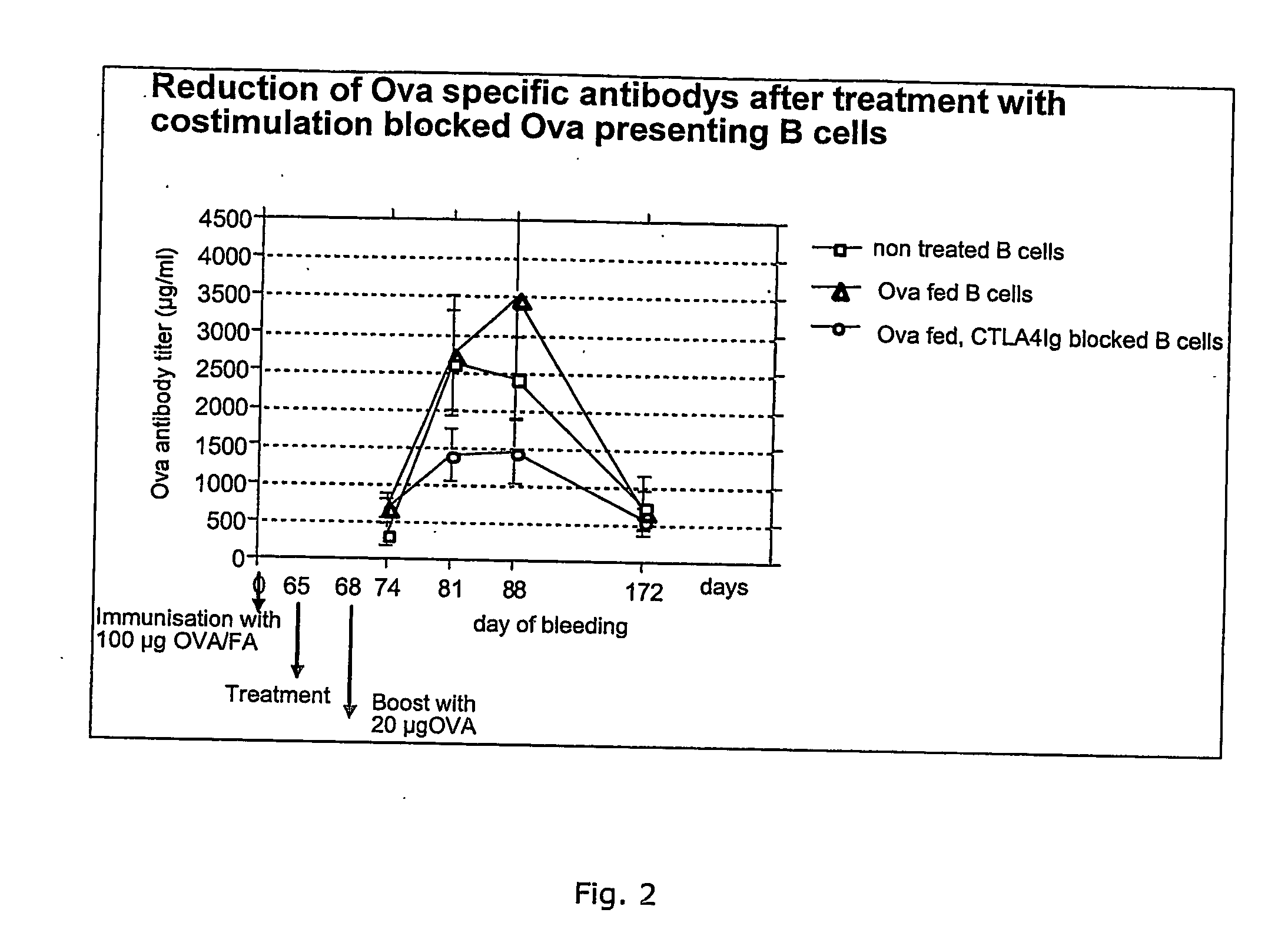
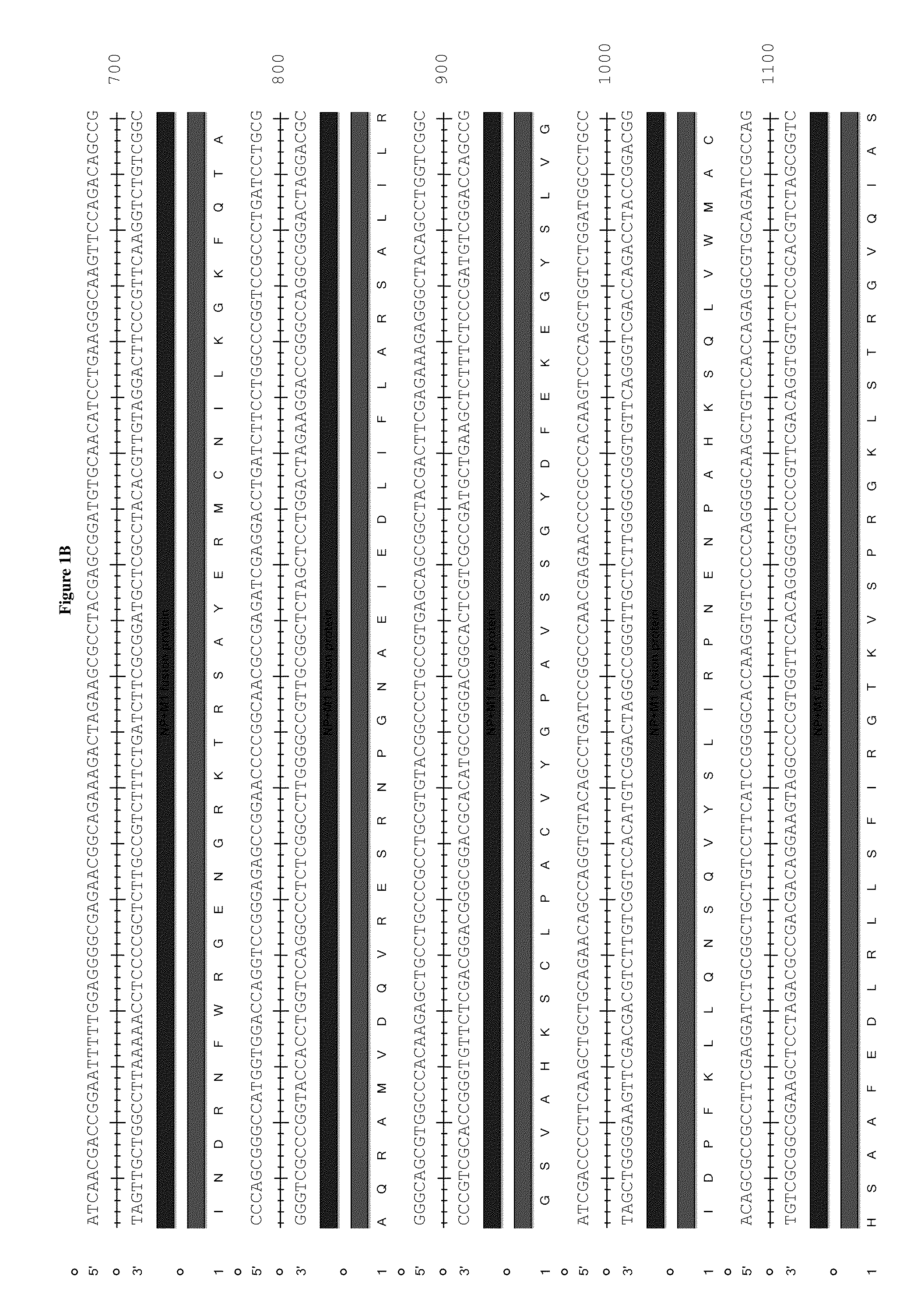
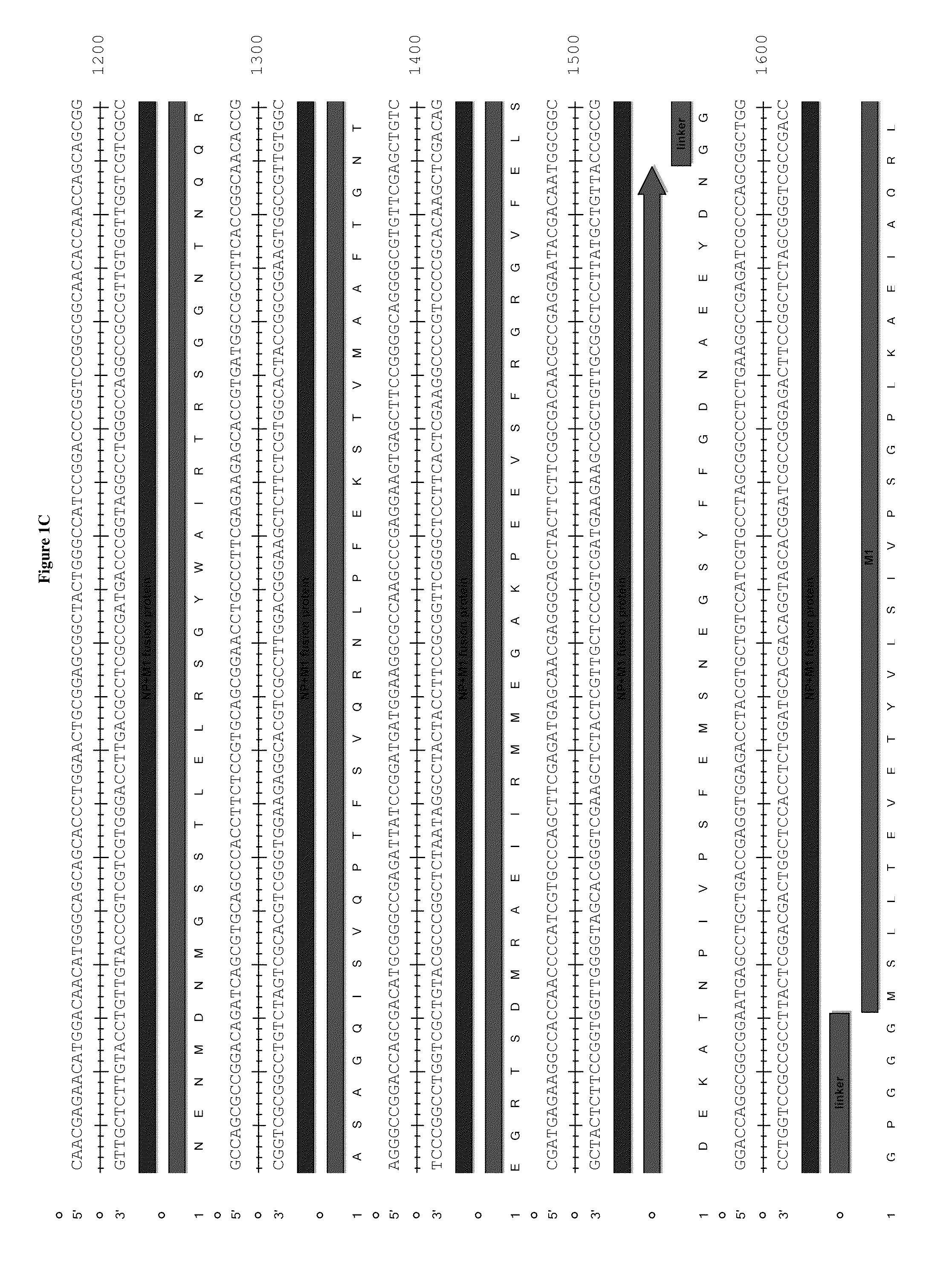
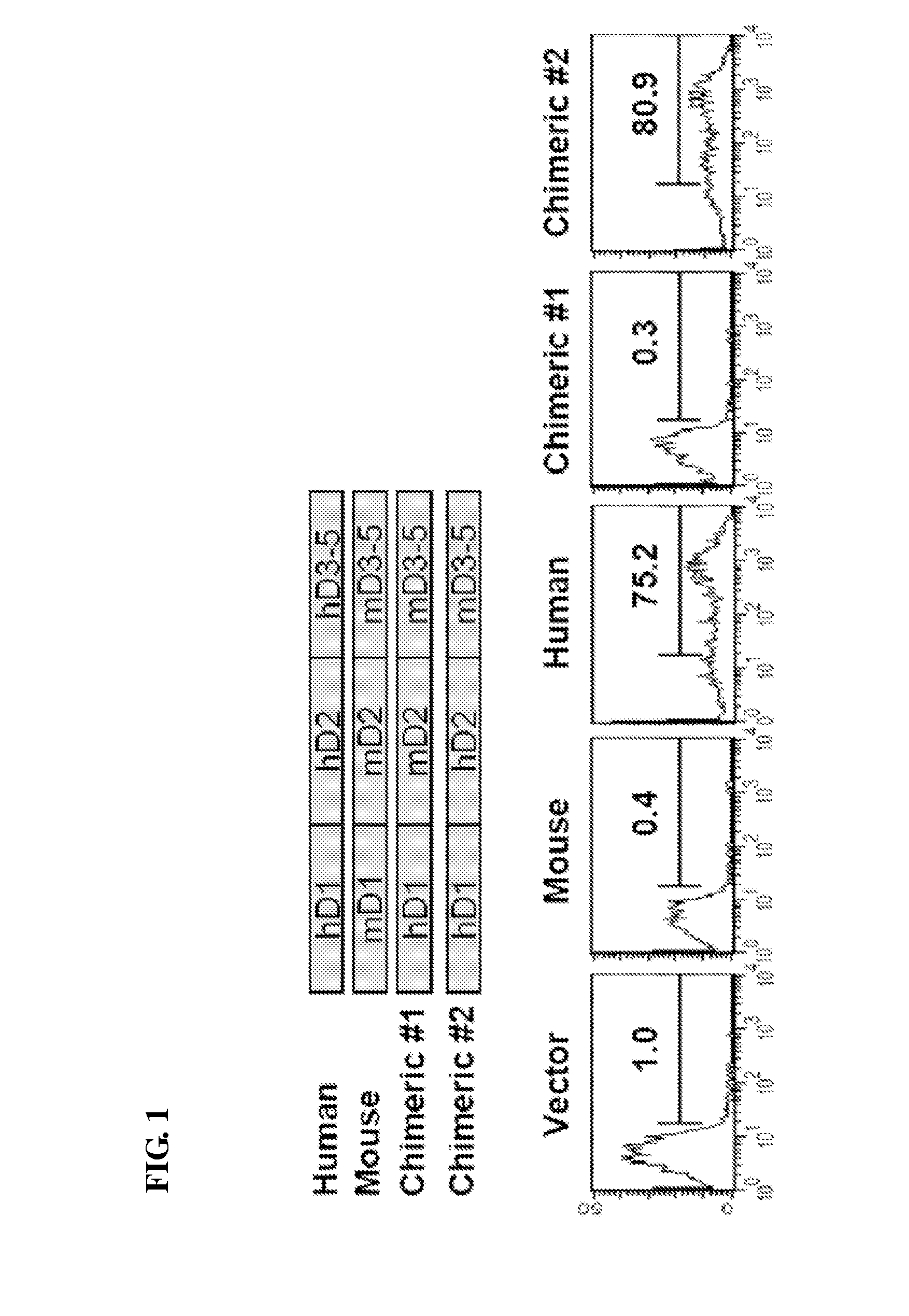
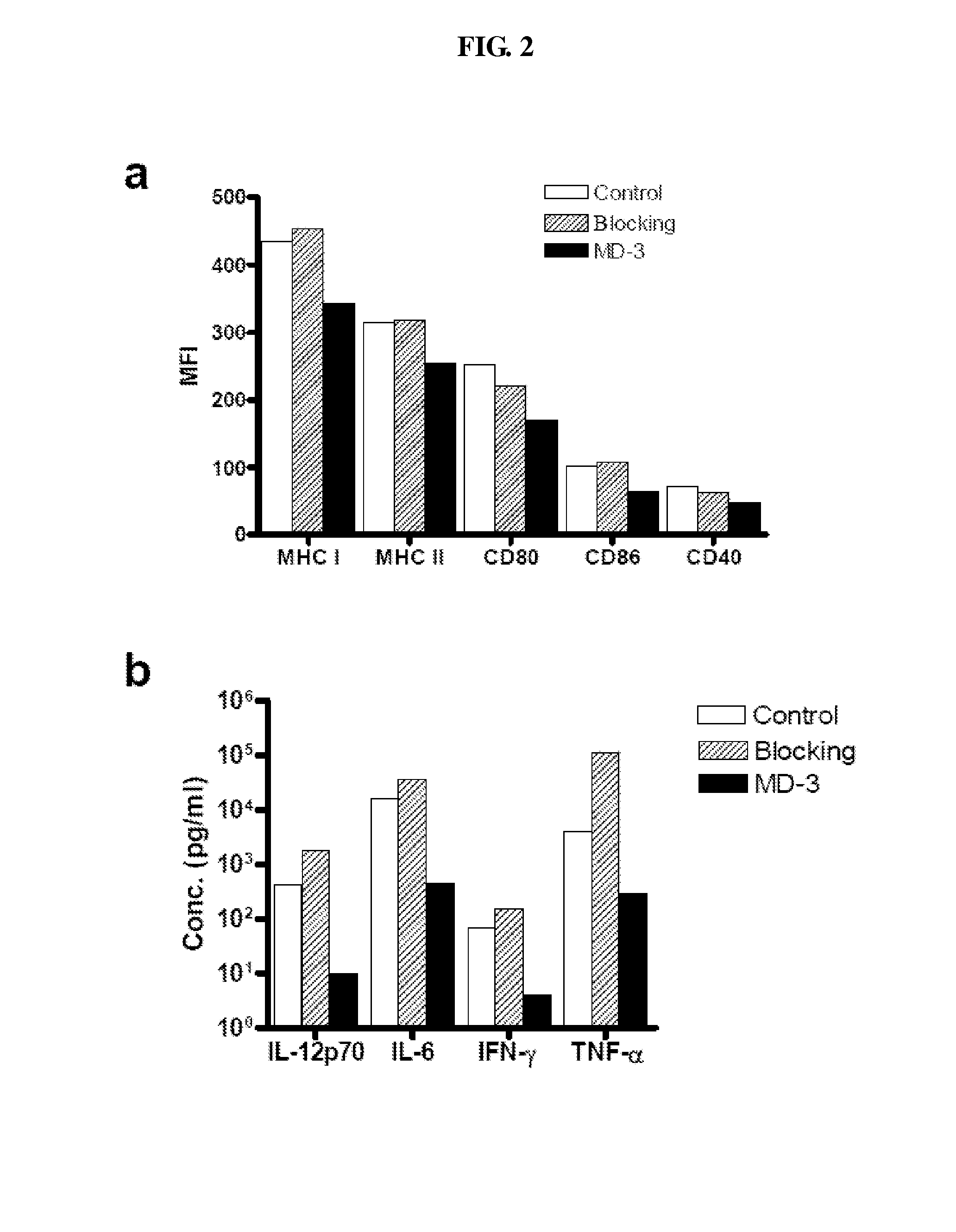
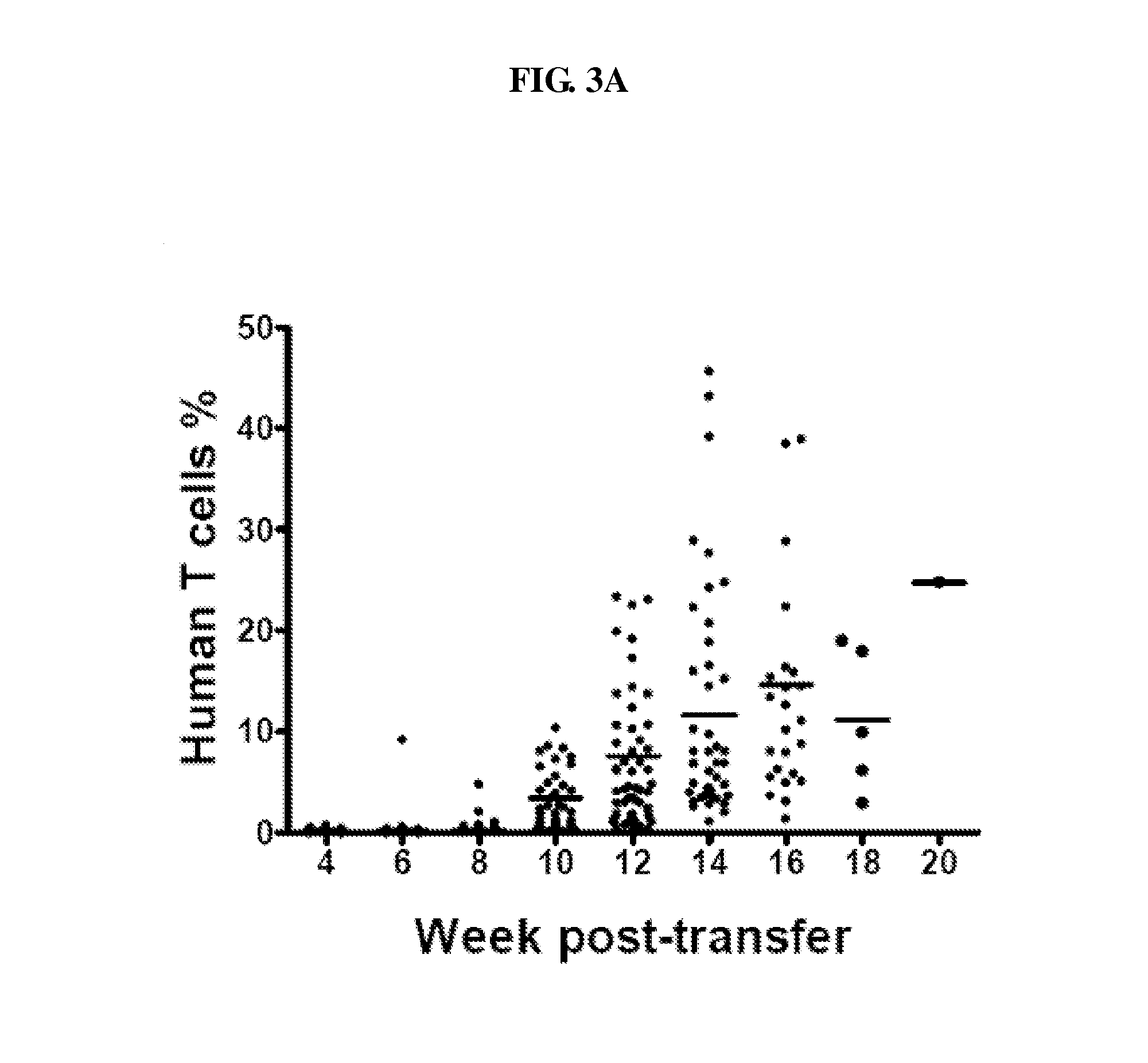
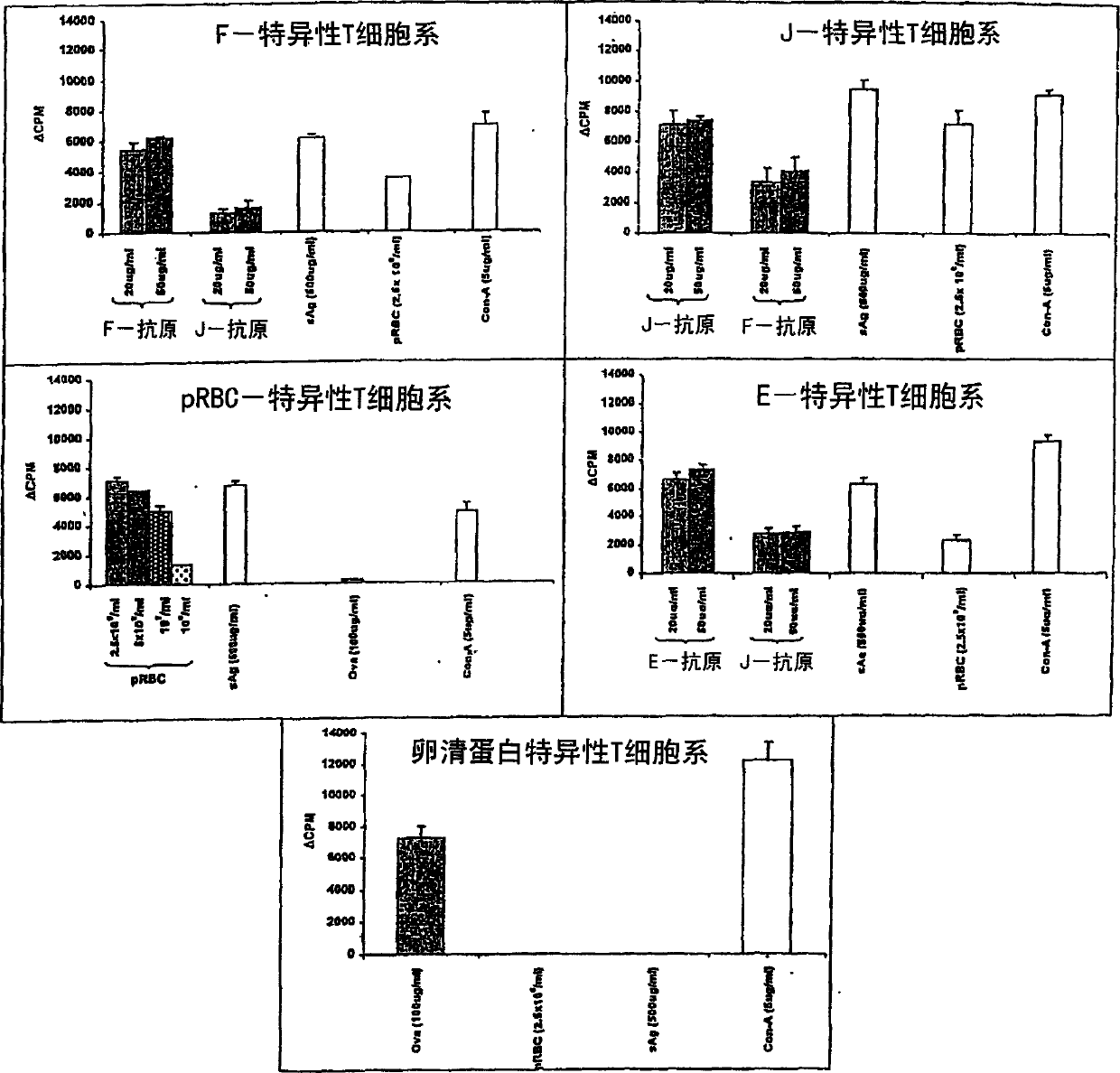
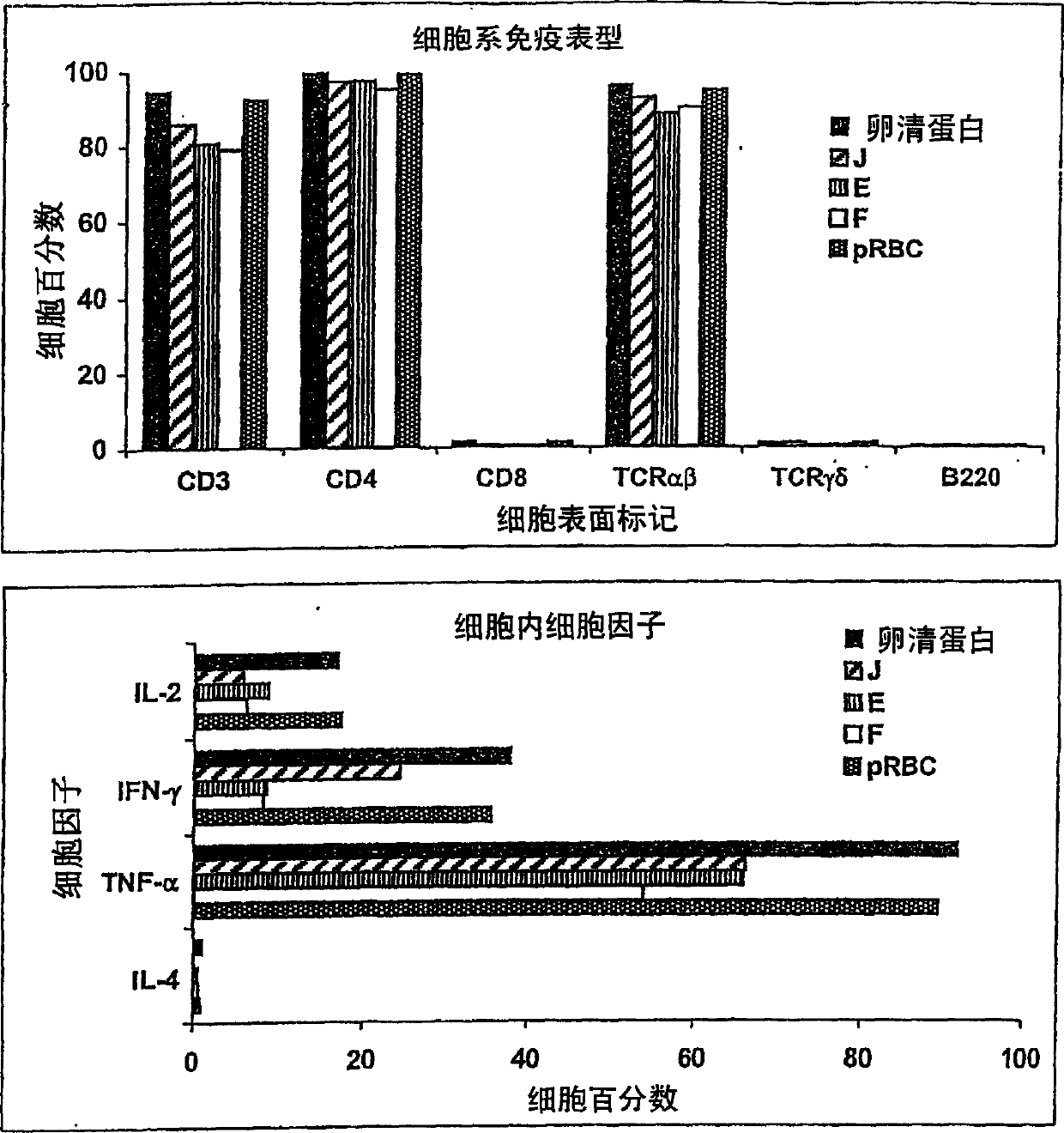
Patents
Literature
55 results about "T cell mediated immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Any process involved in the carrying out of an immune response by a T cell. [GO_REF:0000022, GOC:add, GOC:mtg_15nov05, ISBN:0781735149]
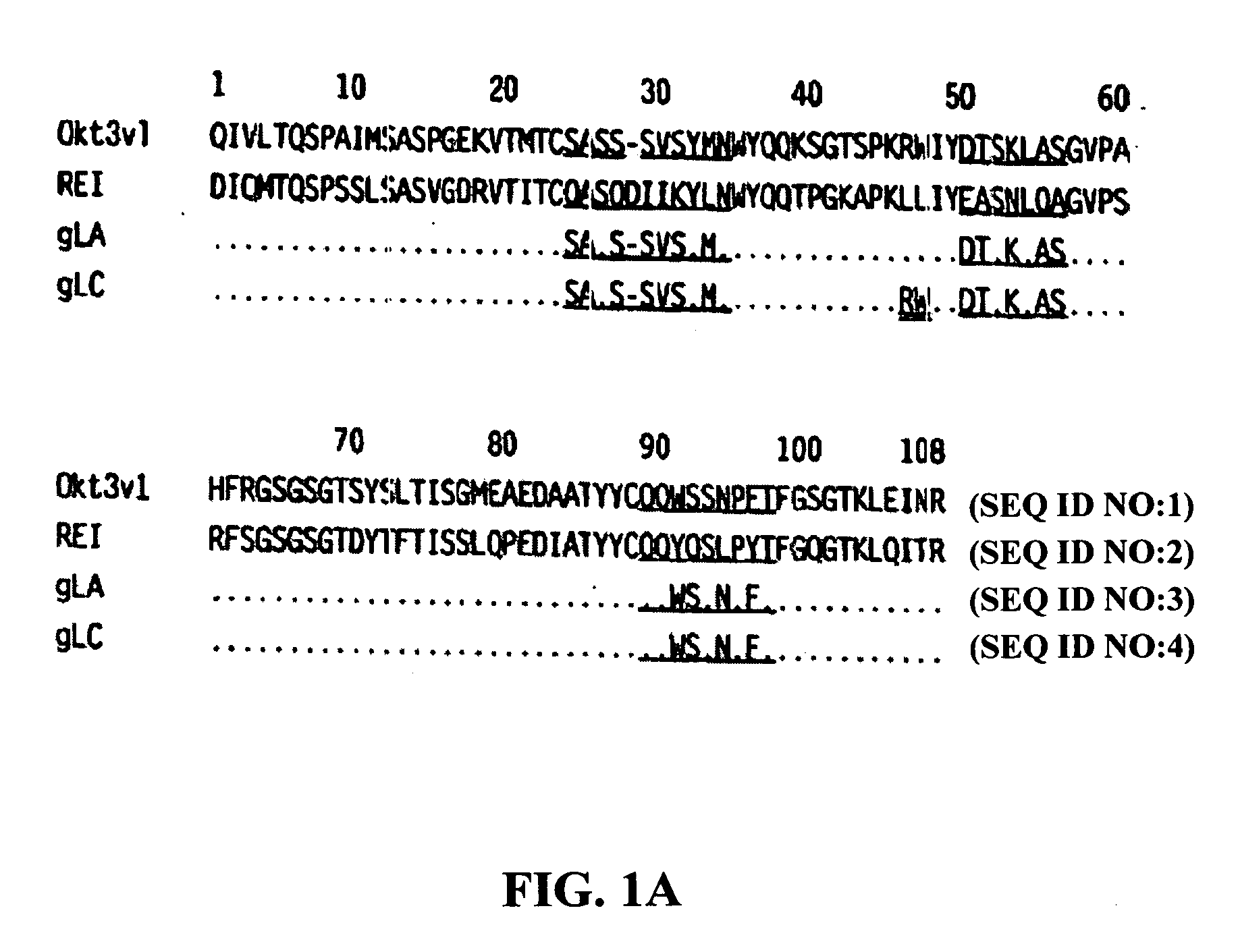
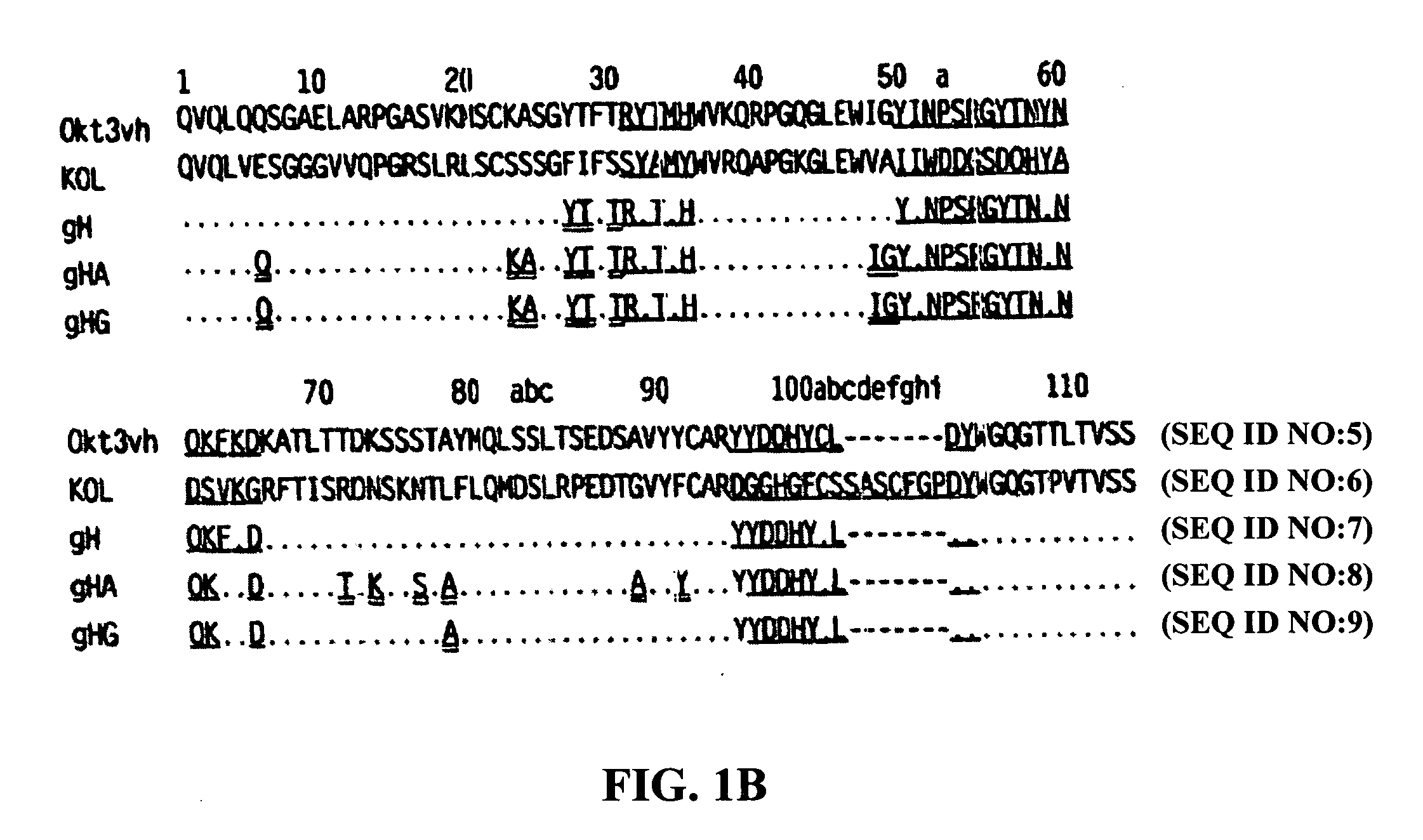
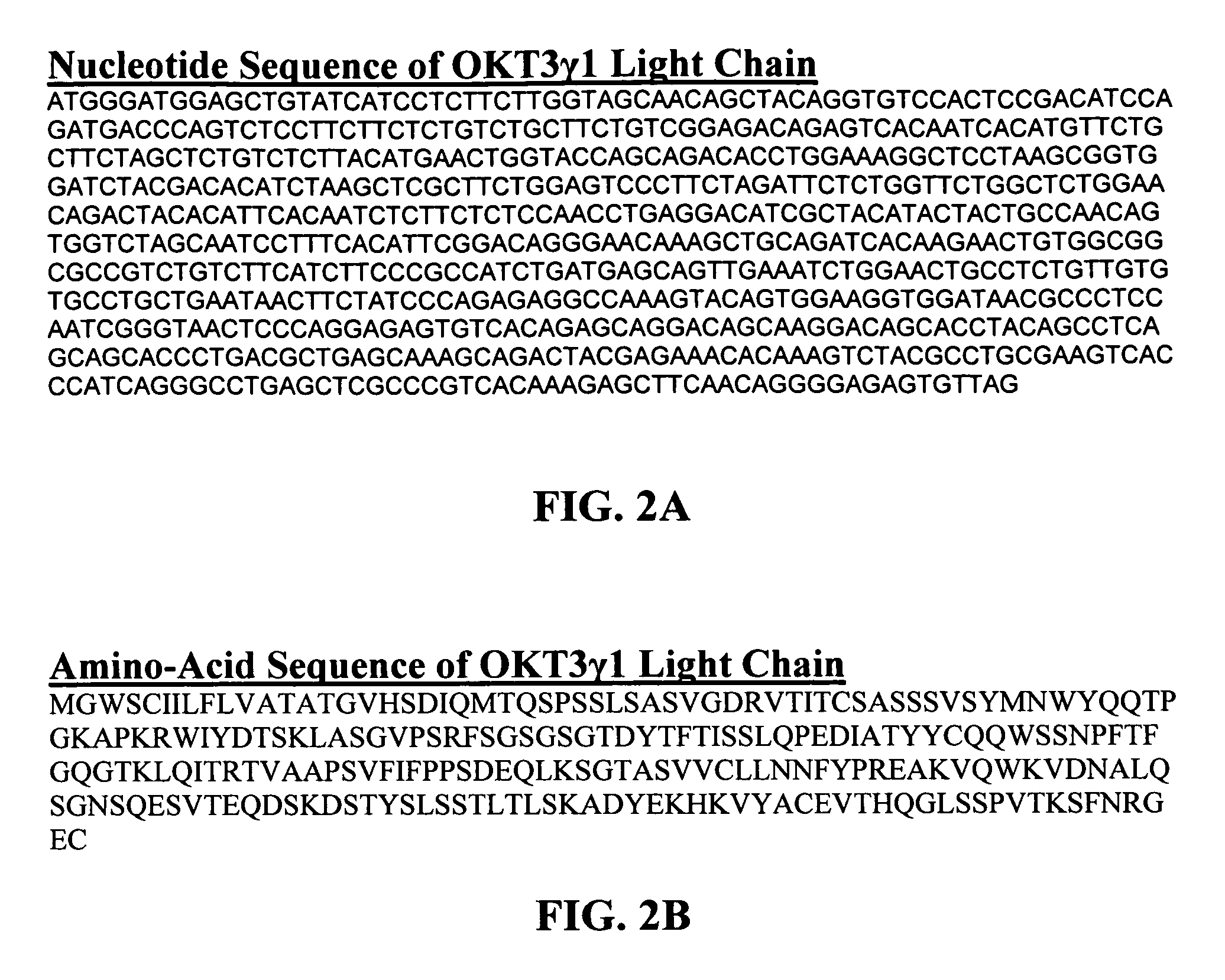
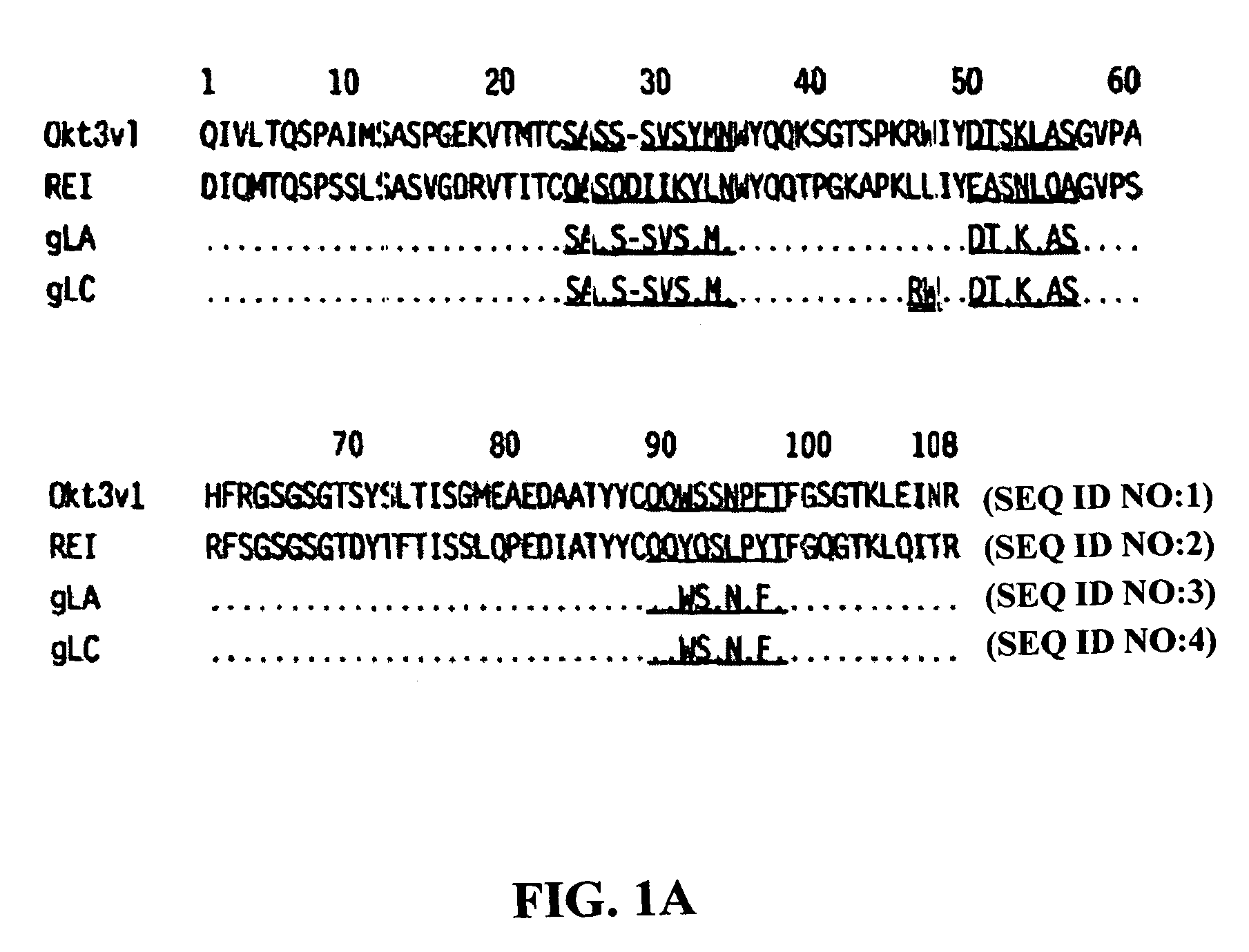
