Improvement of T cell mediated immunity
A cell and cell group technology, which can be used in medical preparations containing active ingredients, endocrine system diseases, peptide/protein components, etc., and can solve problems such as memory cell deviation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
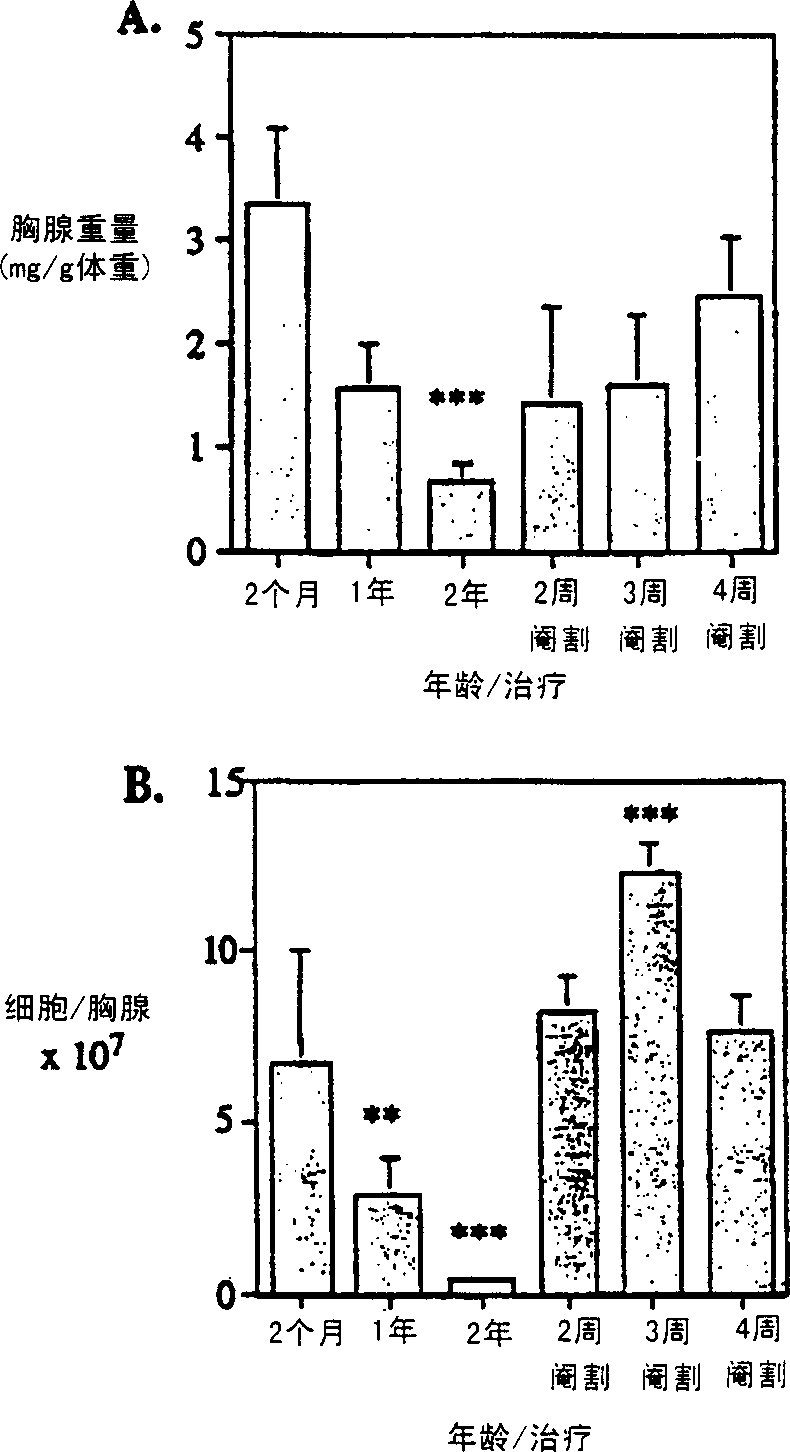
[0071] EXAMPLES Example 1: Reversal of Age-Induced Thymic Atrophy Materials and Methods Animals
[0072] CBA / CAH and C57B16 / J male mice were obtained from CentralAnimal Services, Monash University, and housed under conventional conditions. Ages range from 4-6 weeks to 26 months and are indicated where relevant. castration
[0073] By intraperitoneal injection of 0.3 mL of 0.3 mg xylazine (Xylazine; BayerAustralia Ltd., Botany, New South Wales, Australia) and 1.5 mg of ketamine hydrochloride (Cordamine; Parke-Davis, Caringbah, New South Wales, Australia) Anesthetize the animal with a solution in saline. Surgical castration is performed by incision of the scrotum to expose the testes, which are ligated with sutures and then removed along with the surrounding fatty tissue. Bromodeoxyuridine (BrdU) introduction
[0074] Mice received two intraperitoneal injections (100 mg / kg body weight in 100 microliters of PBS) of BrdU (Sigma Chemical Co., St. Louis, MO) at 4 hour intervals....
Embodiment 2