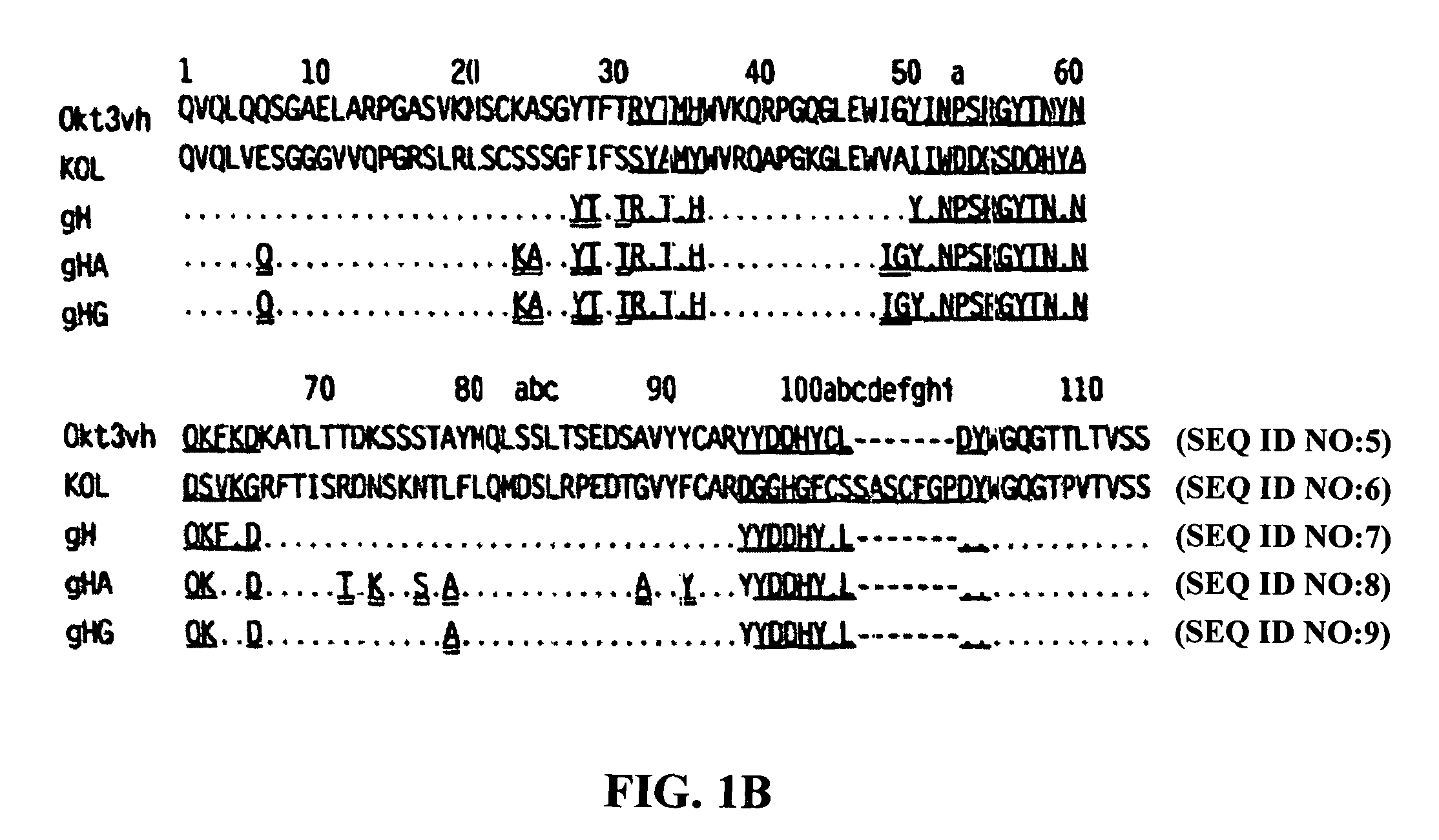
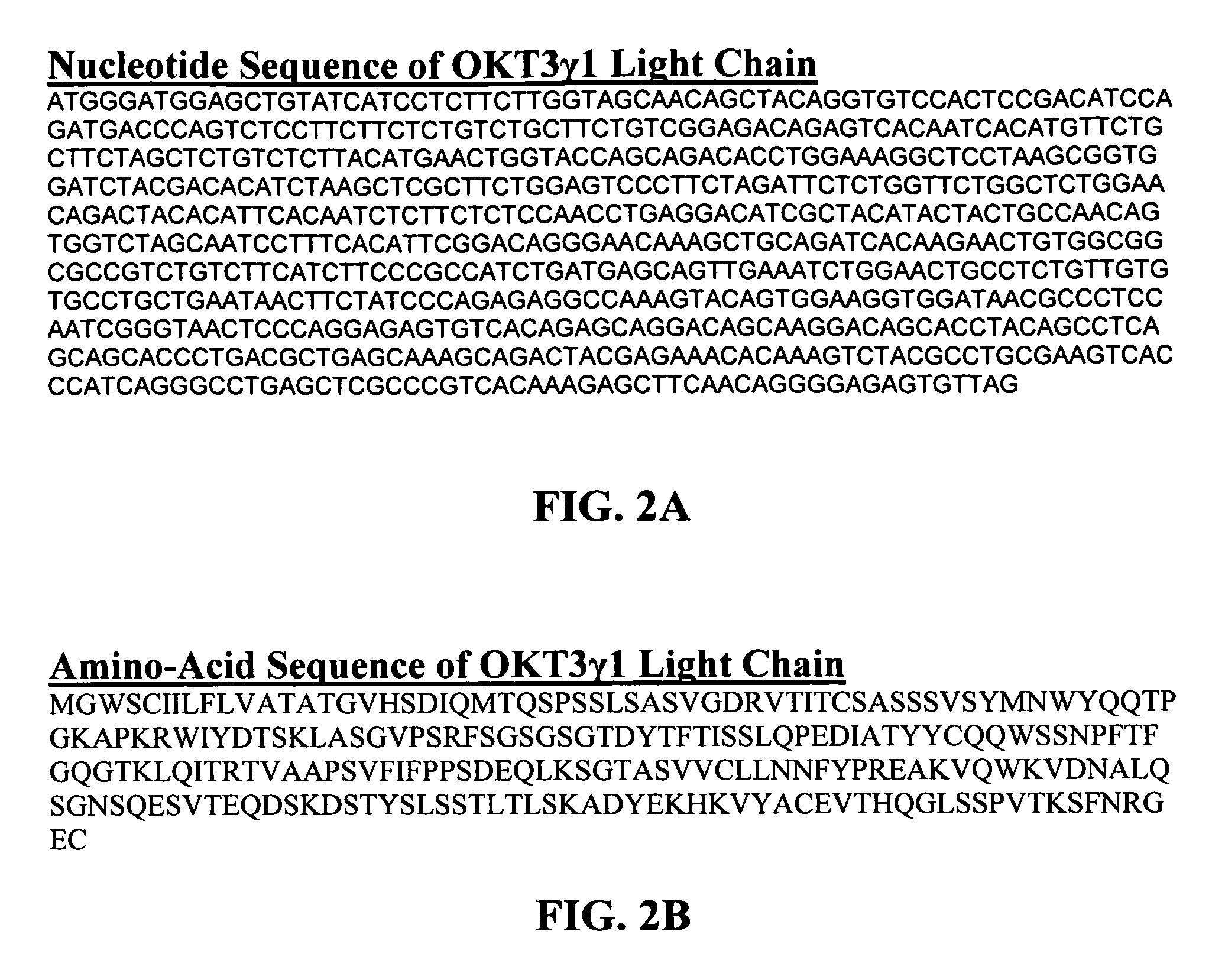
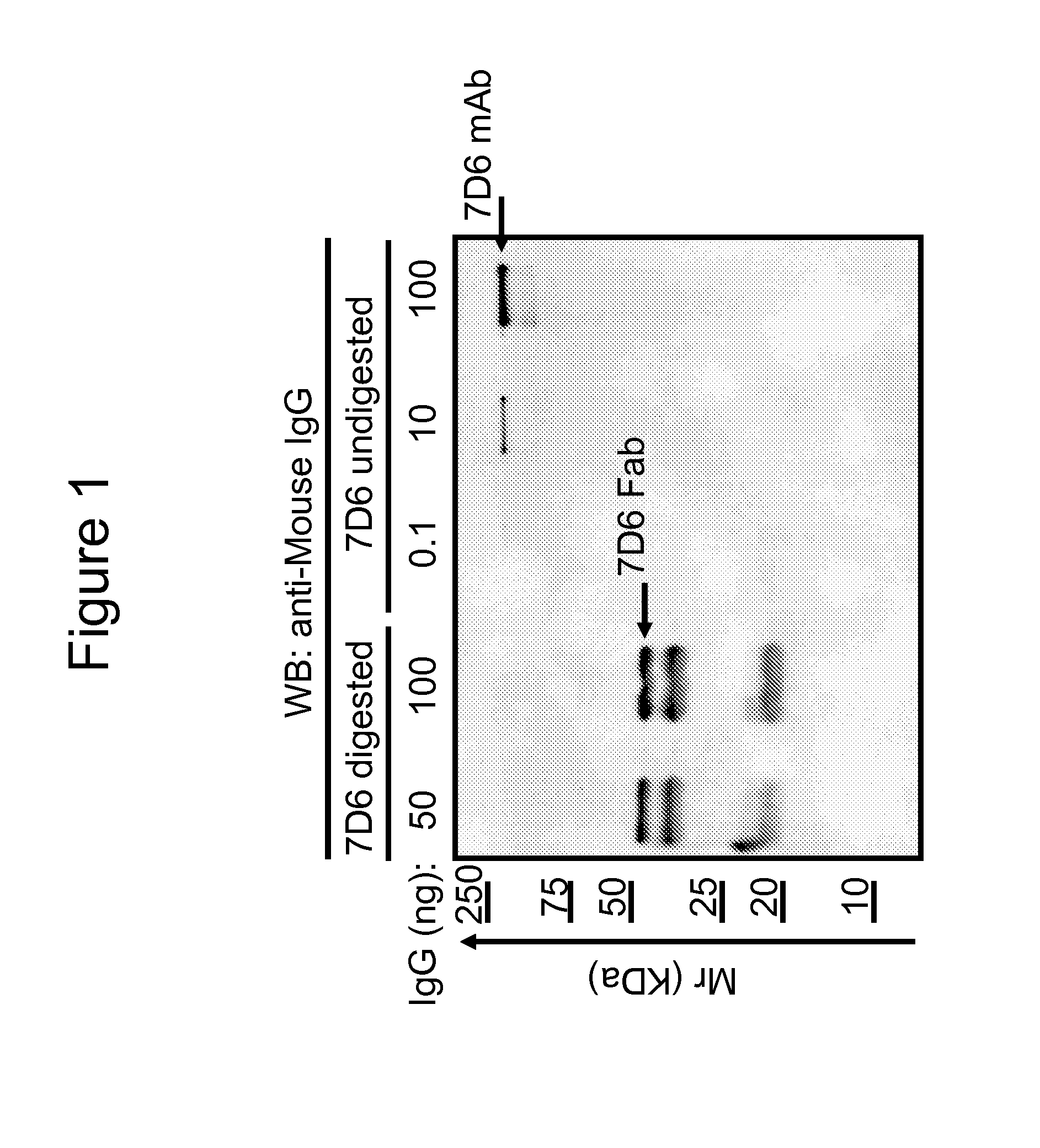
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS20070077246A1Treating and preventing and ameliorating symptomSlow and reduce damagePeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
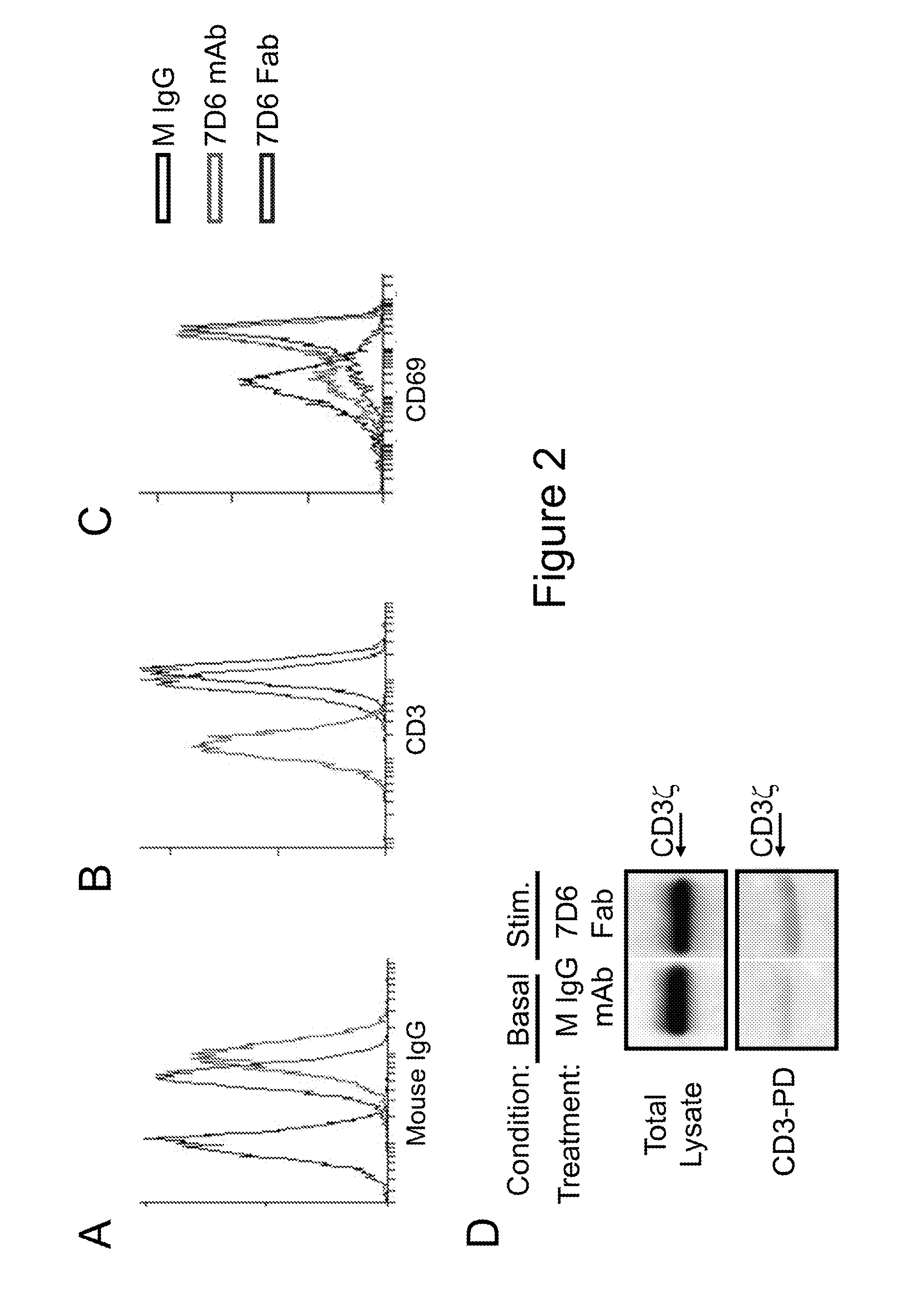
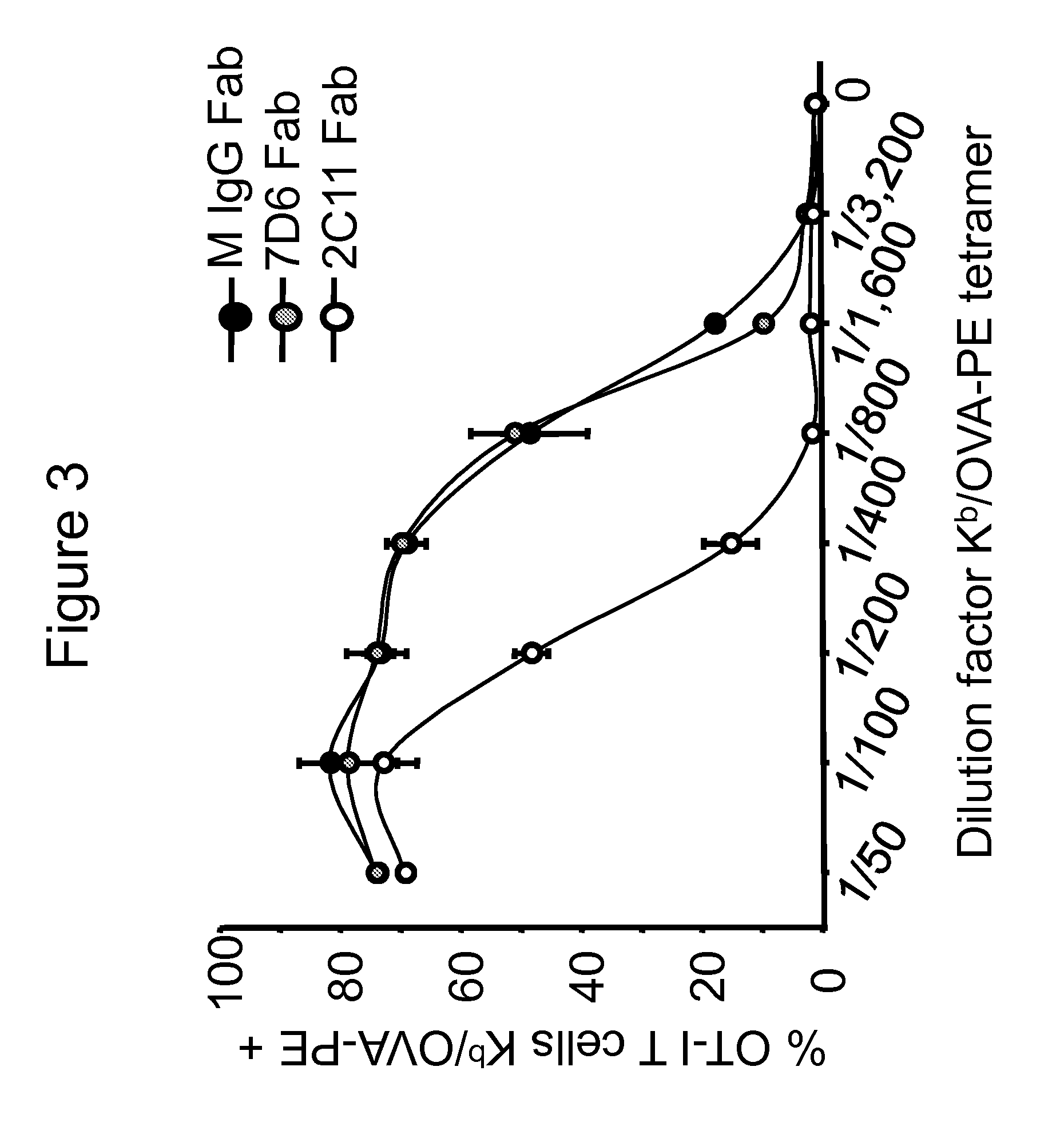
The present invention provides methods of treating, preventing or ameliorating the symptoms of T cell-mediated immunological diseases, particularly autoimmune diseases, through the use of anti-CD3 antibodies. In particular, the methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-CD3 antibodies.
Owner:PROVENTION BIO INC
Redirected, genetically-engineered t regulatory cells and their use in suppression of autoimmune and inflammatory disease
InactiveUS20100135974A1Effective quantityOvercome scarcityBiocideAntipyreticIntracellular signallingInflammatory Bowel Diseases
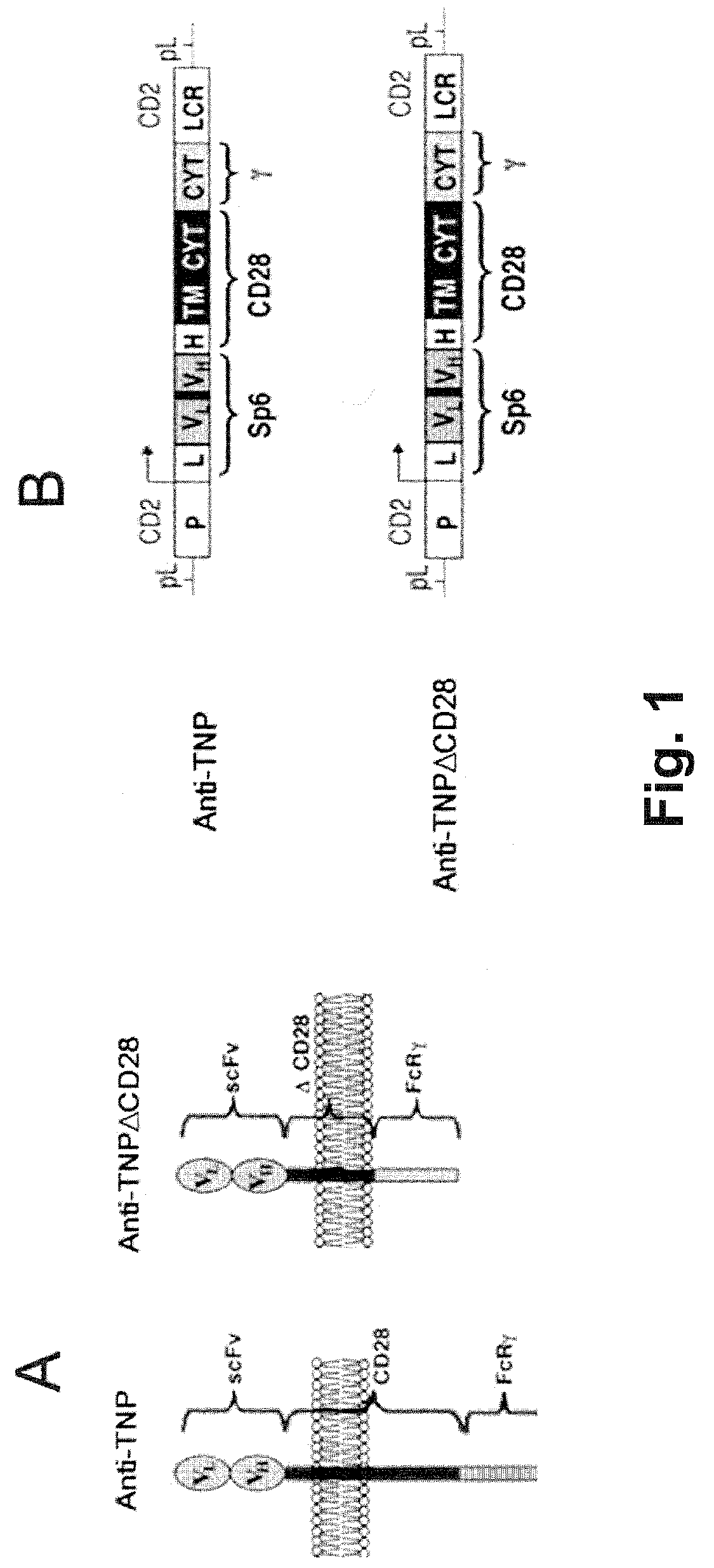
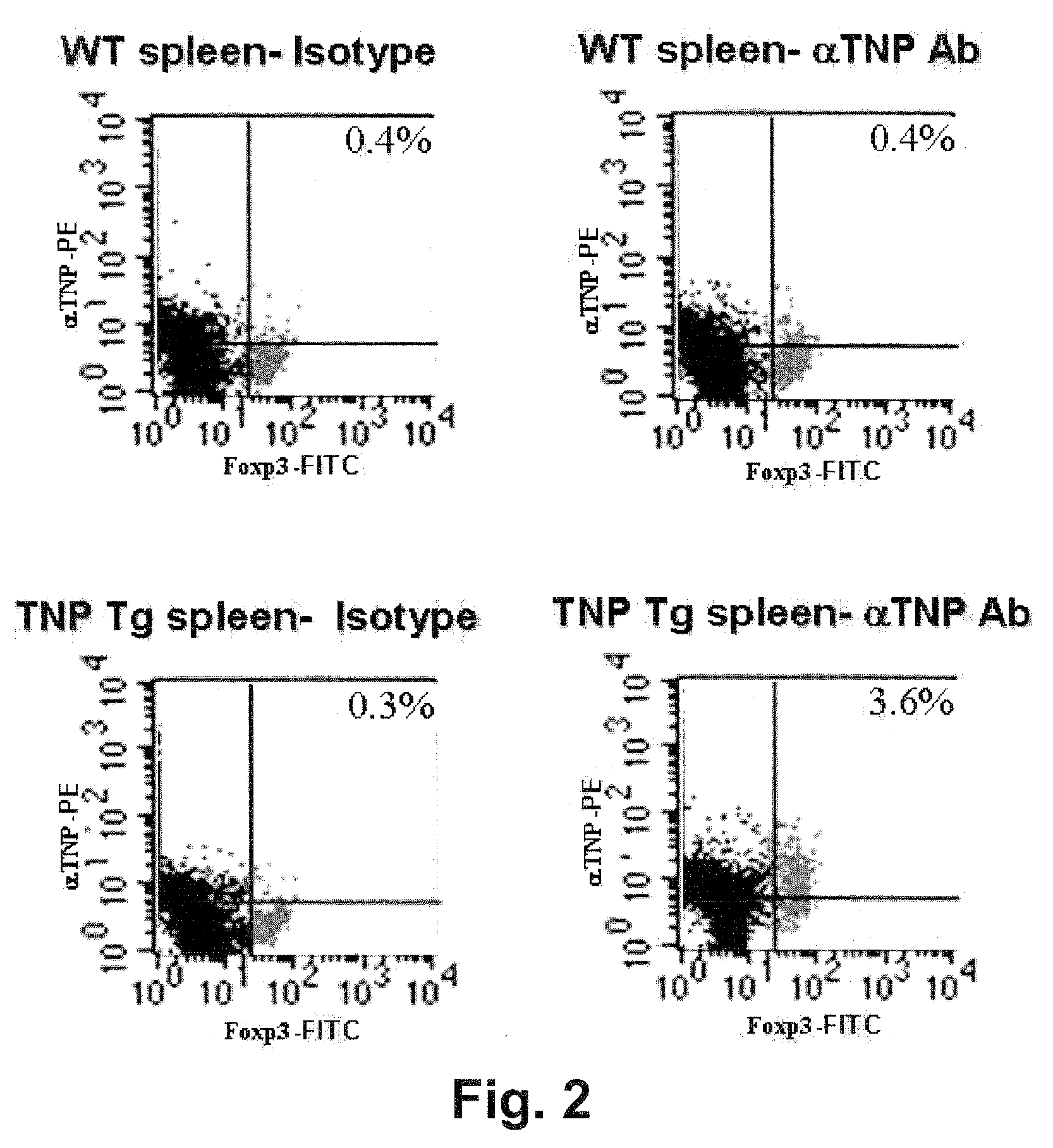
A redirected Treg cell is endowed with specificity toward a selected target antigen or ligand. The cell contains a chimeric receptor polypeptide that is expressed in a single, continuous chain, with an extracellular recognition region displayed on the surface of the cell, a transmembrane region and an intracellular signaling region. The extracellular recognition region is specific for the selected target antigen or ligand. The intracellular signaling region includes a combination of T-cell signaling polypeptide moieties, which combination, upon binding of the extracellular recognition region to the selected target antigen or ligand, triggers activation of the redirected Treg cells to cause suppression of T-cell mediated immunity. Such redirected Treg cells may be used to suppress undesired activity of T effector cells thereby mediating an immune or inflammatory response. They are particularly useful in treating T effector cell-mediated diseases, such as inflammatory bowel disease, transplant rejection and GVH disease.
Owner:YEDA RES & DEV CO LTD
Method to inhibit T cell interactions with soluble B7
InactiveUS6887471B1Suppression problemPeptide/protein ingredientsAntibody mimetics/scaffoldsRegulatory T cellT cell mediated immunity
Owner:BRISTOL MYERS SQUIBB CO
Methods for using dendritic cells to activate gamma/delta-T cell receptor-positive T cells
InactiveUS6821778B1Control progressIncrease the number ofArtificial cell constructsBlood/immune system cellsAdoptive cellular immunotherapyDendritic cell
This invention relates to methods of using human dendritic cells to present antigens for the induction of antigen-specific T cell-mediated immune responses. In particular, it relates to the isolation of dendritic cells from human blood, exposing the cells to antigens, co-culturing the antigen-pulsed dendritic cells with gammadelta-T cell receptor-positive-T cells (gammadelta-TCR<+> T cells) obtained from unprimed or weakly primed individuals for the stimulation of antigen-specific T cell proliferative and cytotoxic activities. The dendritic cell antigen presentation system described herein has a wide range of applications, including but not limited to, activation and expansion of large numbers of antigen-specific major histocompatibility complex-unrestricted T cells for use in adoptive cellular immunotherapy against infectious diseases and cancer.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Molecule which binds cd80 and cd86
InactiveUS20070148162A1Prevent rejectionAvoid immune responseAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenT cell mediated immunity
The present invention relates to the identification of molecules, which are specific to CD80 and CD86 antigens. Also preferably, such antibodies are capable of inhibiting the binding of CD28 and CTLA4 to those receptors. Those molecules are able to inhibit T cell mediated immune reactions.
Owner:THERAVISION
Regulation of T cell-mediated immunity by D isomers of inhibitors of indoleamine-2,3-dioxygenase
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Compositions including different types of transfer factor, methods for making the compositions, and methods of treatment using the compositions
ActiveUS6866868B1Reduce cleaning frequencyLess fatty or substantially fat-freeMammal material medical ingredientsCapsule deliveryT cell mediated immunityComputational biology
A composition for eliciting a T-cell mediated immune response in a subject includes transfer factor from at least two different types of source animals. For example, the composition may include mammalian transfer factor and nonmammalian transfer factor. An example of the composition includes a combination of a colostrum-derived product, which includes the mammalian transfer factor, and an egg-derived product, which includes the nonmammalian transfer factor. Additionally, the egg-derived product may be substantially free of fat. Methods for forming the composition and eliciting T-cell mediated immune responses in subjects that have been treated with the composition are also disclosed.
Owner:4LIFE PATENTS
VISTA regulatory T cell mediator protein, VISTA binding agents and use thereof
The present invention relates to a novel regulatory T cell protein. This protein, designated PD-L3 OR VISTA resembles members of the PD-L1 family, identified a novel and structurally-distinct, Ig-superfamily inhibitory ligand, whose extracellular domain bears homology to the B7 family ligand PD-L1. This molecule is designated as PD-L3 OR VISTA or V-domain Immunoglobulin Suppressor of T cell Activation (VISTA). Expression of VISTA is primarily within the hematopoietic compartment and is highly regulated on myeloid APCs and T cells. Therapeutic intervention of the VISTA inhibitory pathway represents a novel approach to modulate T cell-mediated immunity for the treatment of a wide variety of cancers, e.g., ovarian, bladder cancer and melanomas. Also, VISTA proteins, especially multimeric VISTA proteins and antibodies may be used to suppress T cell immunity in autoimmune disease, allergy, infection and inflammatory conditions, e.g. multiple sclerosis and artritic conditions such as RA.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
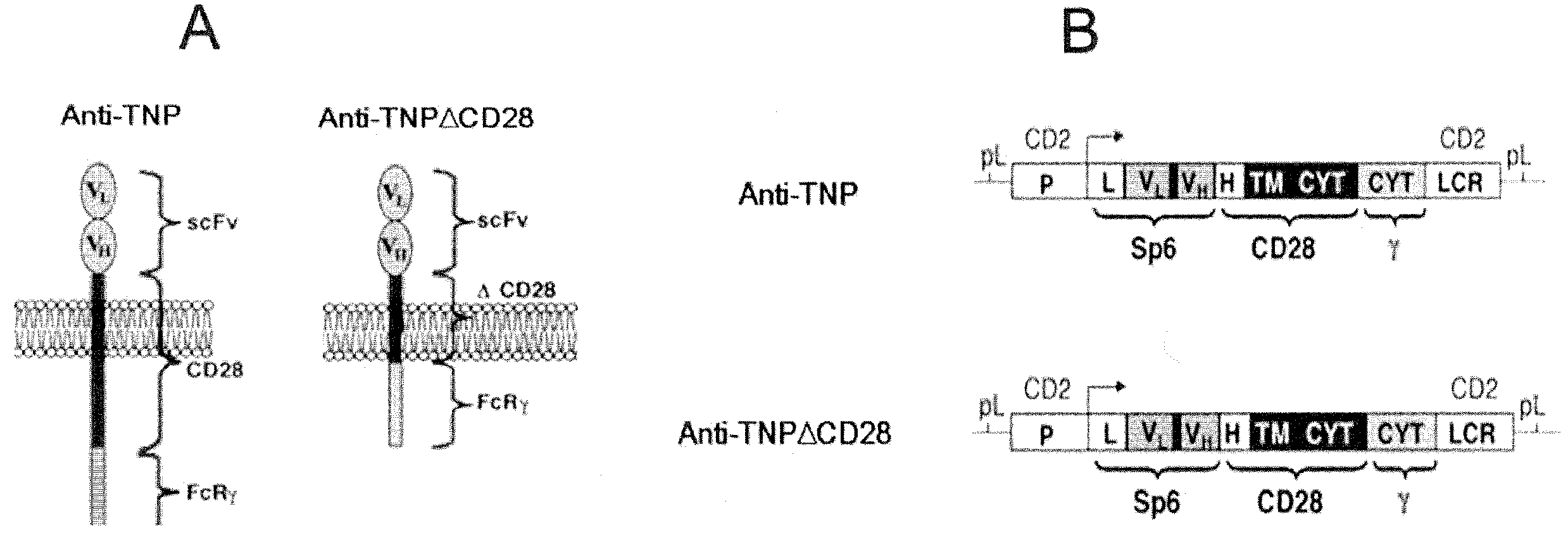
Immune cell compositions and methods of use
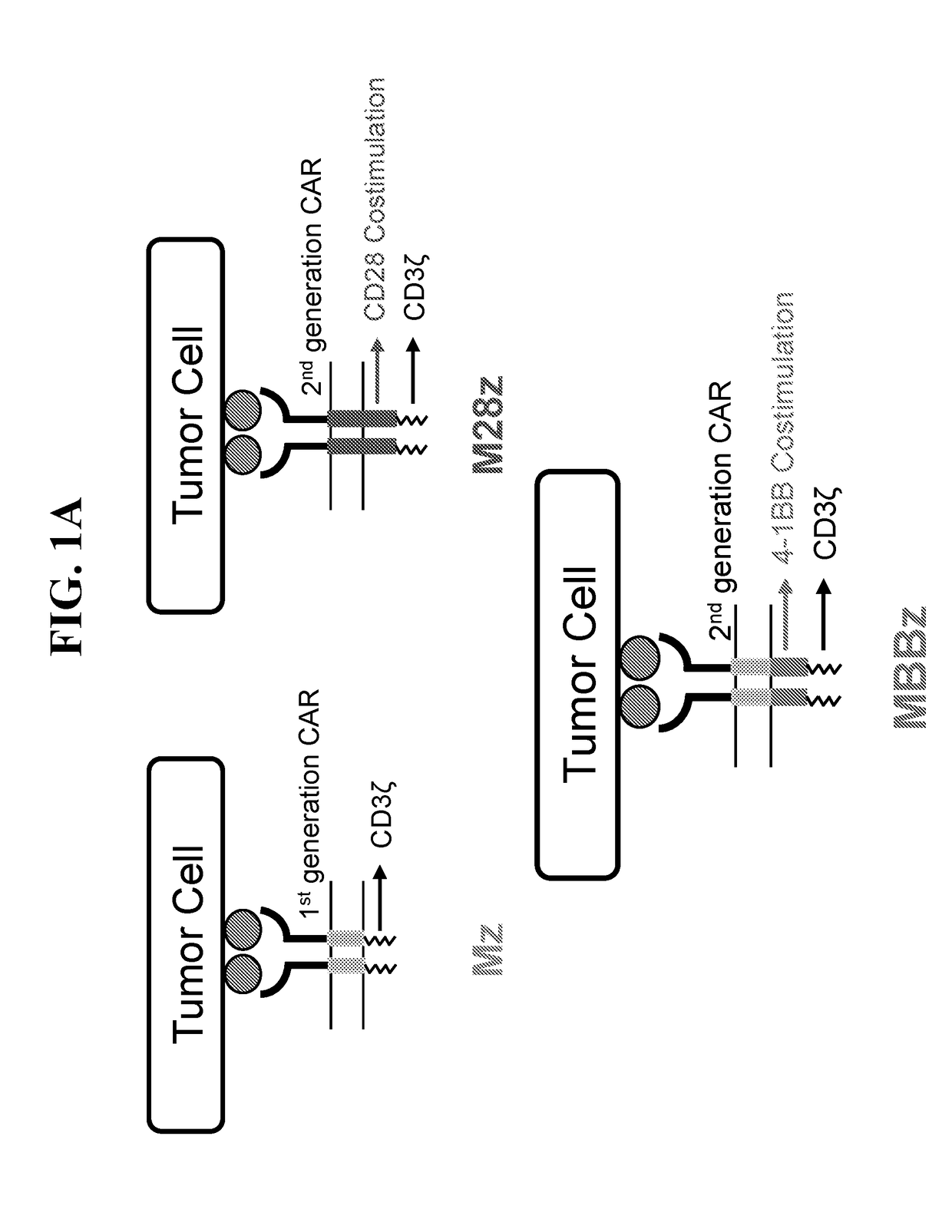
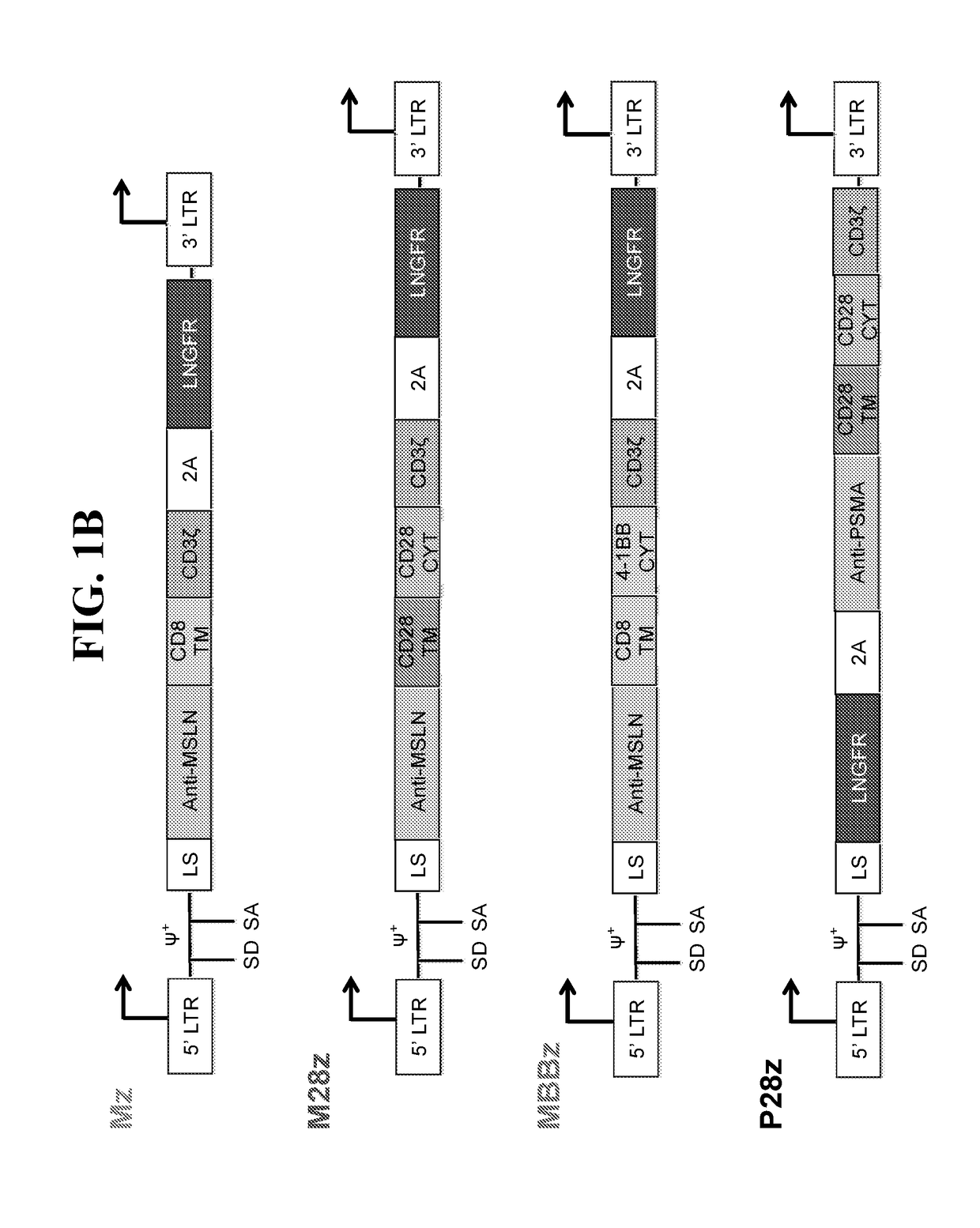
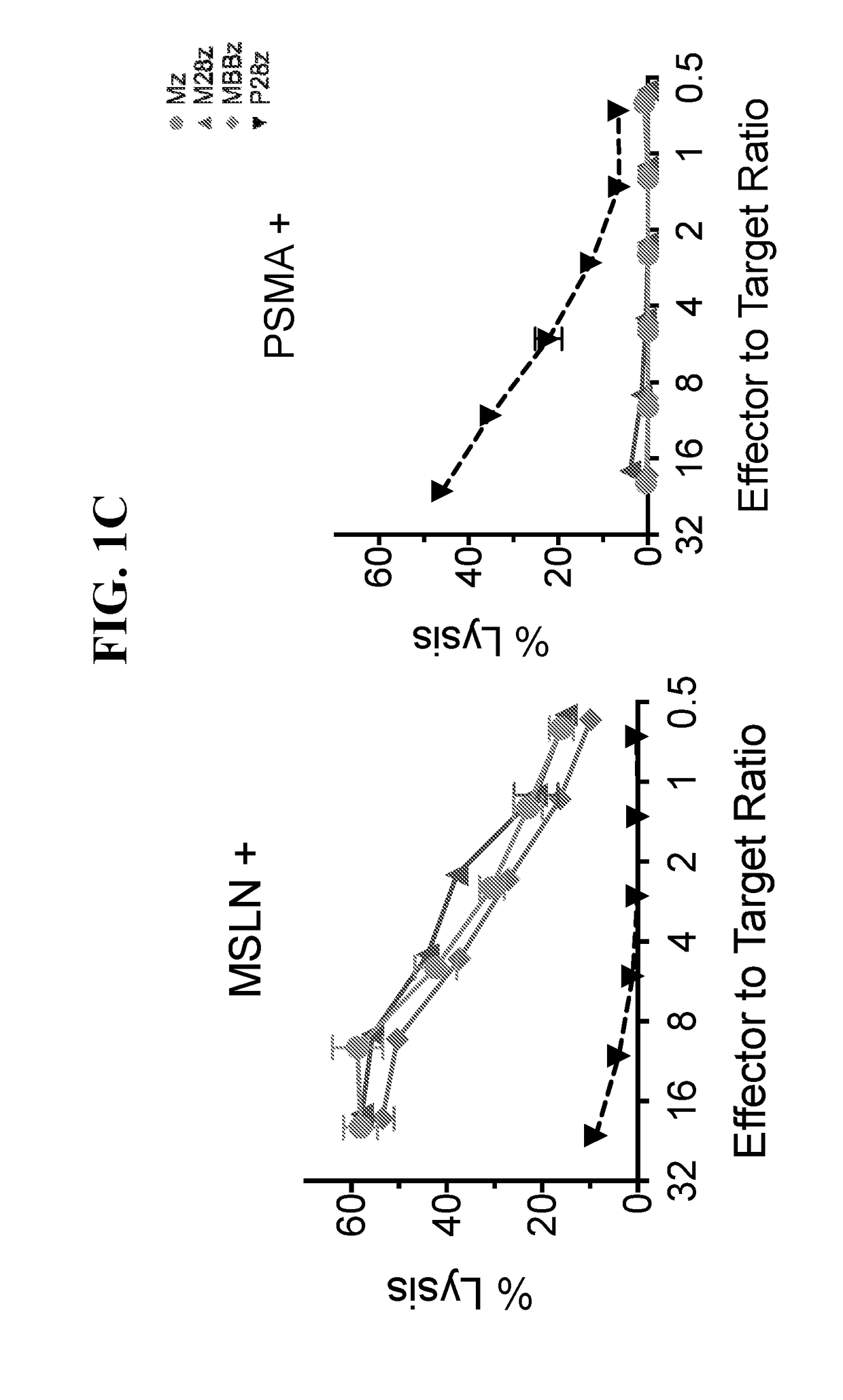
ActiveUS20180273601A1Restores effector functionEnhances tumor burden controlPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorT cell mediated immunity
Disclosed herein are cells that are immune cells or precursor cells thereof, which cells recombinantly express a chimeric antigen receptor (CAR), and a dominant negative form of an inhibitor of a cell-mediated immune response of the immune cell, wherein the CAR binds to a cancer antigen. Also disclosed herein are T cells that recognize and are sensitized to a cancer antigen, which T cells recombinantly express a dominant negative form of an inhibitor of a T cell-mediated immune response. Additionally provided are methods of using such cells to treat cancer in a subject in need thereof.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Compositions and methods for dendritic cell-based immunotherapy
Disclosed are immunostimulatory fusion proteins and methods for generating protective DC-induced, T cell-mediated immune responses in vitro and in vivo. The immunostimulatory fusion proteins comprise a polypeptide antigen component and an immunostimulatory component derived from the intracellular domain of the HER-2 protein. Also disclosed are immunostimulatory compositions comprising dendritic cells pulsed with such an immunostimulatory fusion protein and methods for immunotherapy using the compositions.
Owner:DENDREON PHARMA LLC
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS8663634B2Treating and preventing and ameliorating symptomSlow and reduce damagePeptide/protein ingredientsAntipyreticDiseaseAutoimmune responses
The present invention provides methods of treating, preventing or ameliorating the symptoms of T cell-mediated immunological diseases, particularly autoimmune diseases, through the use of anti-CD3 antibodies. In particular, the methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-CD3 antibodies.
Owner:PROVENTION BIO INC
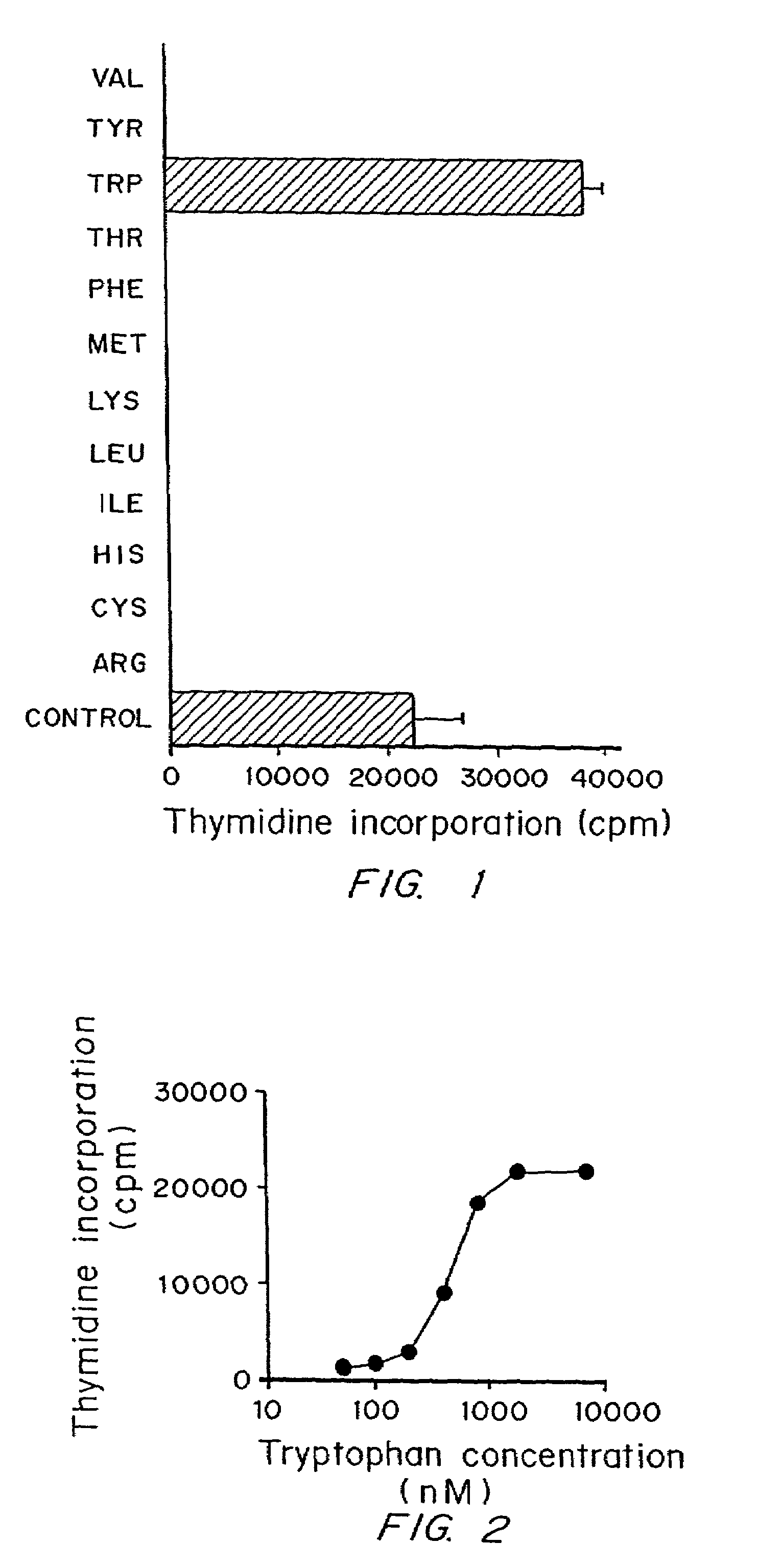
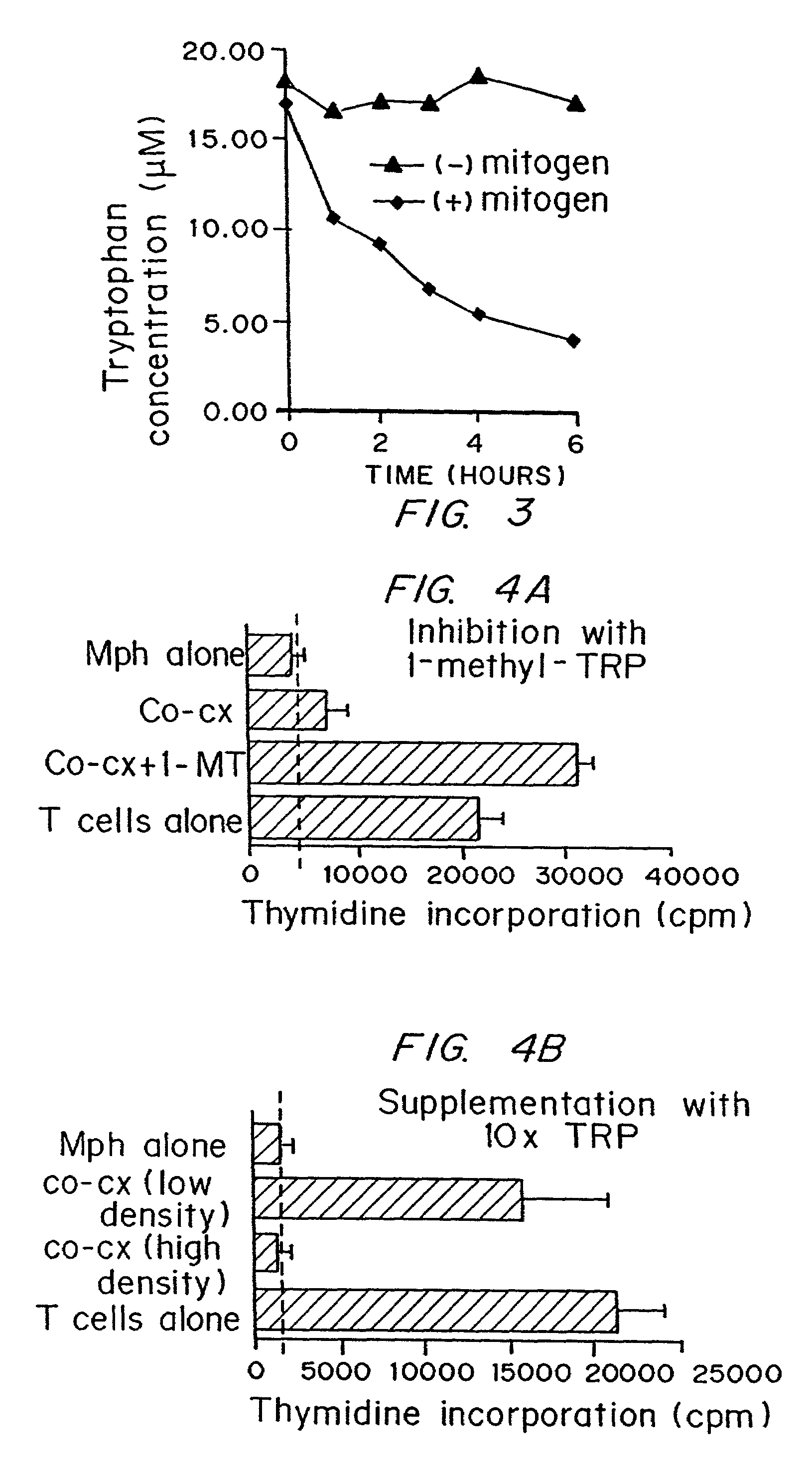
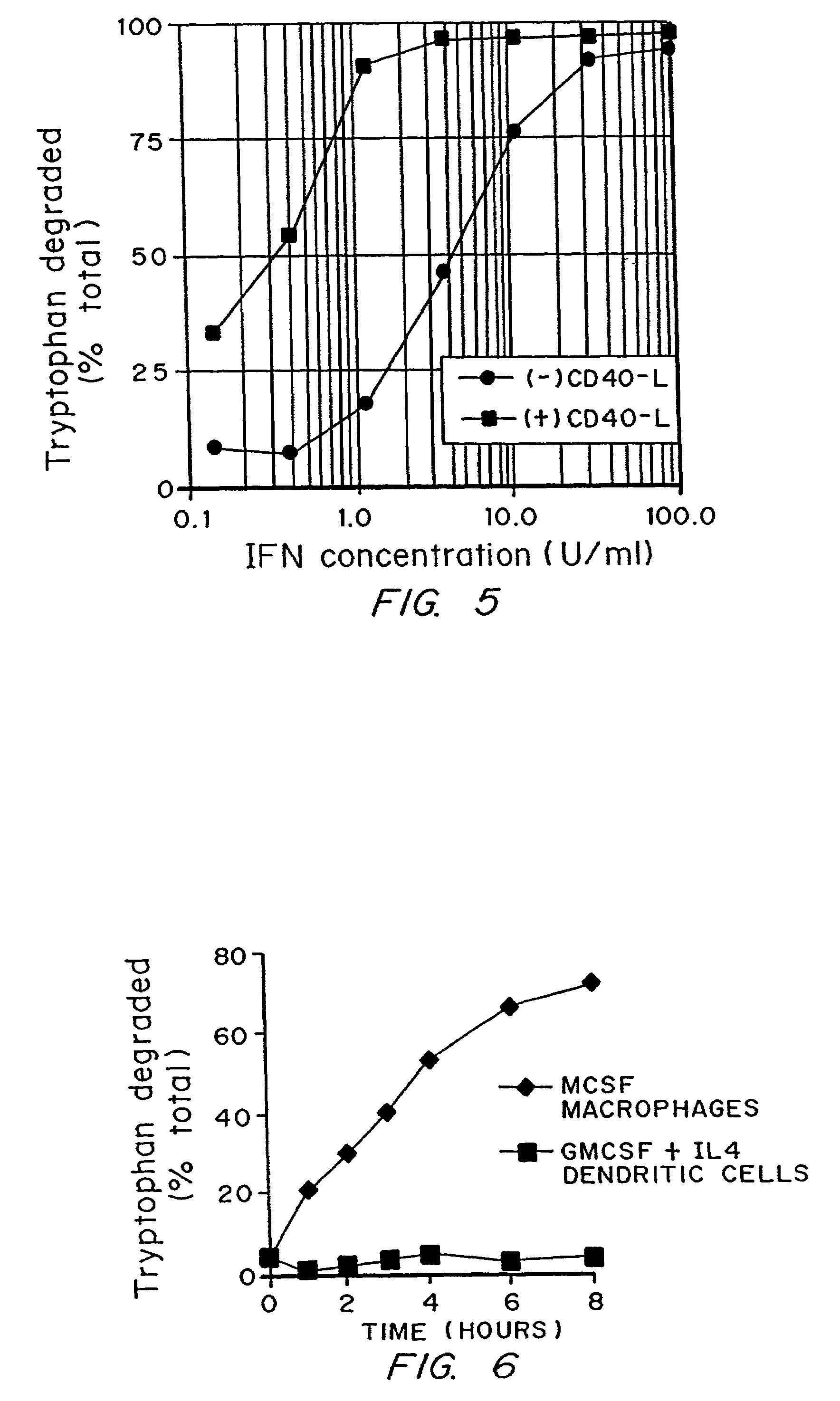
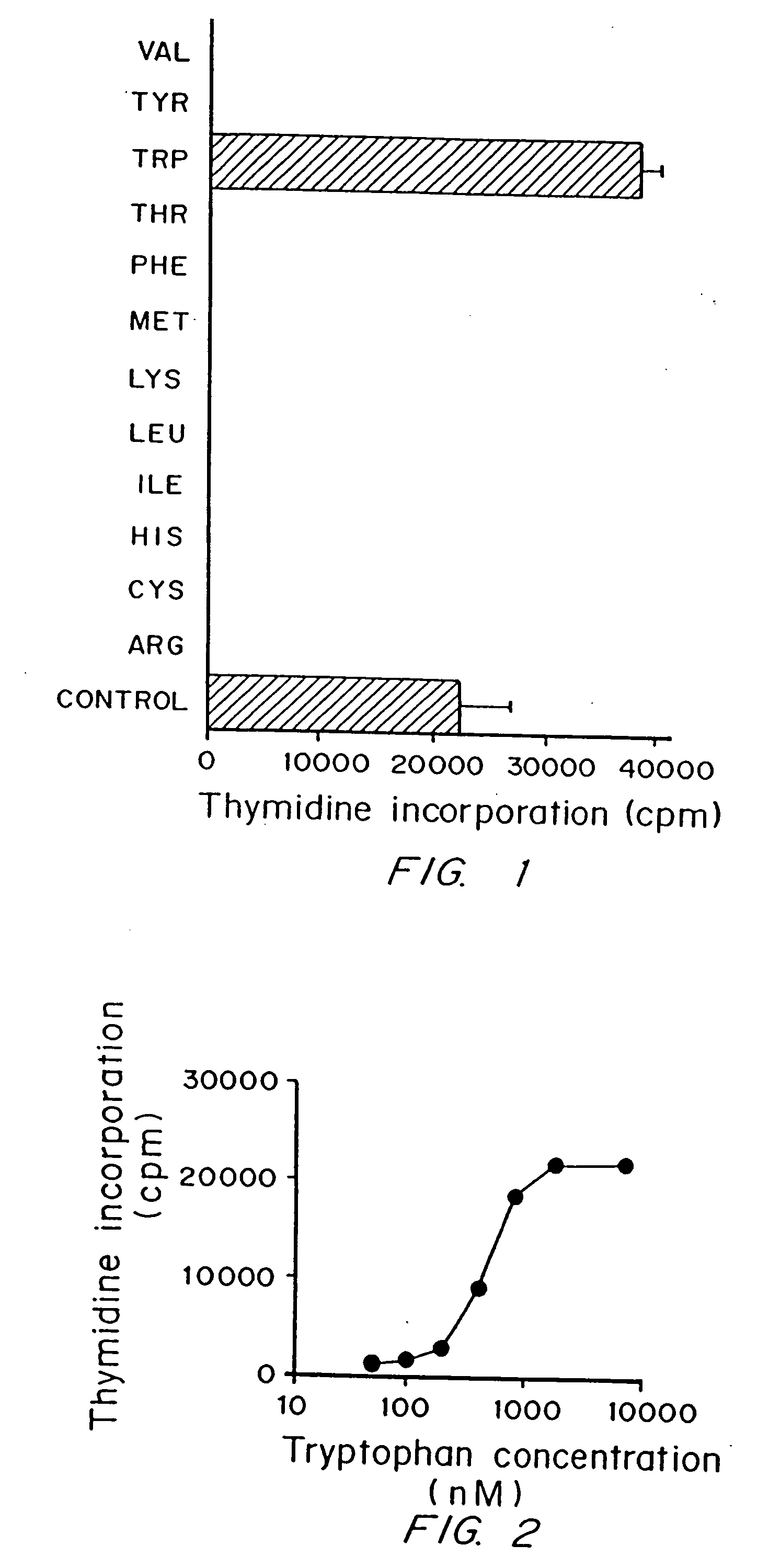
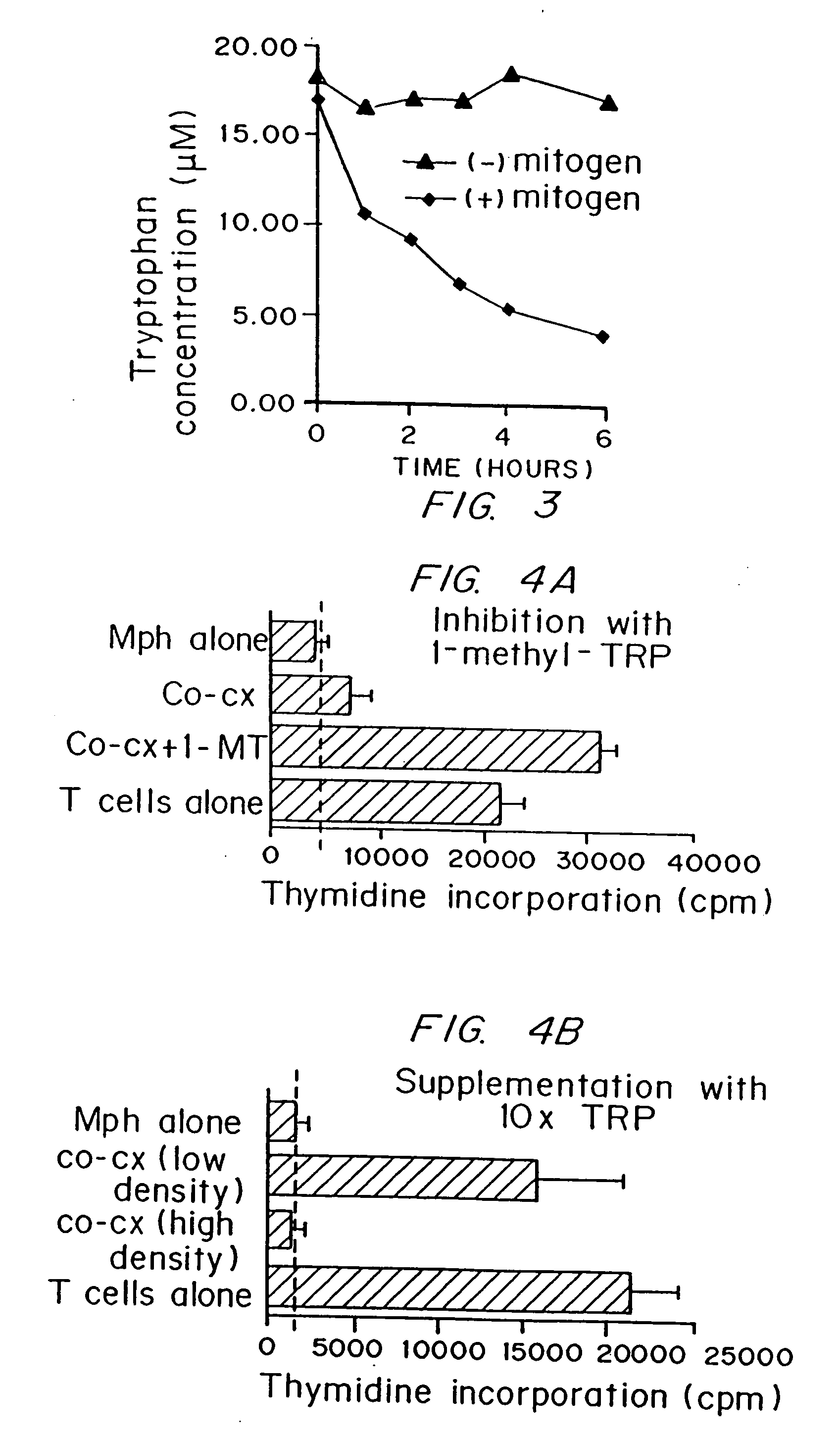
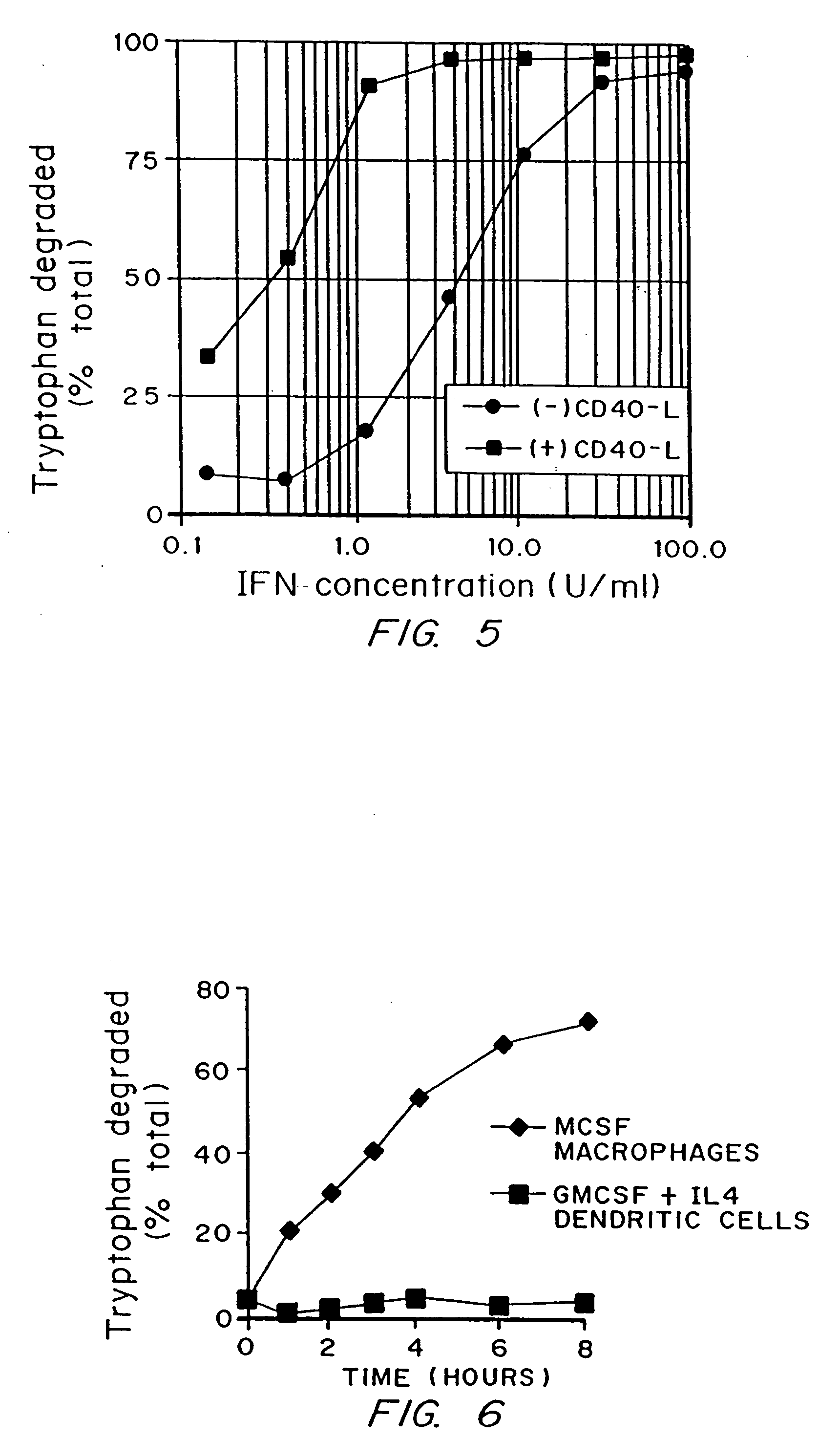
Regulation of T cell-mediated immunity by tryptophan
InactiveUS7160539B2Reduce viral loadAvoid destructionBiocidePeptide/protein ingredientsAntigenPregnancy
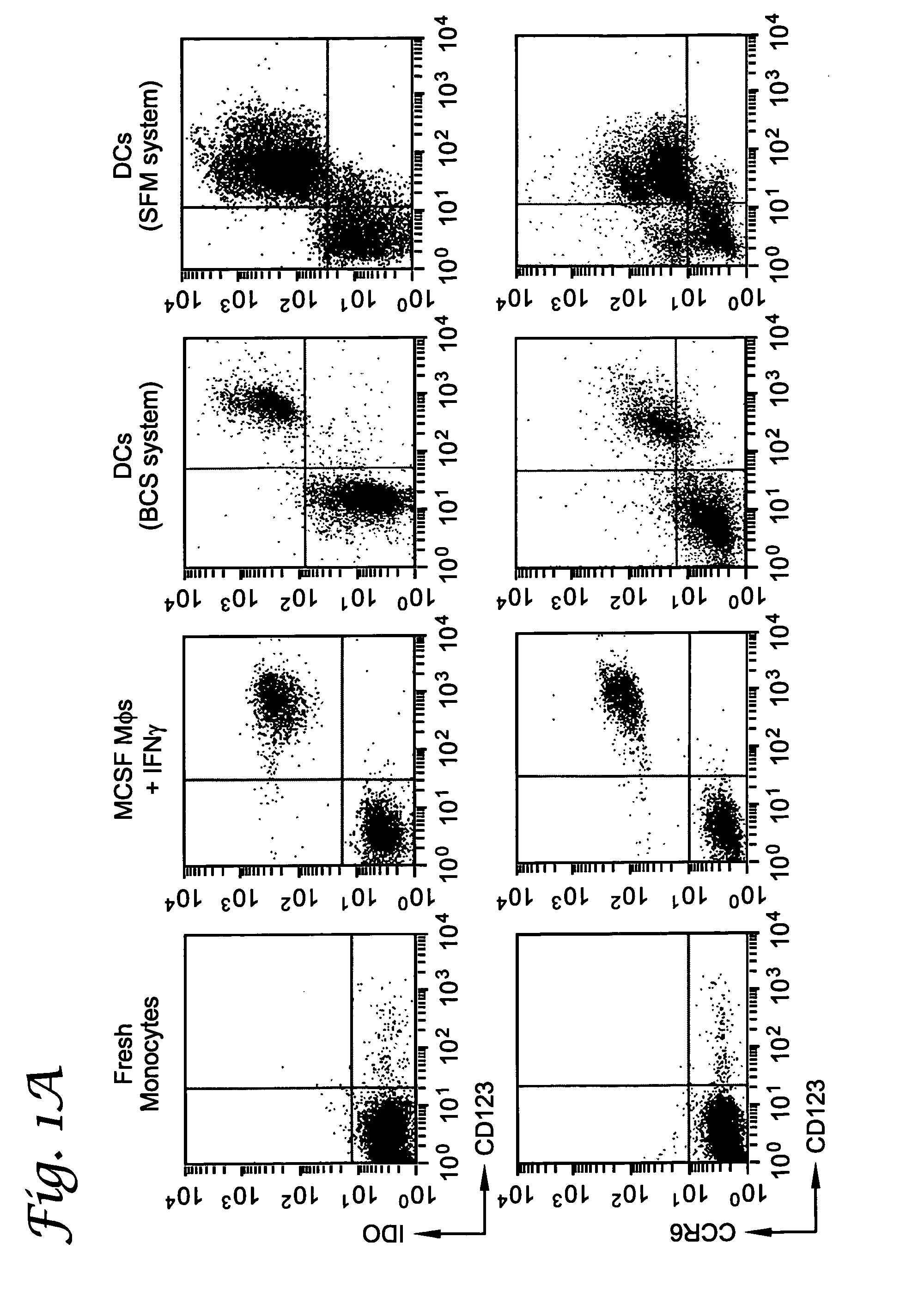
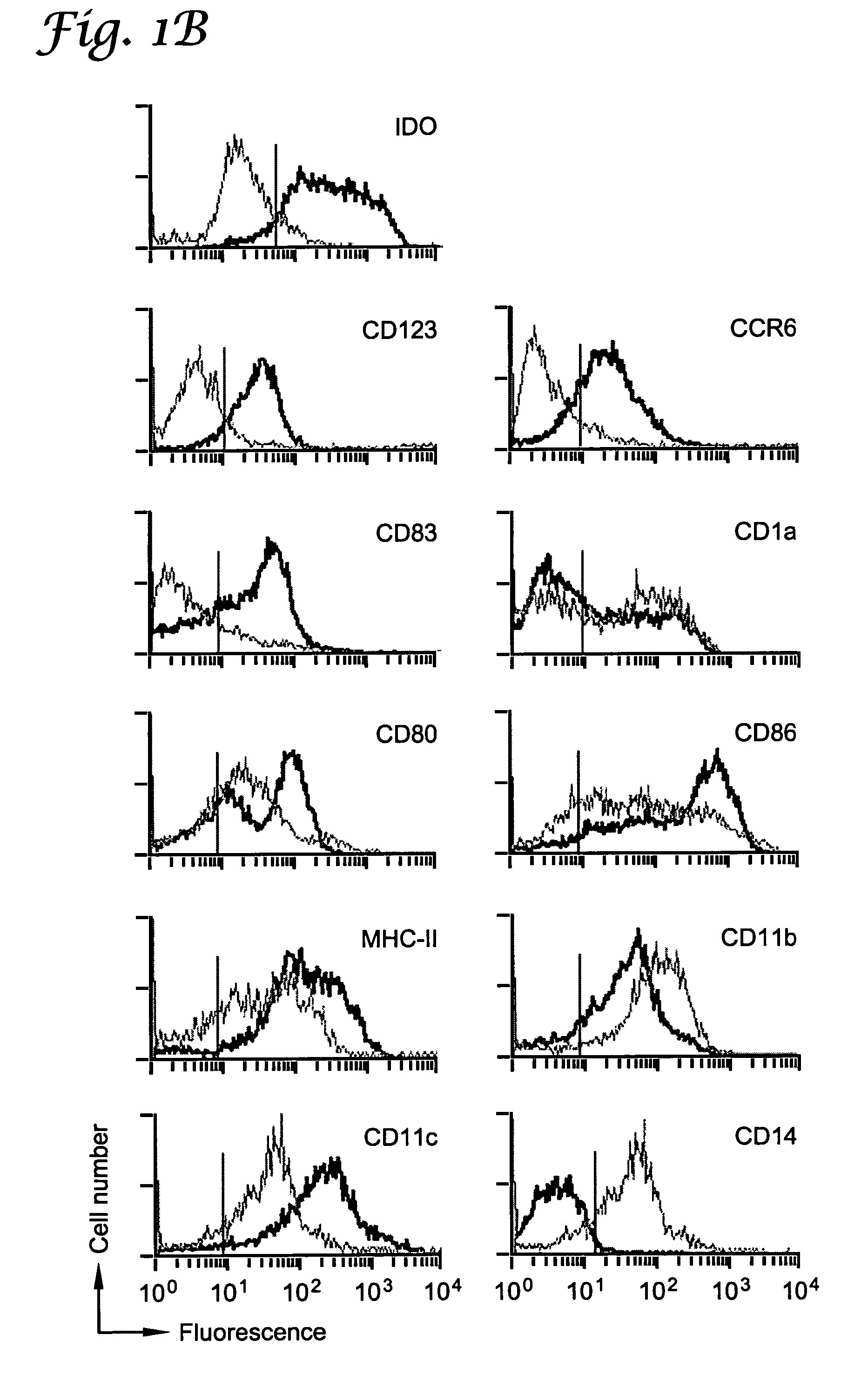
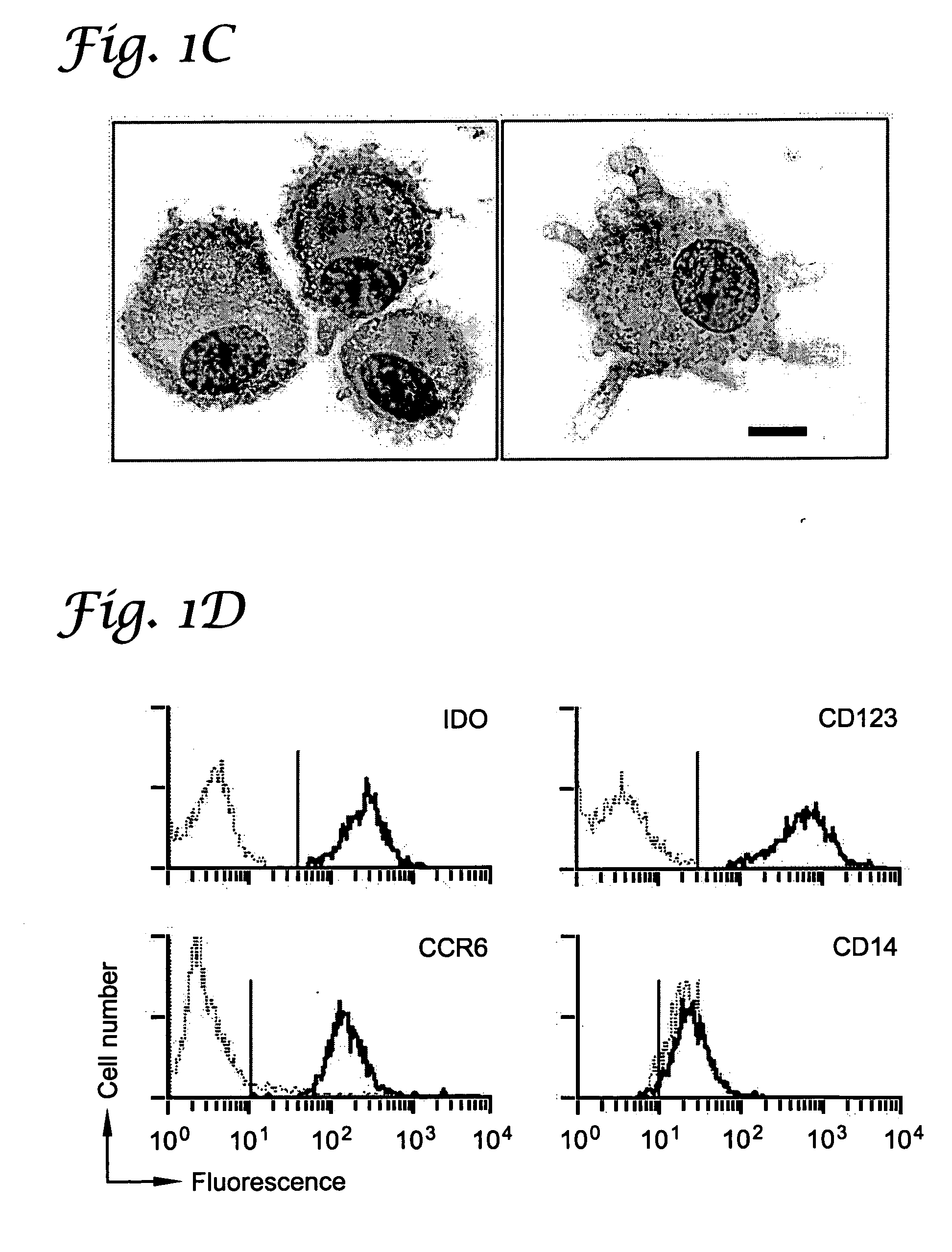
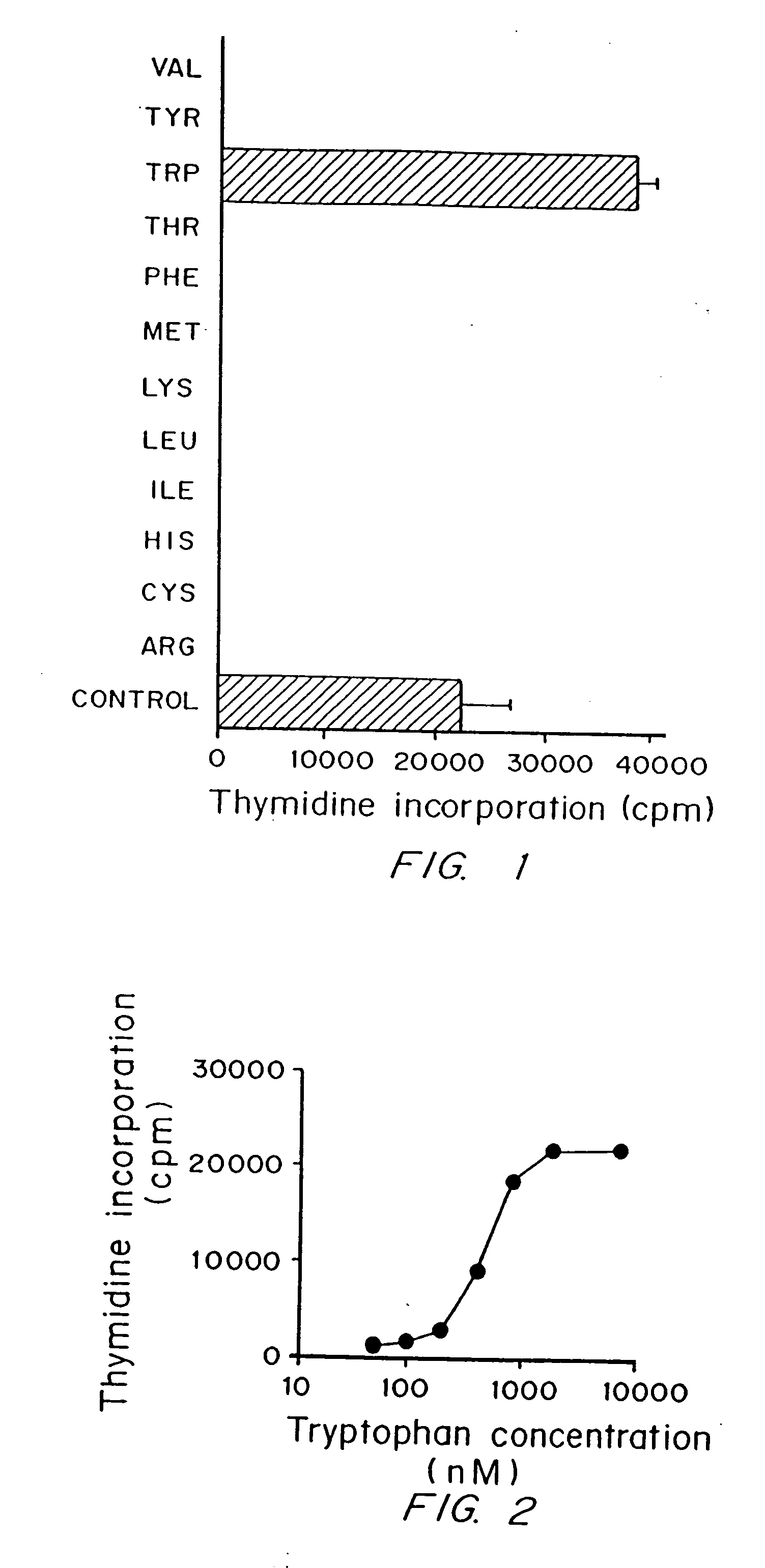
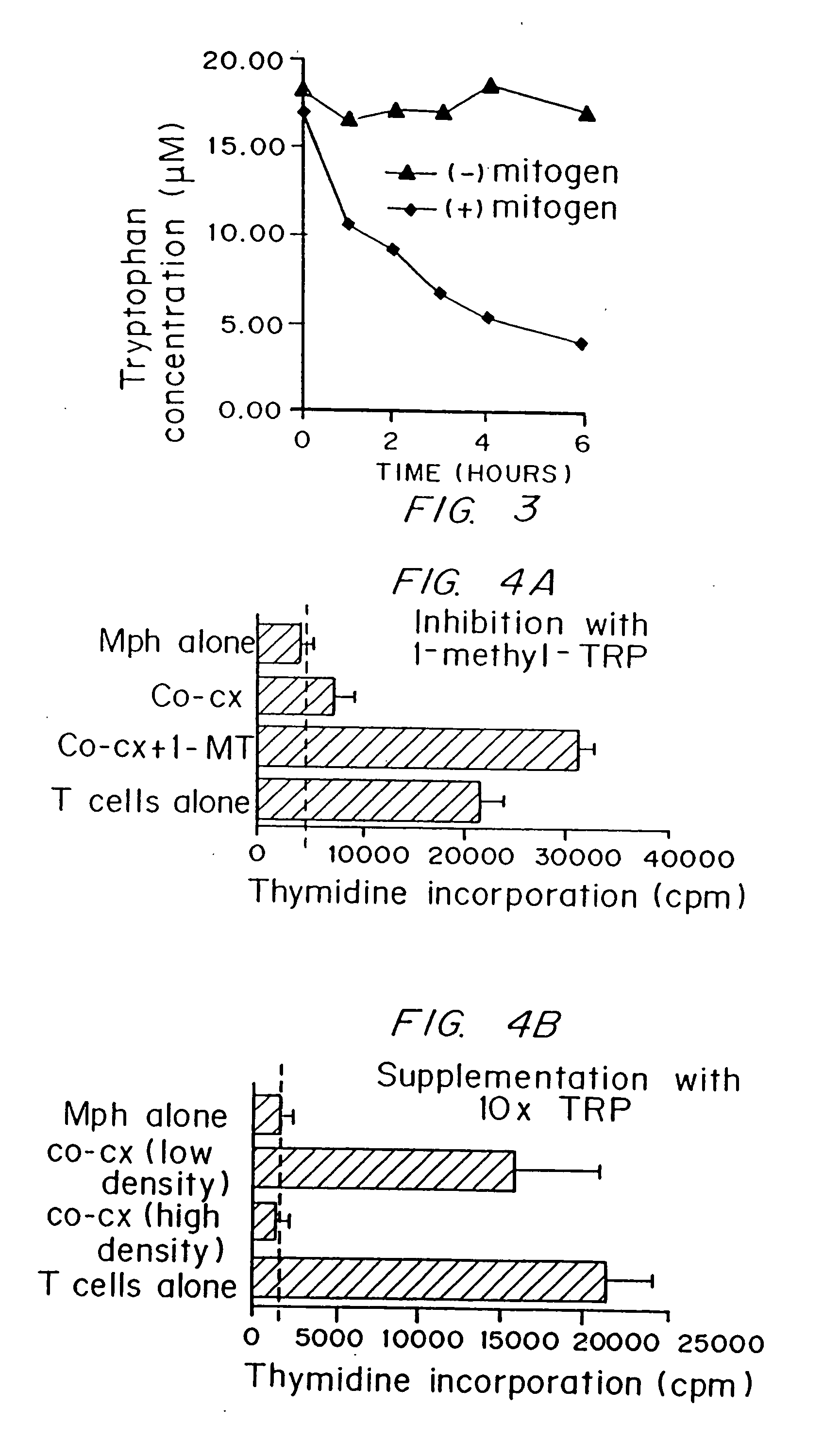
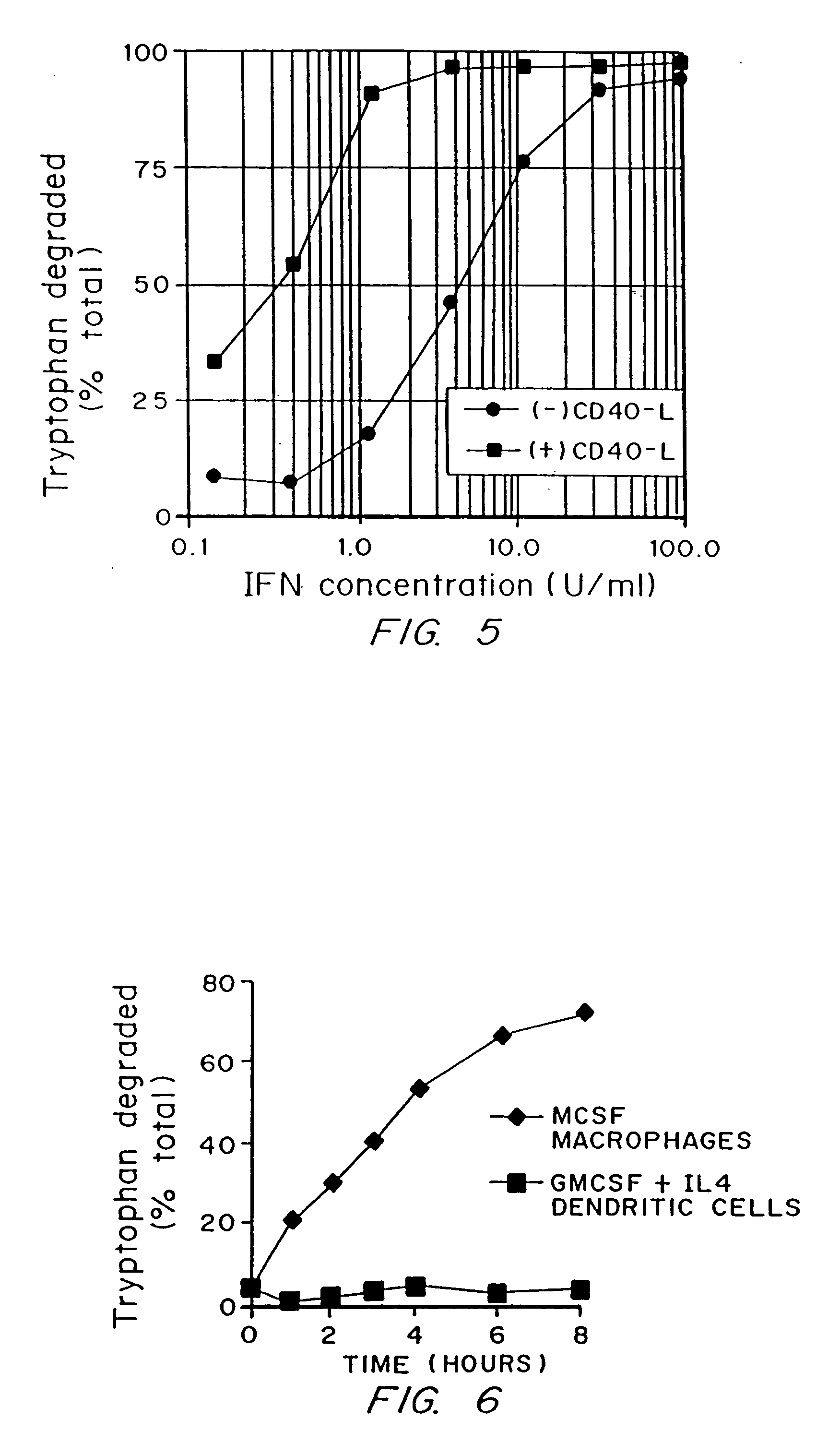
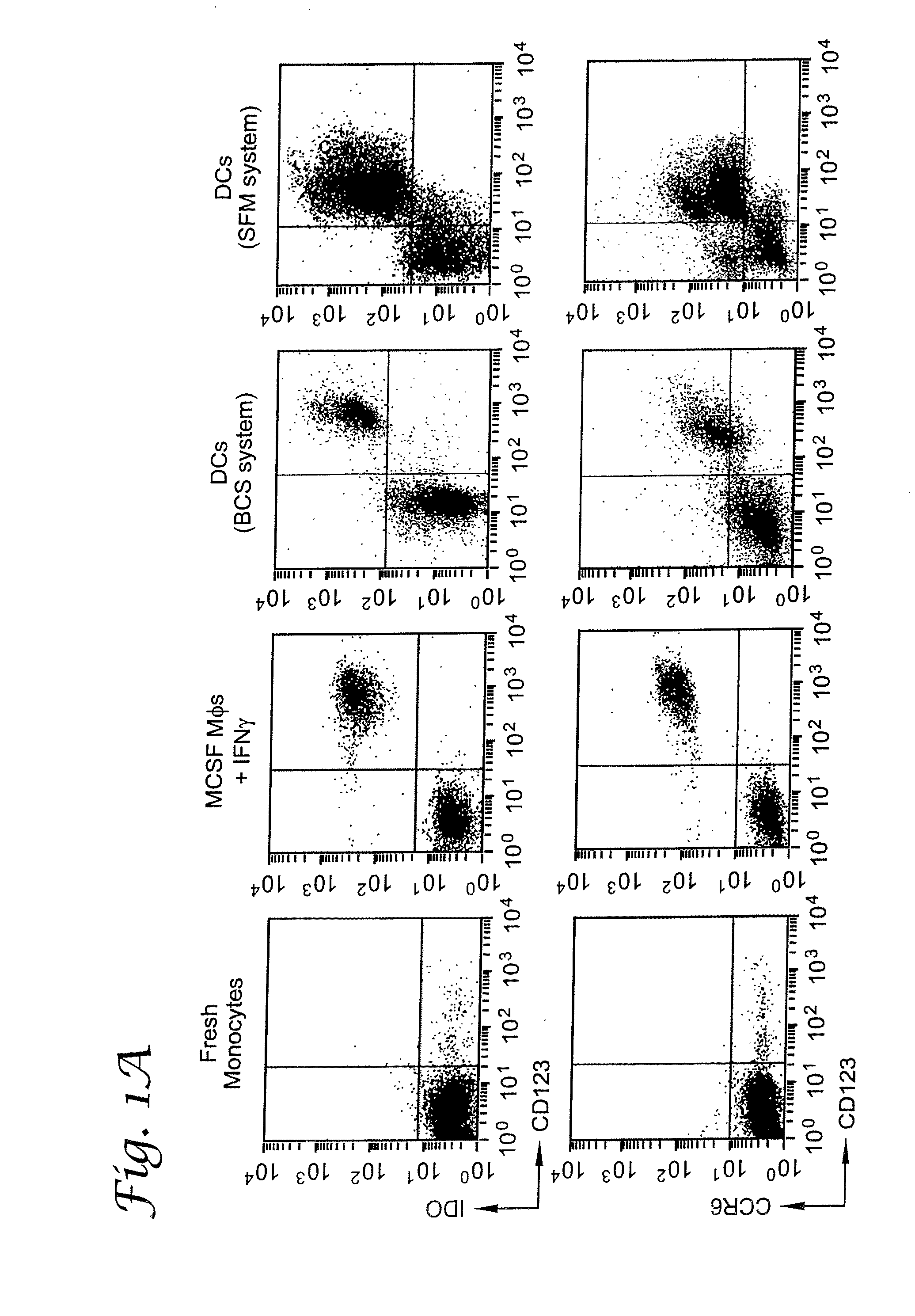
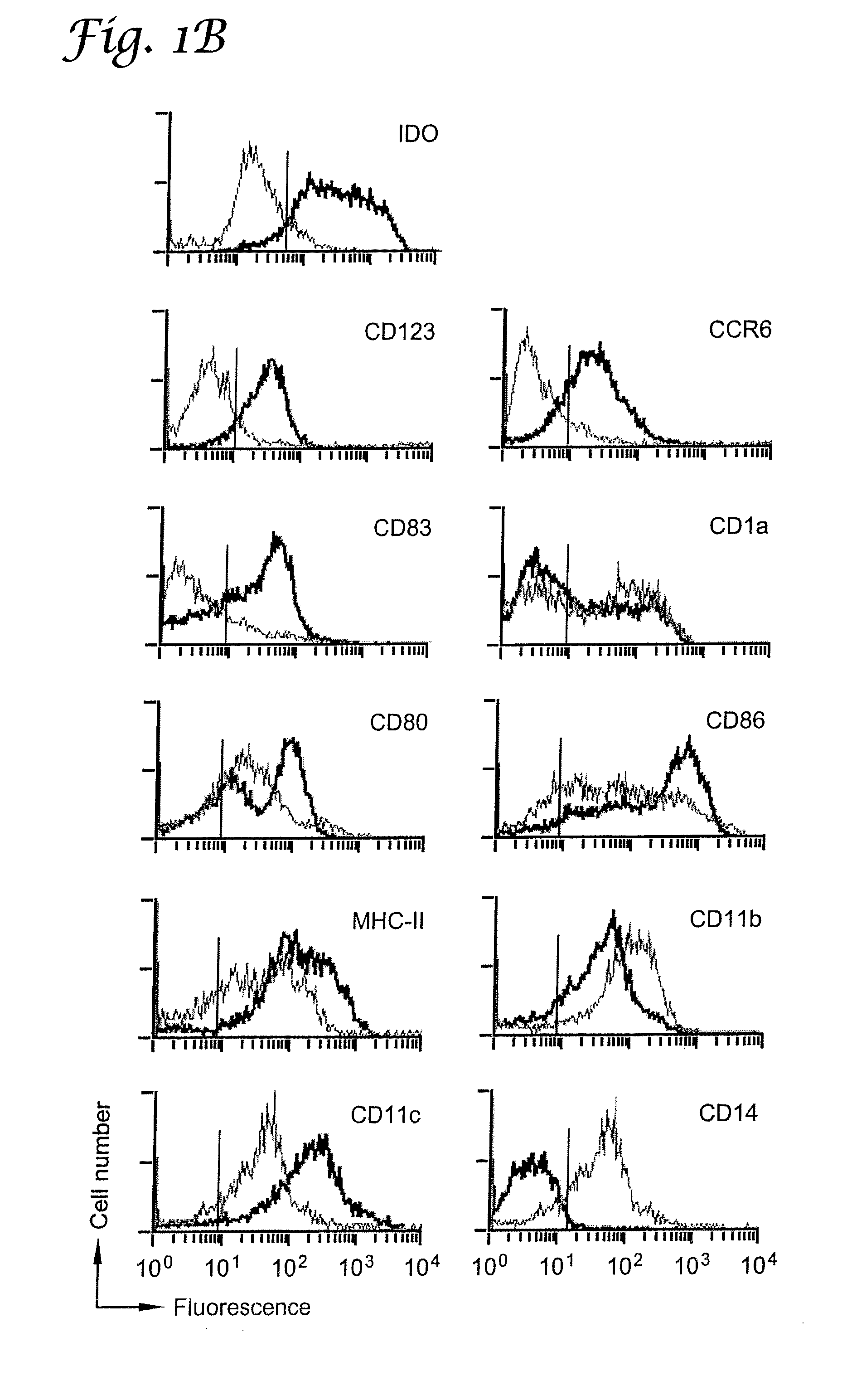
A mechanism of macrophage-induced T cell suppression is the selective elimination of tryptophan and / or increase in one or more tryptophan metabolites within the local macrophage microenvironment Studies demonstrate that expression of IDO can serve as a marker of suppression of T cell activation, and may play a significant role in allogeneic pregnancy and therefore other types of transplantation, and that inhibitors of IDO can be used to activate T cells and therefore enhance T cell activation when the T cells are suppressed by pregnancy, malignancy or a virus such as HIV. Inhibiting tryptophan degradation (and thereby increasing tryptophan concentration while decreasing tryptophan metabolite concentration), or supplementing tryptophan concentration, can therefore be used in addition to, or in place of, inhibitors of IDO. Similarly, increasing tryptophan degradation (thereby , decreasing tryptophan concentration and increasing tryptophan metabolite concentration), for example, by increasing IDO concentration or IDO activity, can suppress T cells. Although described particularly with reference to IDO regulation, one can instead manipulate local tryptophan concentrations, and / or modulate the activity of the high affinity tryptophan transporter, and / or administer other tryptophan degrading enzymes. Regulation can be further manipulated using cytokines such as macrophage colony stimulating factor, interferon gamma, alone or in combination with antigen or other cytokines.
Owner:MEDICAL COLLEGE OF GEORGIA FOUND
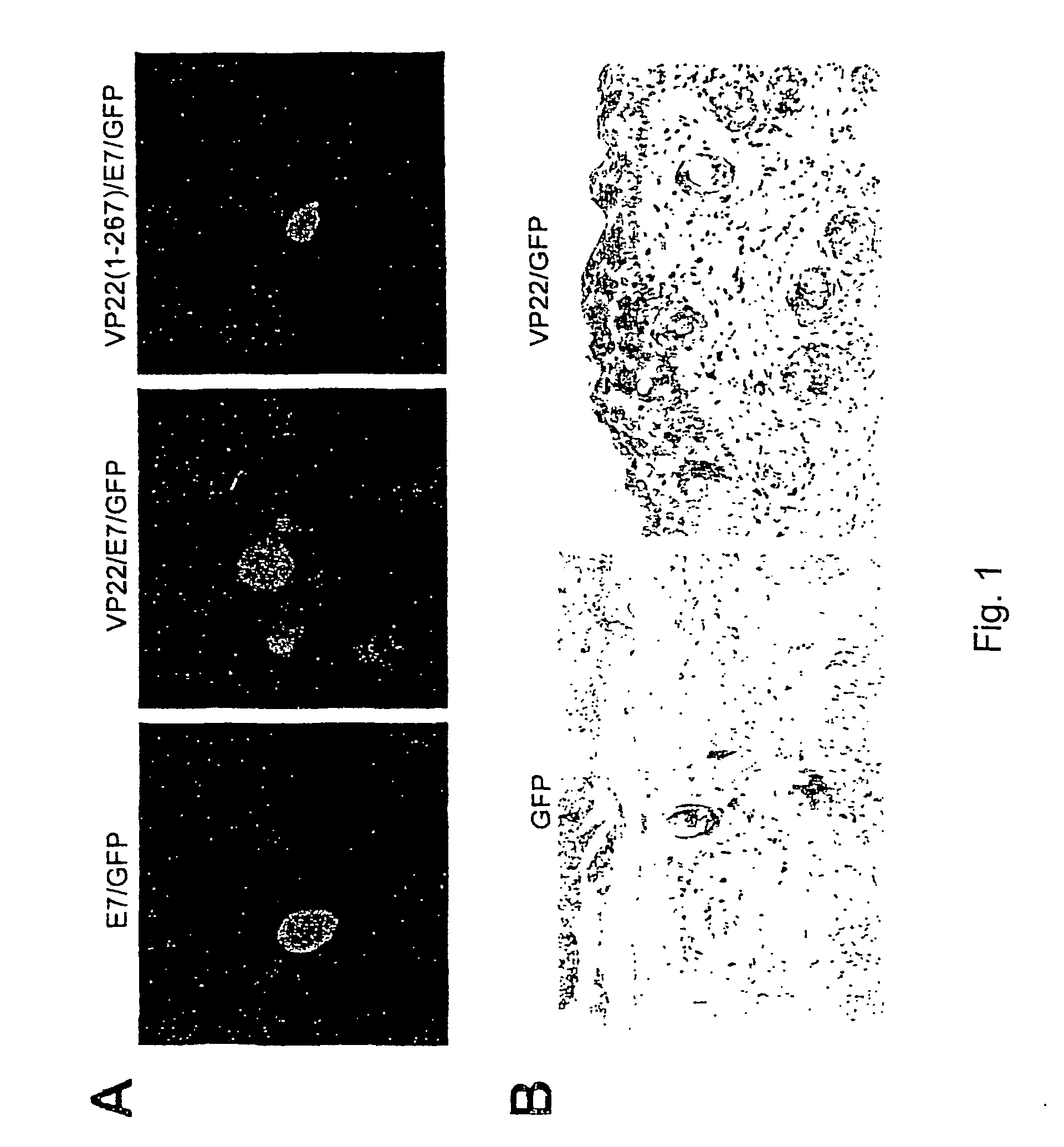
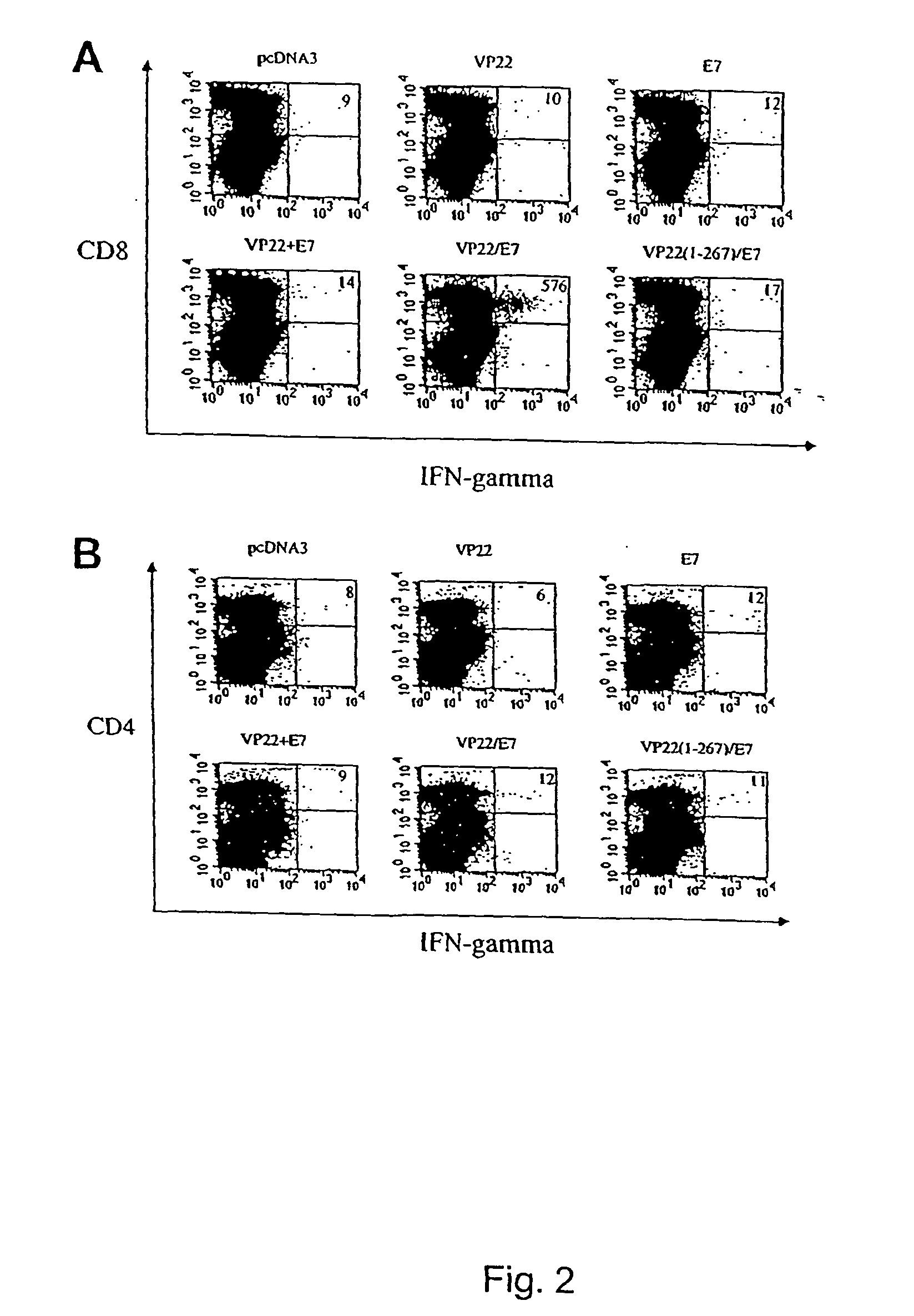
Molecular vaccine linking intercellular spreading protein to an antigen
InactiveUS7318928B2Enhancing vaccine potencyEasy to spreadAntibody mimetics/scaffoldsViral antigen ingredientsT lymphocyteOrganism
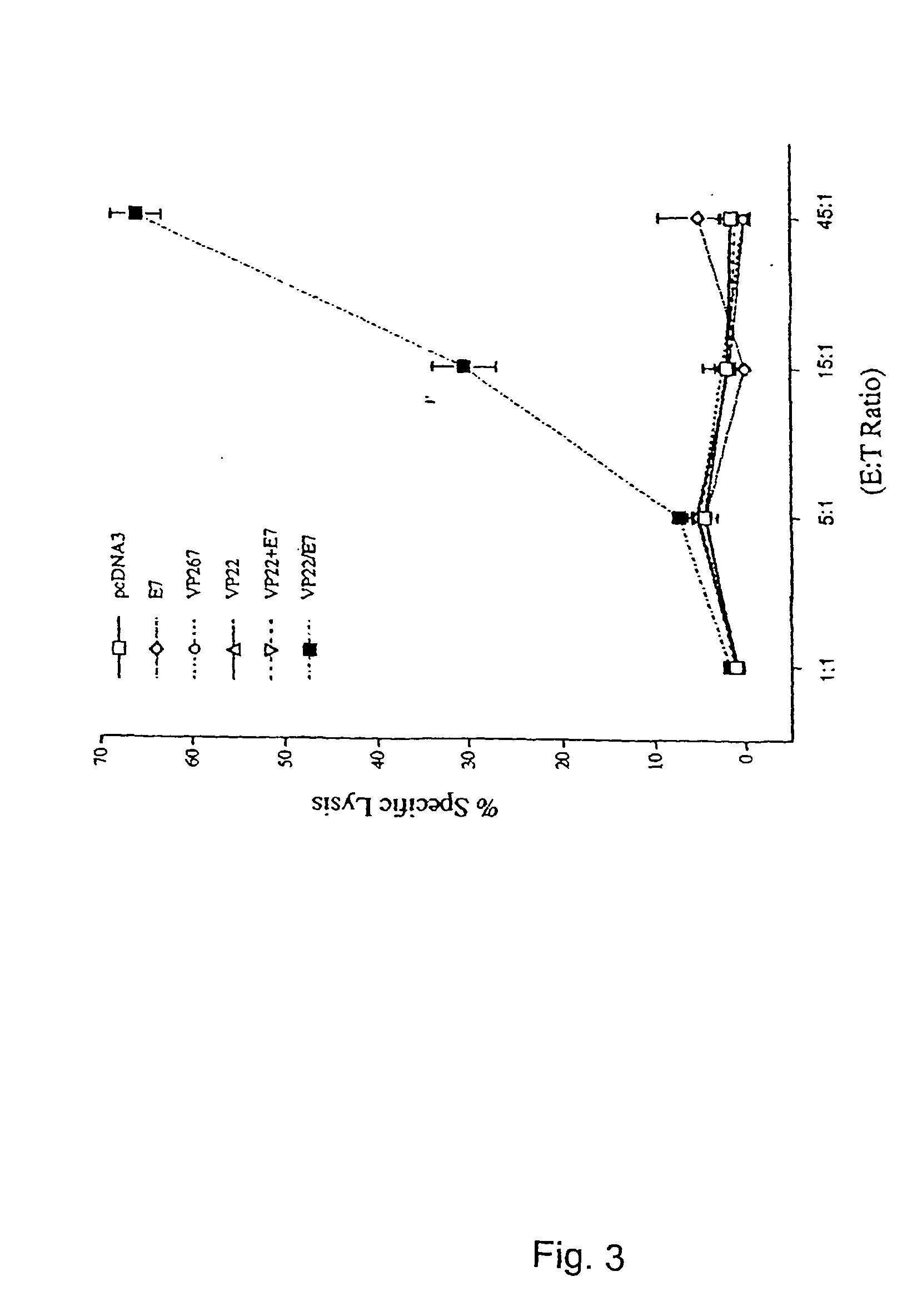
Superior molecular vaccines comprise nucleic acids, including naked DNA and replicon RNA, that encode a fusion polypeptide that includes an antigenic peptide or polypeptide against which an immune response is desired. Fused to the antigenic peptide is an intercellular spreading protein, in particular a herpes virus protein VP22 or a homologue or functional derivative thereof. Preferred spreading proteins are VP22 from HSV-1 and Marek's disease virus. The nucleic acid can encode any antigenic epitope of interest, preferably an epitope that is processed and presented by MHC class I proteins. Antigens of pathogenic organisms and cells such as tumor cells are preferred. Vaccines comprising HPV-16 E7 oncoprotein are exemplified. Also disclosed are methods of using the vaccines to induce heightened T cell mediated immunity, in particular by cytotoxic T lymphocytes, leading to protection from or treatment of a tumor.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Regulation of T cell-mediated immunity by tryptophan
InactiveUS20070077234A1Reduce loadAvoid destructionBiocidePeptide/protein ingredientsAntigenMetabolite
Owner:GEORGIA HEALTH SCI UNIV RES INST
Flavivirus vaccine delivery system
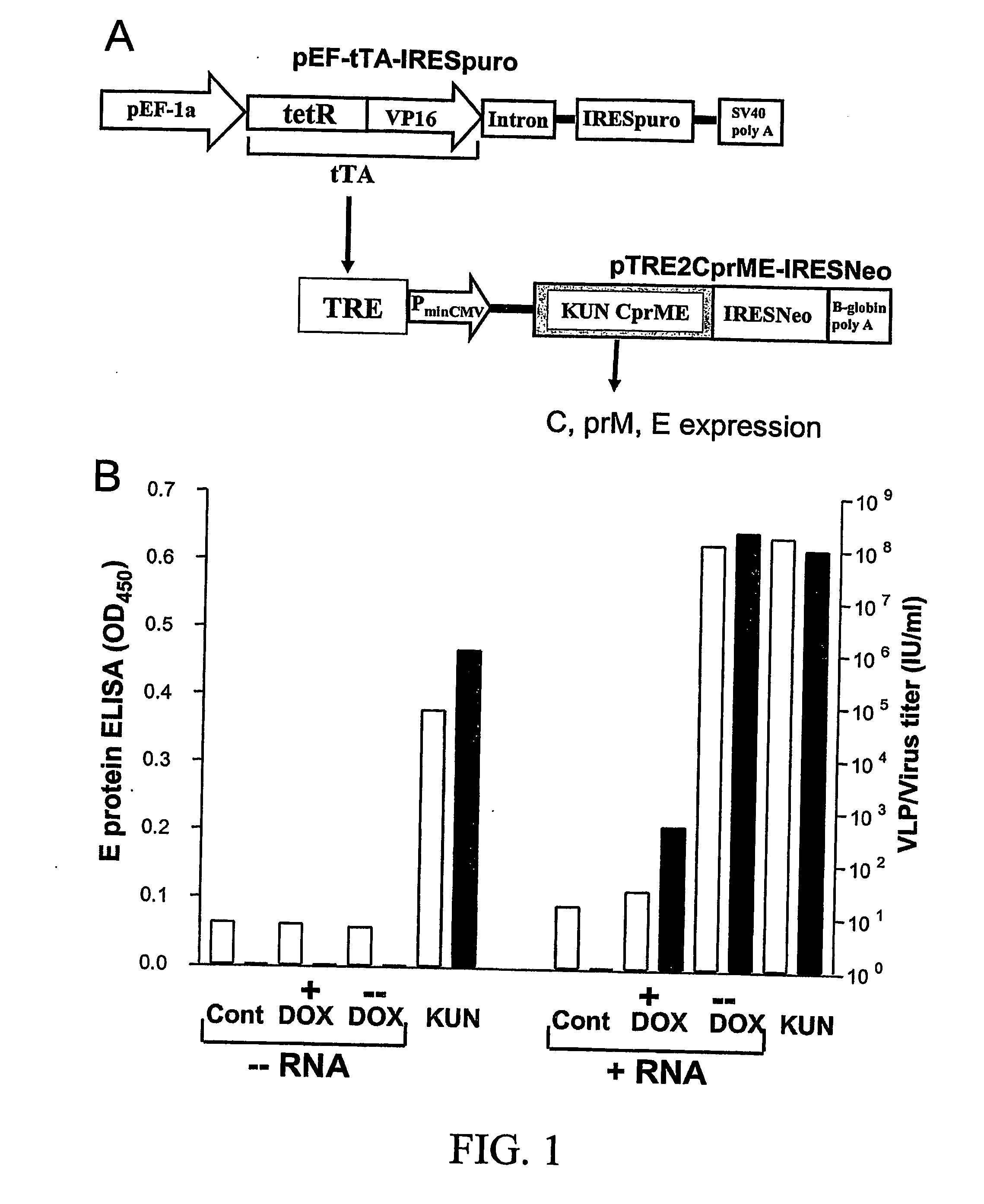
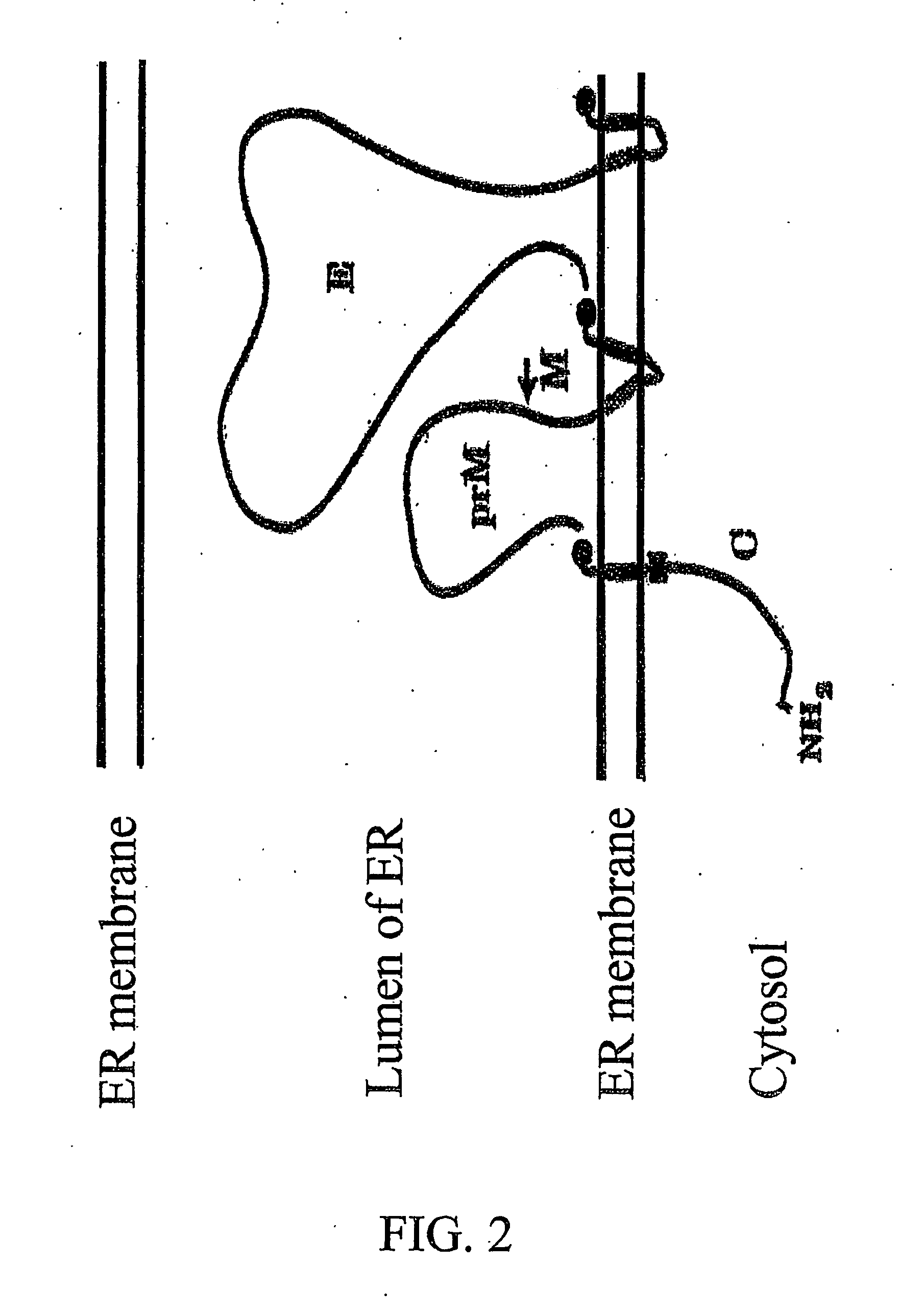
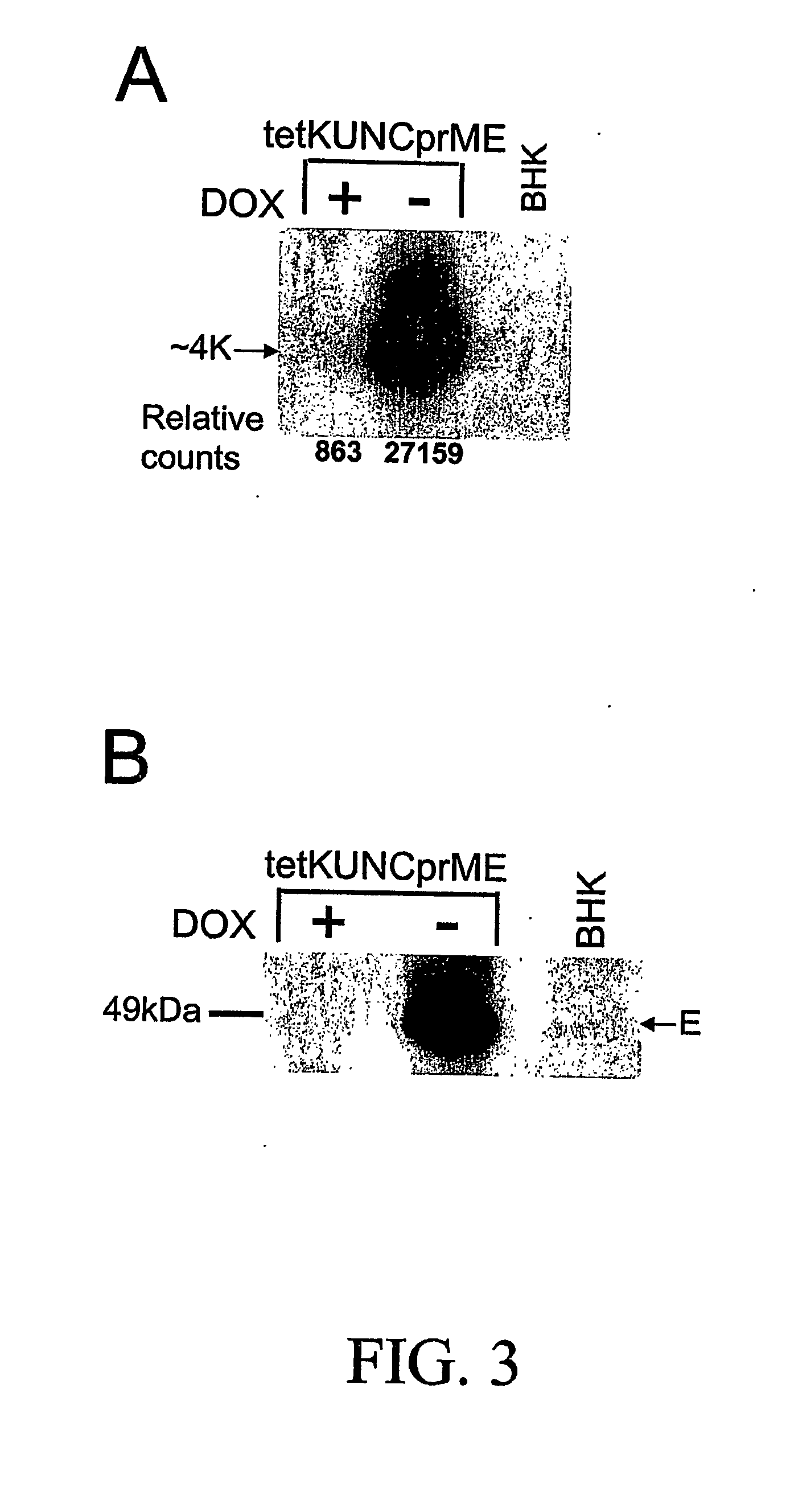
A tetracycline regulatable flaviviral packaging system is provided that facilitates expression of flaviviral structural proteins necessary for flaviviral RNA replicon packaging and virus like particle production in animal cells. This regulatable packaging system is compatible with Kunjin, Dengue and West Nile virus and other flaviviral replicon-based expression systems and produces unexpectedly high titres of virus-like particles. A particular application of this packaging system is the production of virus-like particles that package RNA comprising a flaviviral replicon and encoding a heterologous protein or peptide for expression in animal cells. Even more particularly, the packaging system is capable of delivering immunogens that induce a protective CD8 T cell-mediated immune response.
Owner:REPLIKUN BIOTECH
Regulation of T Cell-Mediated Immunity by D Isomers of Inhibitors of Indoleamine-2,3-Dioxygenase
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Compositions and Methods
InactiveUS20100285050A1High reactivityWell representedSsRNA viruses negative-senseViral antigen ingredientsEpitopeT cell mediated immunity
The invention relates to a composition suitable for inducing a T cell mediated immune response against an influenza virus in a vertebrate, said composition comprising nucleic acid encoding one or more epitopes of one or more internal proteins of influenza virus, wherein said composition comprises nucleic acid encoding at least two said epitopes, at least one epitope being from each of two or more internal proteins of influenza virus. The invention also relates to uses of same and to methods involving same.
Owner:ISIS INNOVATION LTD
Methods for the treatment and prevention of inflammatory diseases
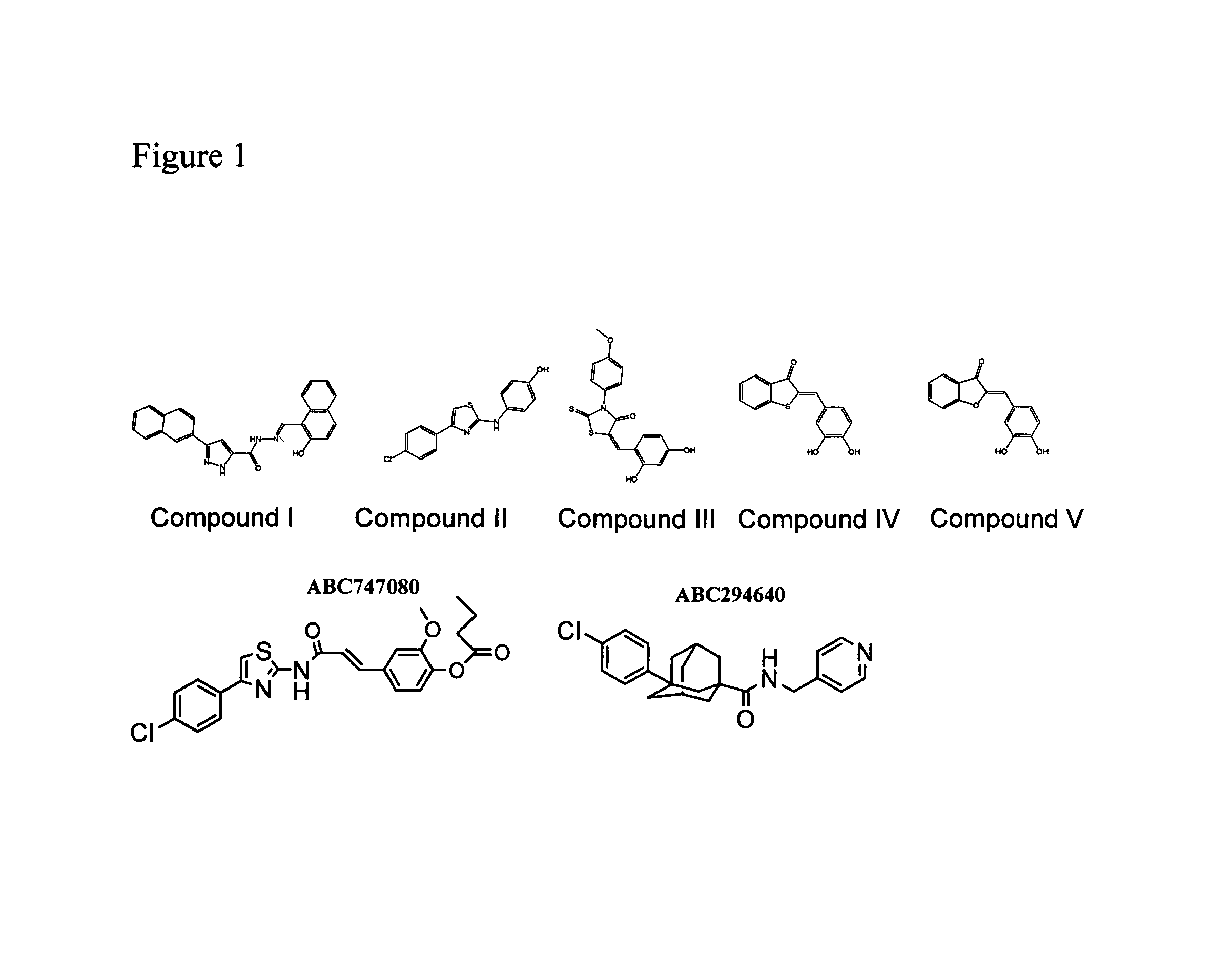
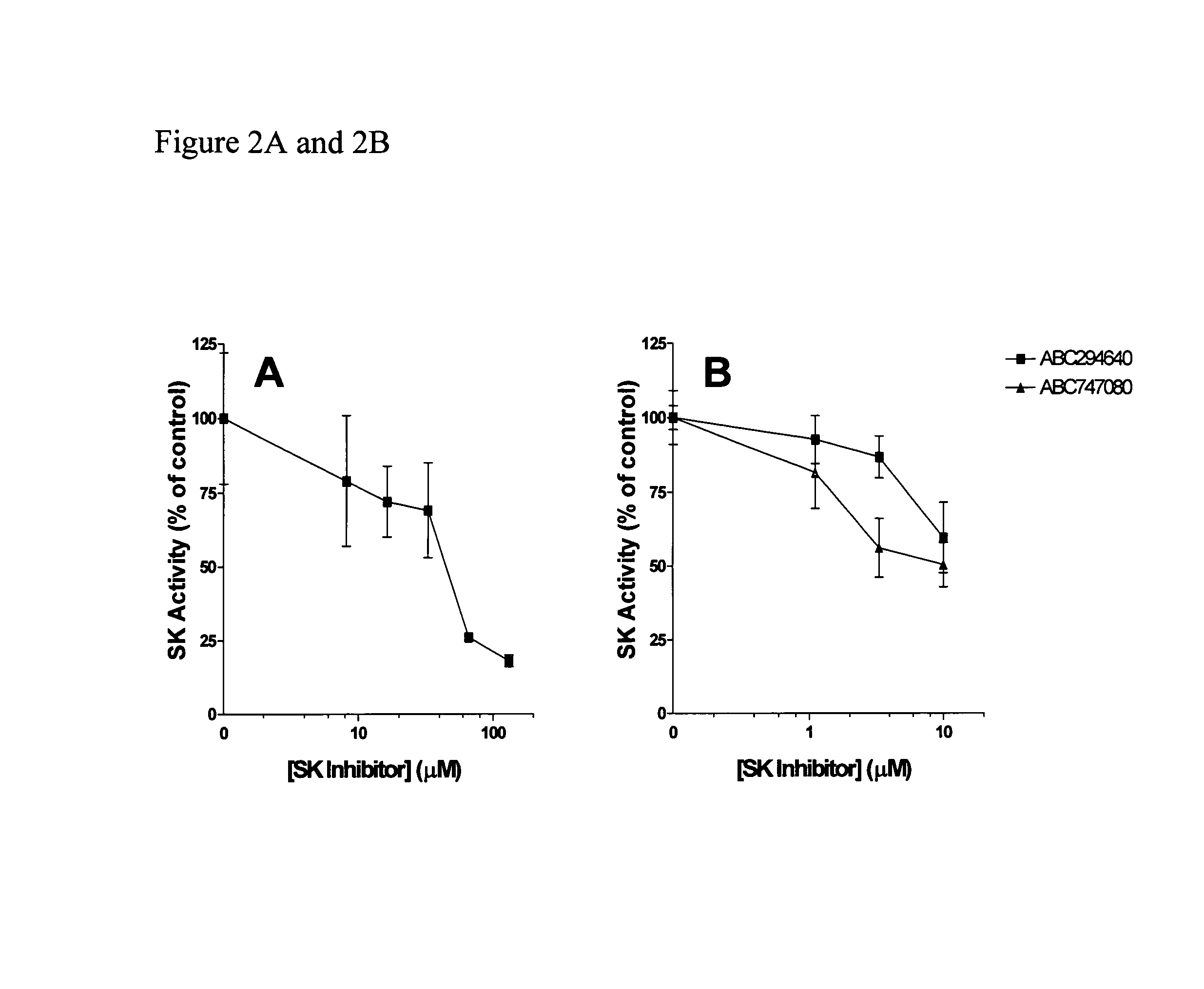
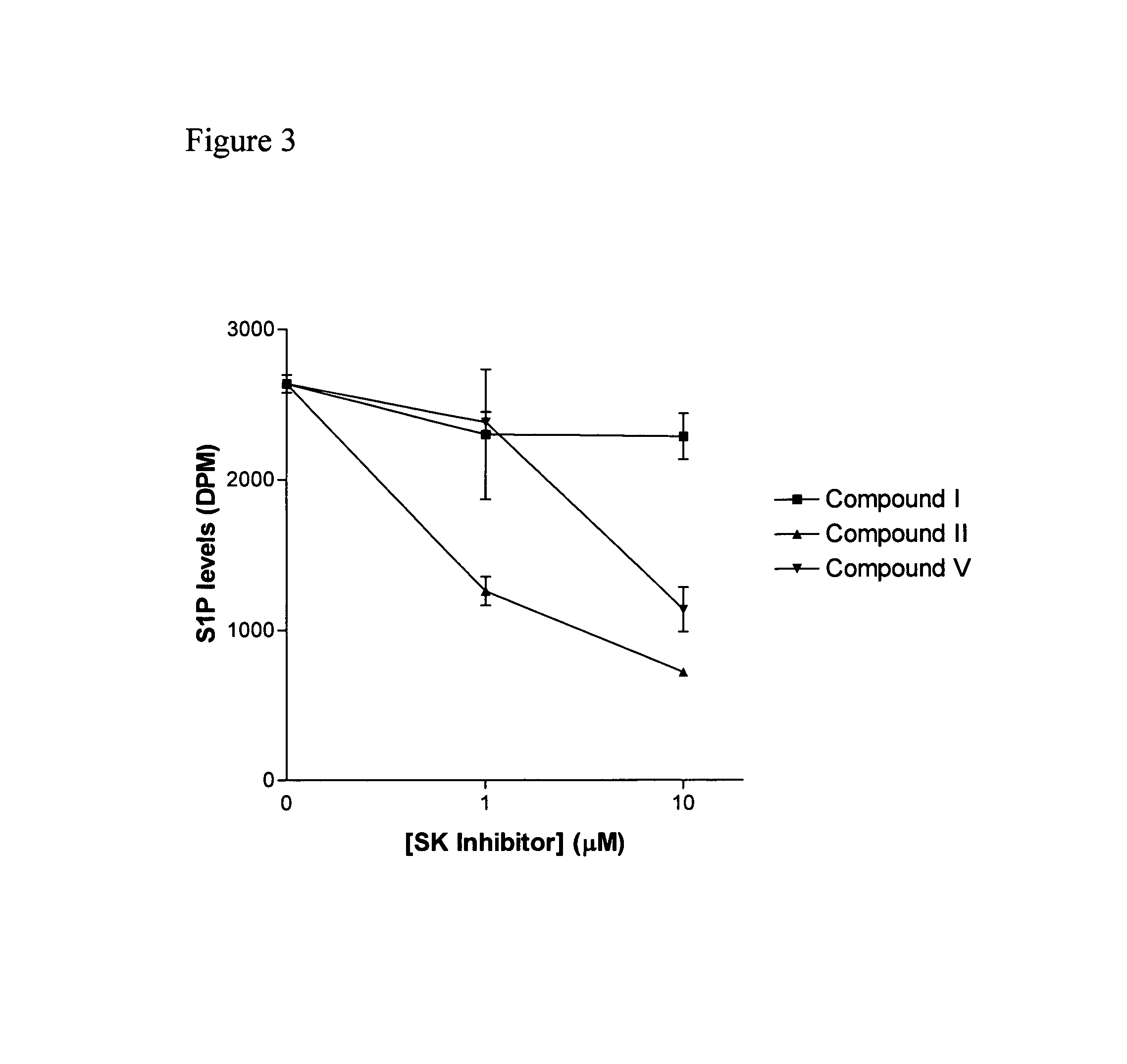
The invention includes processes mainly for the treatment of a inflammatory diseases, such as inflammatory bowel disease, arthritis, atherosclerosis, asthma, allergy, inflammatory kidney disease, circulatory shock, multiple sclerosis, chronic obstructive pulmonary disease, skin inflammation, periodontal disease, psoriasis and T cell-mediated diseases of immunity, including allergic encephalomyelitis, allergic neuritis, transplant allograft rejection, graft versus host disease, myocarditis, thyroiditis, nephritis, systemic lupus erthematosus, and insulin-dependent diabetes mellitus. The processes involve treating a patient with a pharmaceutical composition containing an active ingredient that inhibits the activity of sphingosine kinase.
Owner:APOGEE BIOTECHNOLOGY CORP
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS9056906B2Treating and preventing and slowing progression of and ameliorating symptomSlow and reduce autoimmunitySenses disorderNervous disorderDosing regimenInsulin dependent diabetes
The present invention provides methods of treating, preventing, slowing the progression of, or ameliorating the symptoms of T cell mediated immunological diseases, particularly autoimmune diseases (e.g., autoimmune diabetes (i.e. type 1 diabetes or insulin-dependent diabetes mellitus (IDDM)) and multiple sclerosis) through the use of anti-human CD3 antibodies. The antibodies of the invention of the invention are preferably used in low dose dosing regimens, chronic dosing regimens or regimens that involve redosing after a certain period of time. The methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the methods of the invention provide for use of anti-human CD3 antibodies modified such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-human CD3 antibodies.
Owner:PROVENTION BIO INC
Regulation of T cell-mediated immunity by tryptophan
InactiveUS20070077224A1Reduce loadAvoid destructionBiocidePeptide/protein ingredientsAntigenMetabolite
A mechanism of macrophage-induced T cell suppression is the selective elimination of tryptophan and / or increase in one or more tryptophan metabolites within the local macrophage microenvironment. Studies demonstrate that expression of IDO can serve as a marker of suppression of T cell activation, and may play a significant role in allogeneic pregnancy and therefore other types of transplantation, and that inhibitors of IDO can be used to activate T cells and therefore enhance T cell activation when the T cells are suppressed by pregnancy, malignancy or a virus such as HIV. Inhibiting tryptophan degradation (and thereby increasing tryptophan concentration while decreasing tryptophan metabolite concentration), or supplementing tryptophan concentration, can therefore be used in addition to, or in place of, inhibitors of IDO. Similarly, increasing tryptophan degradation (thereby, decreasing tryptophan concentration and increasing tryptophan metabolite concentration), for example, by increasing IDO concentration or IDO activity, can suppress T cells. Although described particularly with reference to IDO regulation, one can instead manipulate local tryptophan concentrations, and / or modulate the activity of the high affinity tryptophan transporter, and / or administer other tryptophan degrading enzymes. Regulation can be further manipulated using cytokines such as macrophage colony stimulating factor, interferon gamma, alone or in combination with antigen or other cytokines.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
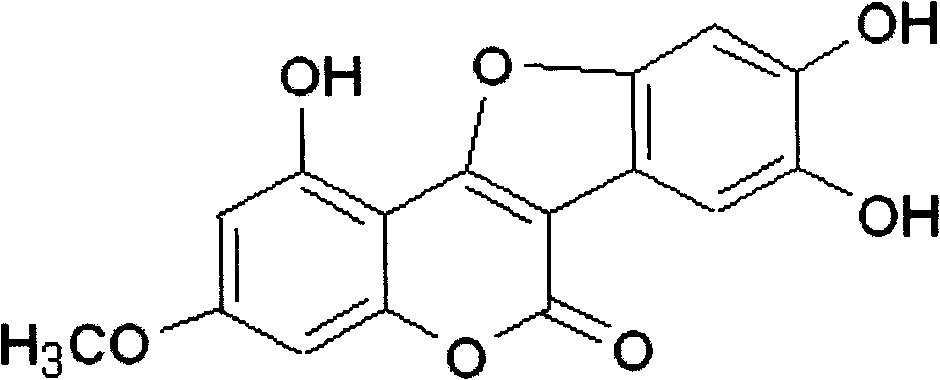
Pharmaceutical use of wedelolactone and its derivative
InactiveCN101259124APrevent proliferationNo inhibitionOrganic active ingredientsDigestive systemDiseaseWedelolactone
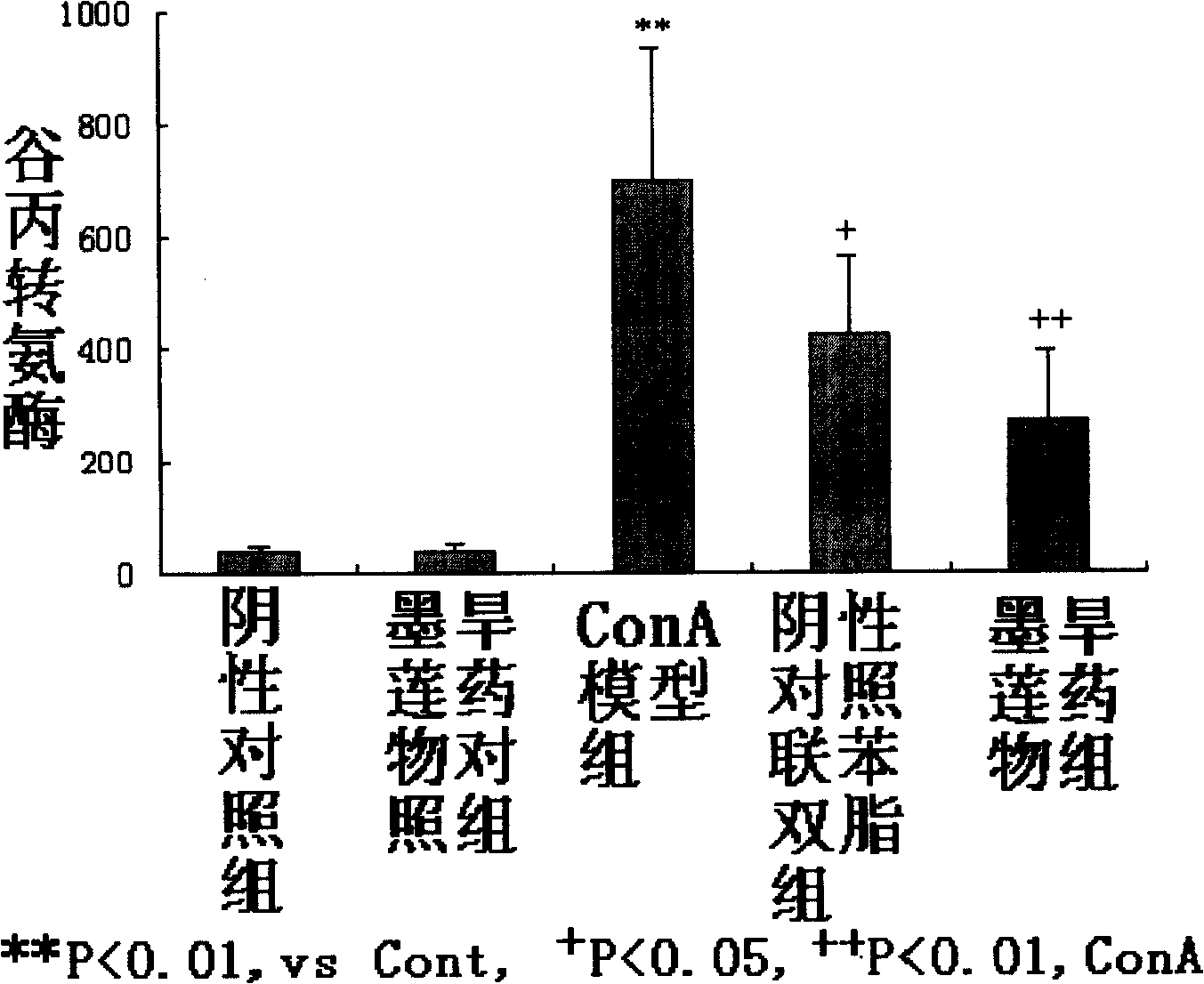
The invention relates to medical applications of wedelolactone and derivatives thereof, in particular to the applications in preparing medicines for treating T-cell mediated immunologic injury diseases (chronic hepatitis or autoimmune hepatitis). Traditional Chinese Medicine eclipta has a function of liver protection; wedelolactone is prepared after the active ingredient of eclipta is traced and separated, so the pharmaceutical function is proved to be produced by wedelolactone; the structure of wedelolactone is determined through the spectrum properties and physicochemical properties. Wedelolactone is provided with biological activities: high selective inhibition to ConA stimulated T lymphocyte proliferation, no inhibition to normal peripheral blood lymphocyte (NPBL), and no cytotoxicity; wedelolactone is the active ingredient of Traditional Chinese Medicine with liver protection function; the method for preparing a novel medicine from the active ingredient of eclipta, wedelolactone, has the advantages of discarding the dross and selecting the essential, clear medicinal ingredients, definite function, safety and effectiveness, controllable quality, reducing the adverse drug reaction, and can be suitable for mass production.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Immune cell compositions and methods of using same
ActiveUS20180360884A1Reduce riskShorten the construction periodPeptide/protein ingredientsAntibody mimetics/scaffoldsRegulatory T cellT cell mediated immunity
Disclosed herein are cells that are immunoinhibitory cell, which cells recombinantly express a dominant negative form of an inhibitor of a cell-mediated immune response of the cell. In certain embodiments, the immunoinhibitory cell is a regulatory T cell. In another aspect, provided herein is a regulatory T cell that recombinantly expresses a dominant negative form of an inhibitor of a regulatory T cell-mediated immune response. The cells can be sensitized to an antigen that is the target of a pathologic immune response associated with an immune-mediated disorder. Additionally provided are methods of using such cells to treat an immune-mediated disorder in a subject in need thereof.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
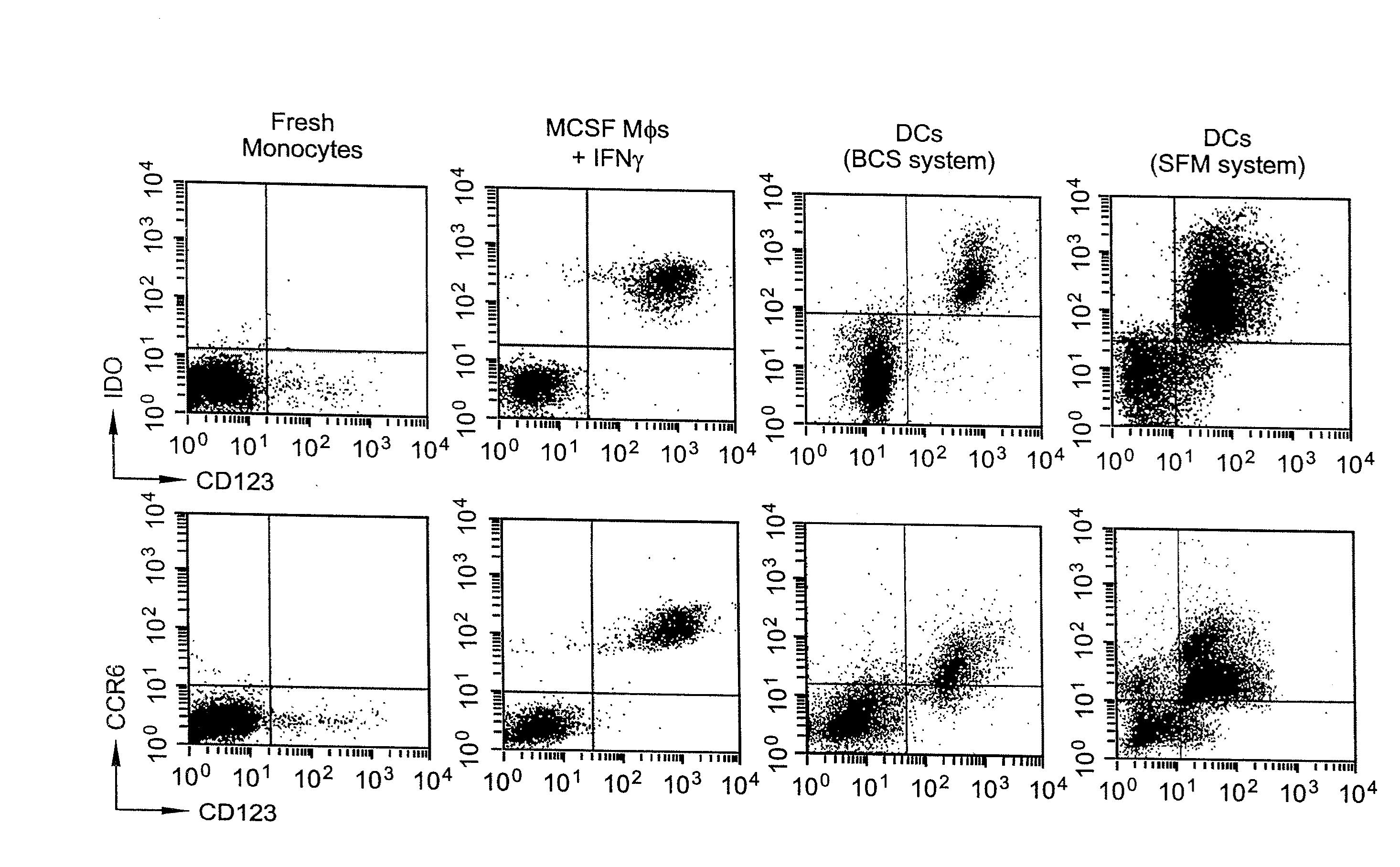
RNA Interference That Blocks Expression of Pro-Apoptotic Proteins Potentiates Immunity Induced by DNA and Transfected Dendritic Cell Vaccines
InactiveUS20080069840A1Enhance antigen presentationConducive to survivalOrganic active ingredientsBiocideAbnormal tissue growthImmunotherapeutic agent
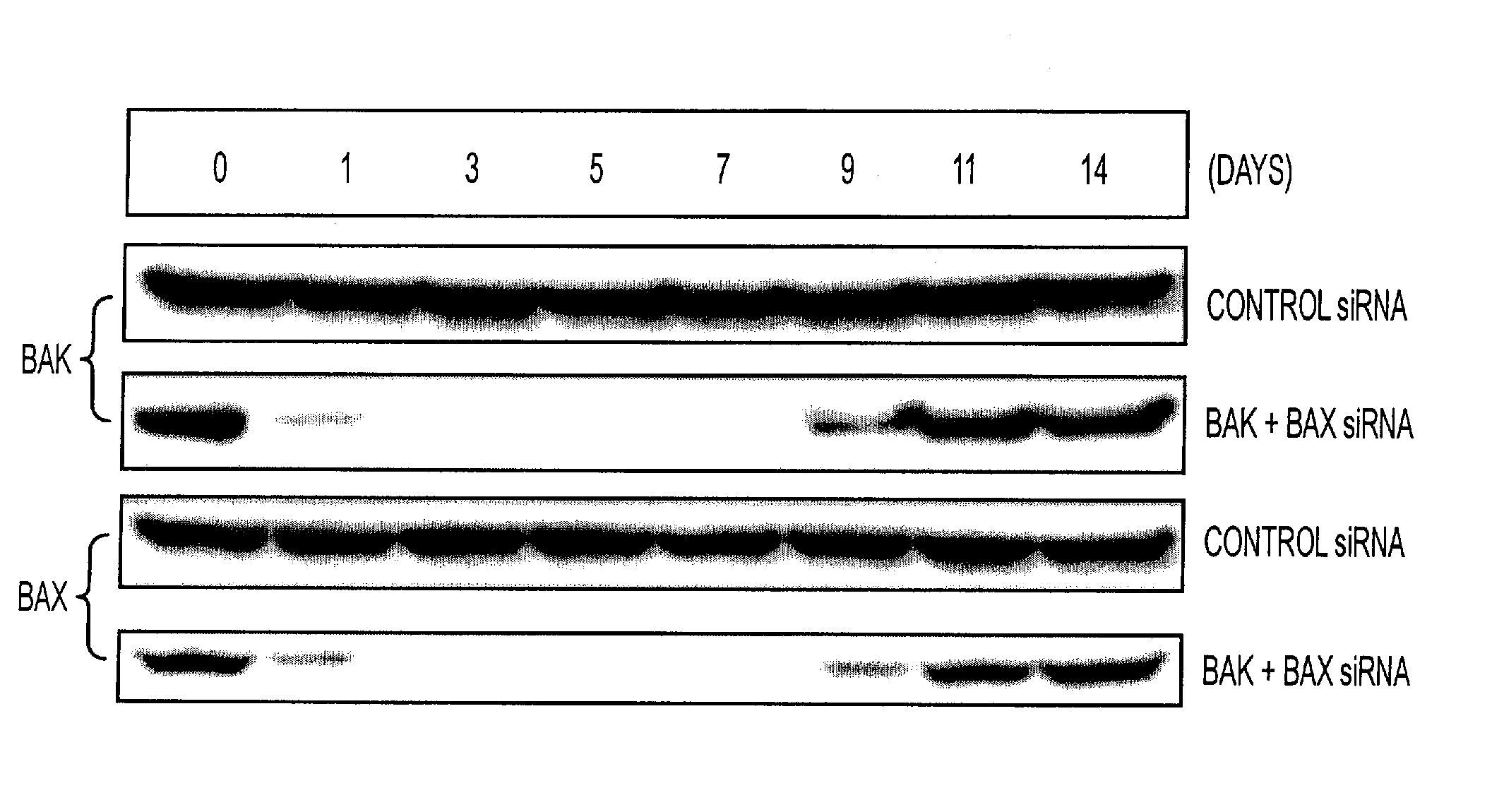
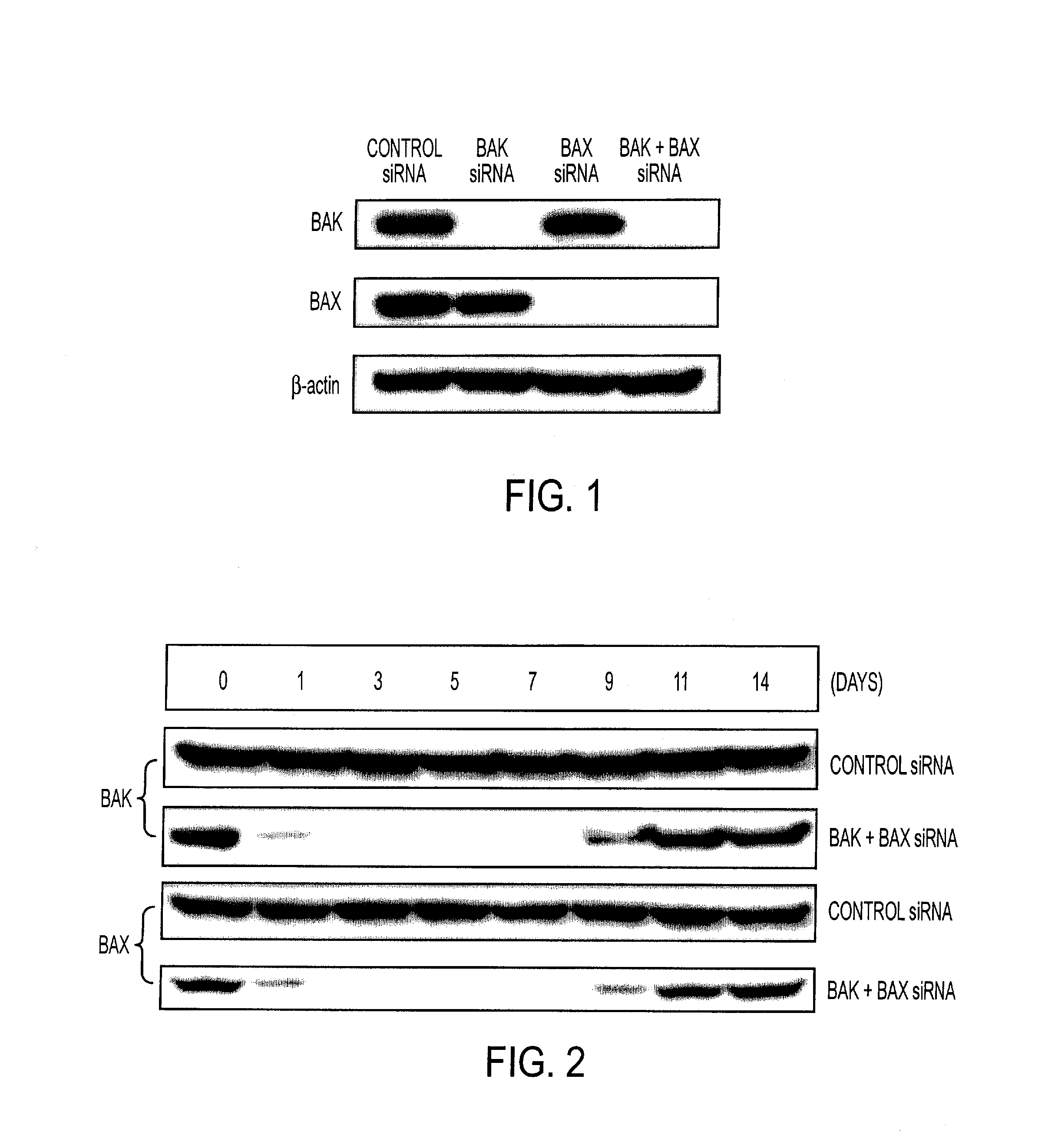
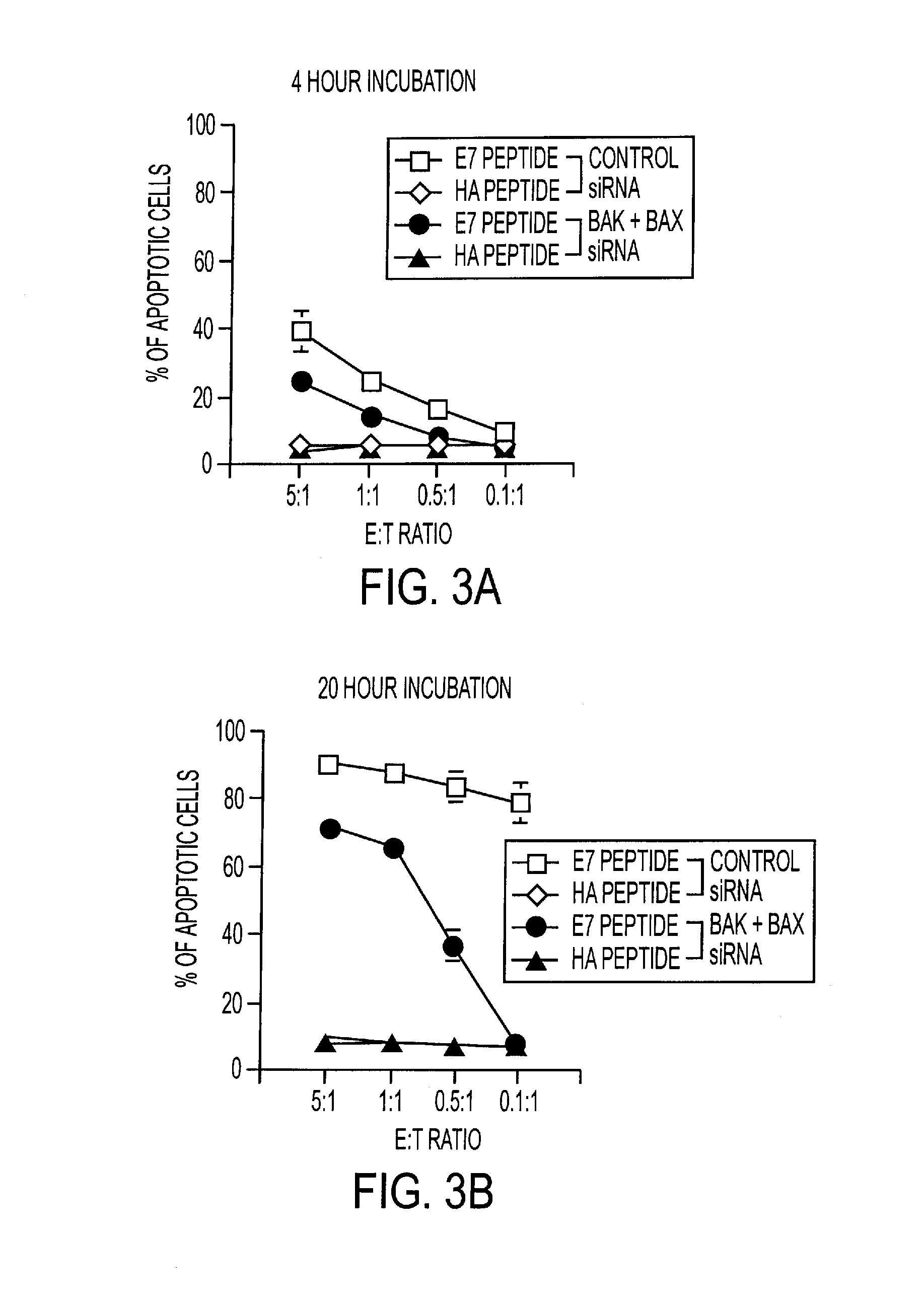
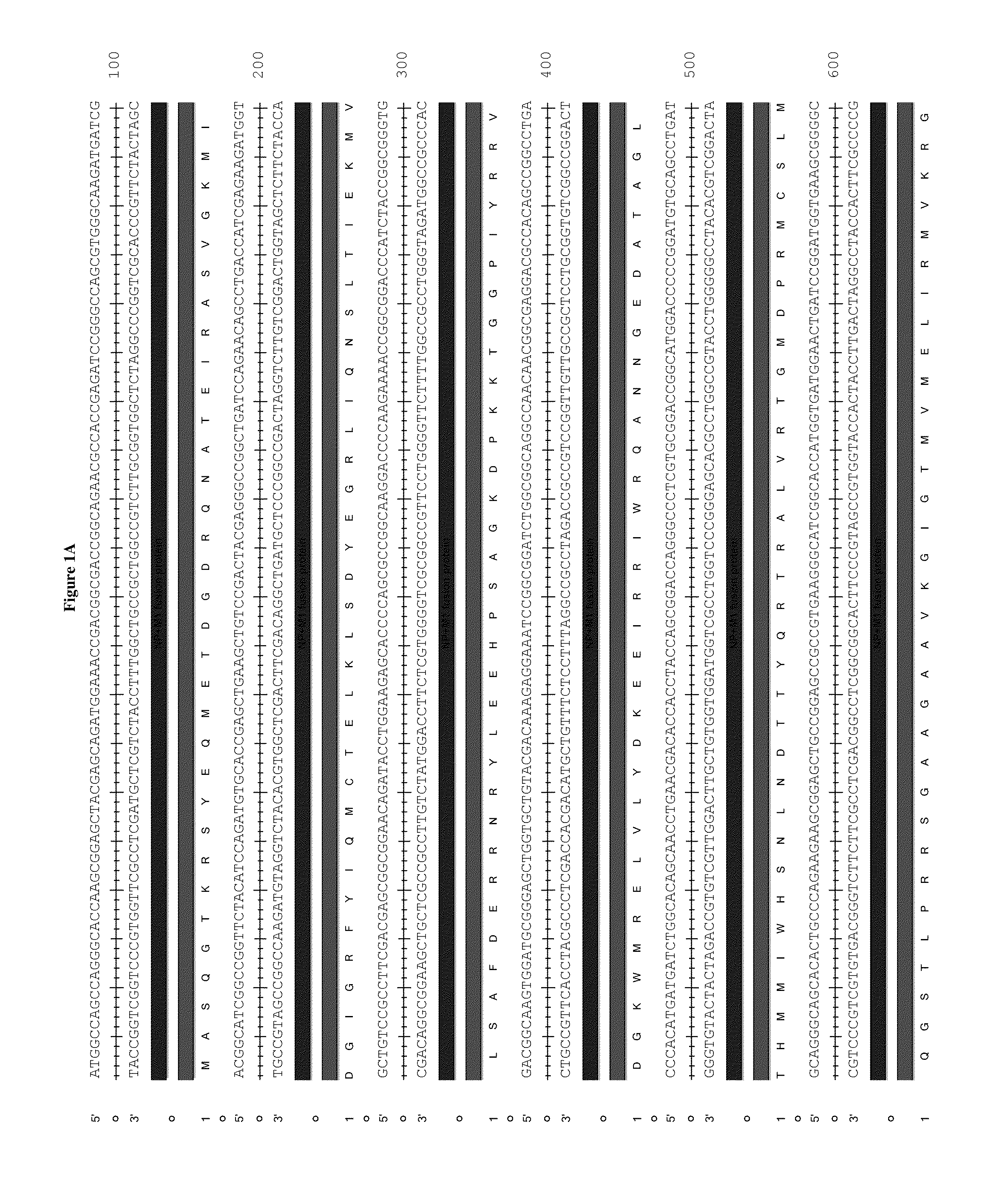
An immunotherapeutic strategy is disclosed that combines antigen-encoding DNA vaccine compositions combined with siRNA directed to pro-apoptotic genes, primarily Bak and Bax, the products of which are known to lead to apoptotic death. Gene gun delivery (particle bombardment) of siRNA specific for Bak and / or Bax to antigen-expressing DCs prolongs the lives of such DCs and lead to enhanced generation of antigen-specific CD8+ T cell-mediated immune responses in vivo. Similarly, antigen-loaded DC's transfected with siRNA targeting Bak and / or Bax serve as improved immunogens and tumor immunotherapeutic agents.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compositions and methods
ActiveUS20150110831A1High reactivityWell representedSsRNA viruses negative-senseViral antigen ingredientsEpitopeT cell mediated immunity
The invention relates to a composition suitable for inducing a T cell mediated immune response against an influenza virus in a vertebrate, said composition comprising nucleic acid encoding one or more epitopes of one or more internal proteins of influenza virus, wherein said composition comprises nucleic acid encoding at least two said epitopes, at least one epitope being from each of two or more internal proteins of influenza virus. The invention also relates to uses of same and to methods involving same.
Owner:OXFORD UNIV INNOVATION LTD
Compositions including different types of transfer factor, methods for making the compositions, and methods of treatment using the compositions
InactiveUS20050058716A1Reduce cleaning frequencyLess fattyMammal material medical ingredientsCapsule deliveryT cell mediated immunityComputational biology
A composition for eliciting a T-cell mediated immune response in a subject includes transfer factor from at least two different types of source animals. For example, the composition may include mammalian transfer factor and nonmammalian transfer factor. An example of the composition includes a combination of a colostrum-derived product, which includes the mammalian transfer factor, and an egg-derived product, which includes the nonmammalian transfer factor. Additionally, the egg-derived product may be substantially free of fat. Methods for forming the composition and eliciting T-cell mediated immune responses in subjects that have been treated with the composition are also disclosed.
Owner:4LIFE PATENTS
Antibody that binds domain 2 of ICAM-1 and methods of treatment
ActiveUS8900586B2Effective prevention and treatmentMinimize adverse effectsAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsDendritic cellT cell mediated immunity
The present invention relates to an antibody binding to the domain 2 of human intercellular adhesion molecules-1 (ICAM-1) where the antibody is able to modulate the differentiation status of dendritic cells and induce antigen-specific T cell tolerance, thereby be effective in the prevention and / or treatment of T cell-mediated immune disorders such as transplantation rejection, graft-versus-host disease, and autoimmune disease. In addition, the present invention provides a pharmaceutical composition comprising the antibody, and method of using them for the treatment of disease.
Owner:KUMHO HT +1
Anti-protozoal vaccine
Immunotherapy of protozoal diseases is provided by use of hypoxanthine guanine xanthine phosphoribosyl transferase protein, or peptide fragments thereof, as an immunogen in vaccines effective against protozoal diseases such as malaria and babesiosis. In particular, immunization with hypoxanthine guanine xanthine phosphoribosyl transferase or peptide fragments thereof, induces T cell immunity to blood stage malaria. In particular embodiments, the invention provides protein and DNA malaria vaccines and methods of prophylactic and therapeutic immunization that elicit T cell-mediated immune responses broadly applicable to protozoal diseases including malaria.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Methods of reducing T cell-mediated immune responses with multimeric P-selectin and/or E-selectin compounds
InactiveUS8628775B2Induce depletionCauses depletionPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseP-selectin
Multimeric compounds that bind to P-Selectin Glycoprotein 1 (PSGL-1) on the surface of T cells or natural killer (NK) cells can be used to induce T cell or NK cell depletion and / or to induce T cell or NK cell apoptosis. The multimeric compounds and methods of the invention can be used to control unwanted T cell- or NK cell-mediated immune responses in conditions such as inflammatory diseases, autoimmune diseases, transplant rejection, and allergic diseases.
Owner:ABGENOMICS COOPERATIEF U A
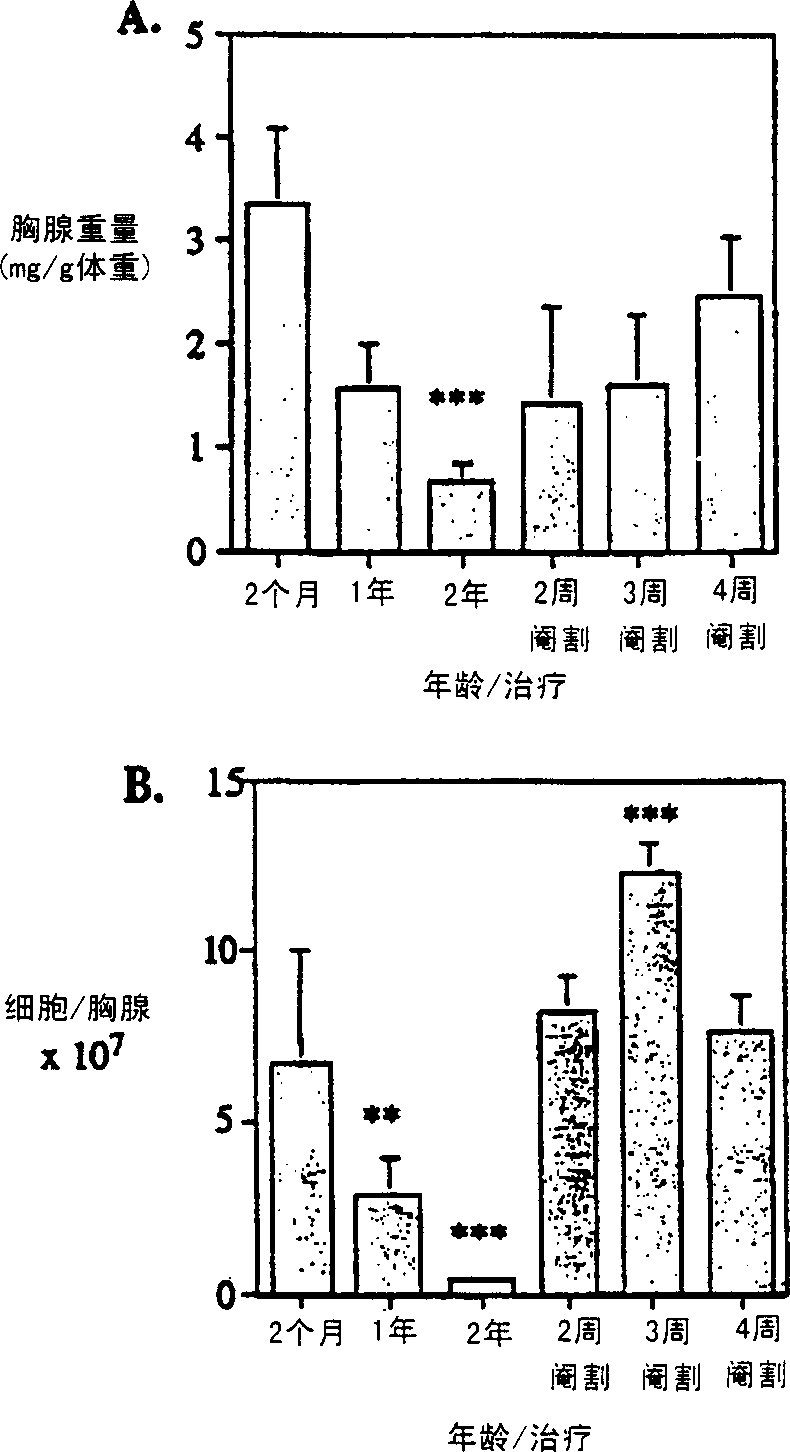
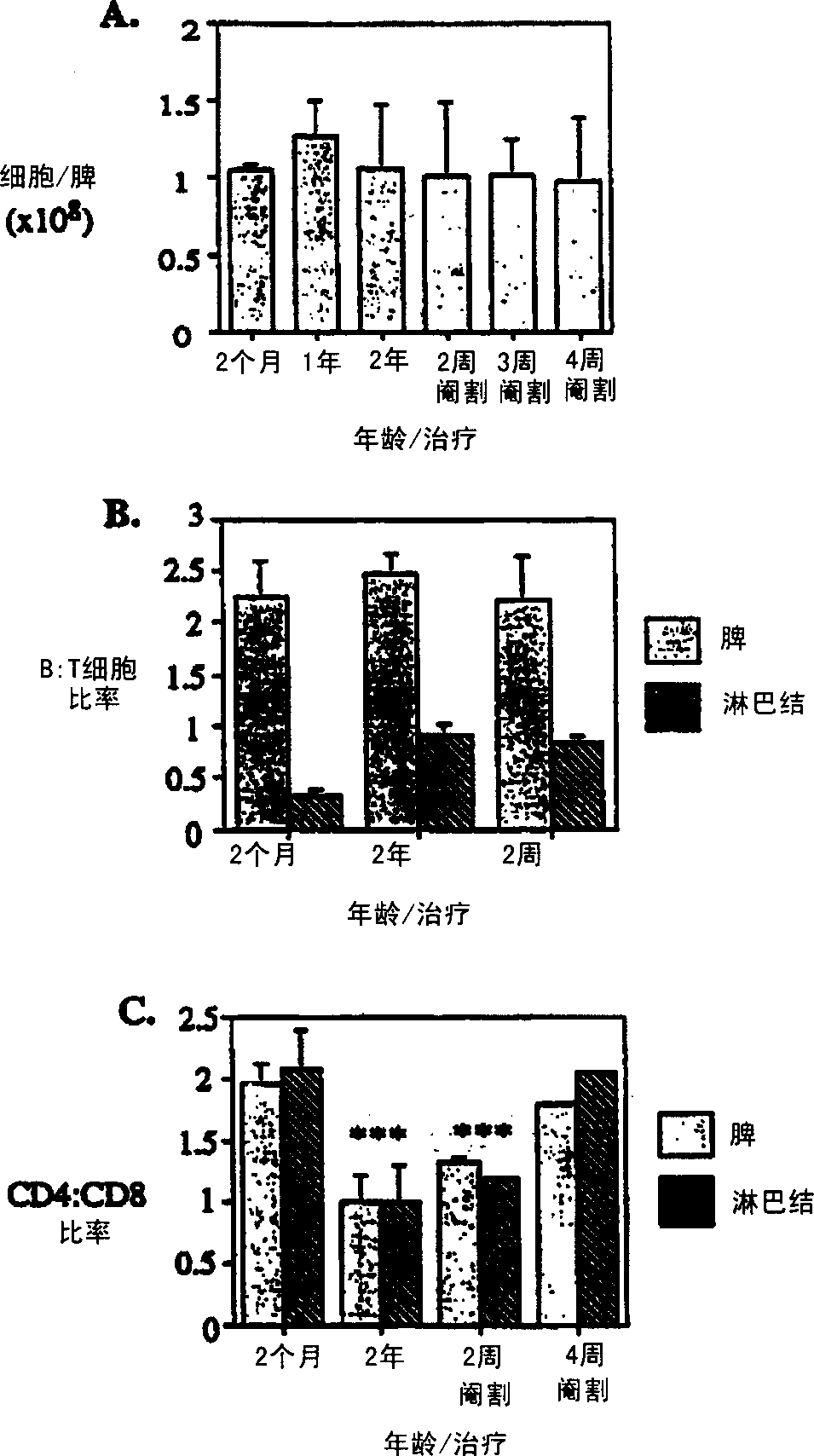
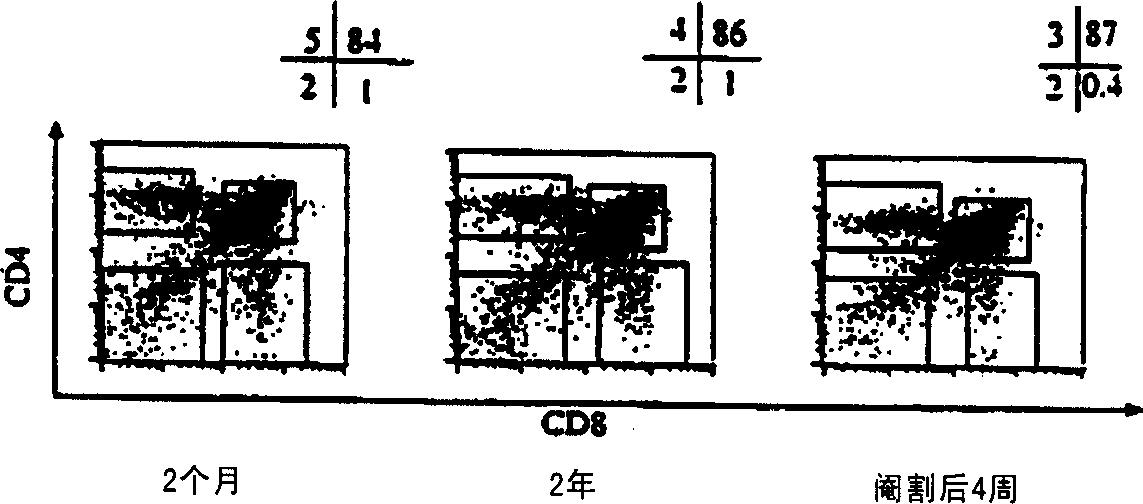
Improvement of T cell mediated immunity
InactiveCN1364093ATake good carePromote differentiationPeptide/protein ingredientsAntiviralsAntigenSterol
The present invention provides a method of modifying the T-cell population makeup or increasing the number of T-cells in a subject having depressed or abnormal T-cell population or function, the method comprising disrupting sex steroid signalling to the thymus in the subject. The invention can be used to treat a subject suffering from a wide array of diseases, for example, cancer, HIV infection, autoimmunity and hypersensitivity. In addition, the present invention provides methods for enhancing an immune response to an antigen, treating an autoimmune disease, and decreasing a host-vs-graft reaction in a transplantation donor.
Owner:NORWOOD IMMUNOLOGY
Anti-cd3 therapies
InactiveUS20140141020A1Reduce severityReduce the possibilityMetabolism disorderAntibody ingredientsT cell mediated immunityDocument preparation
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com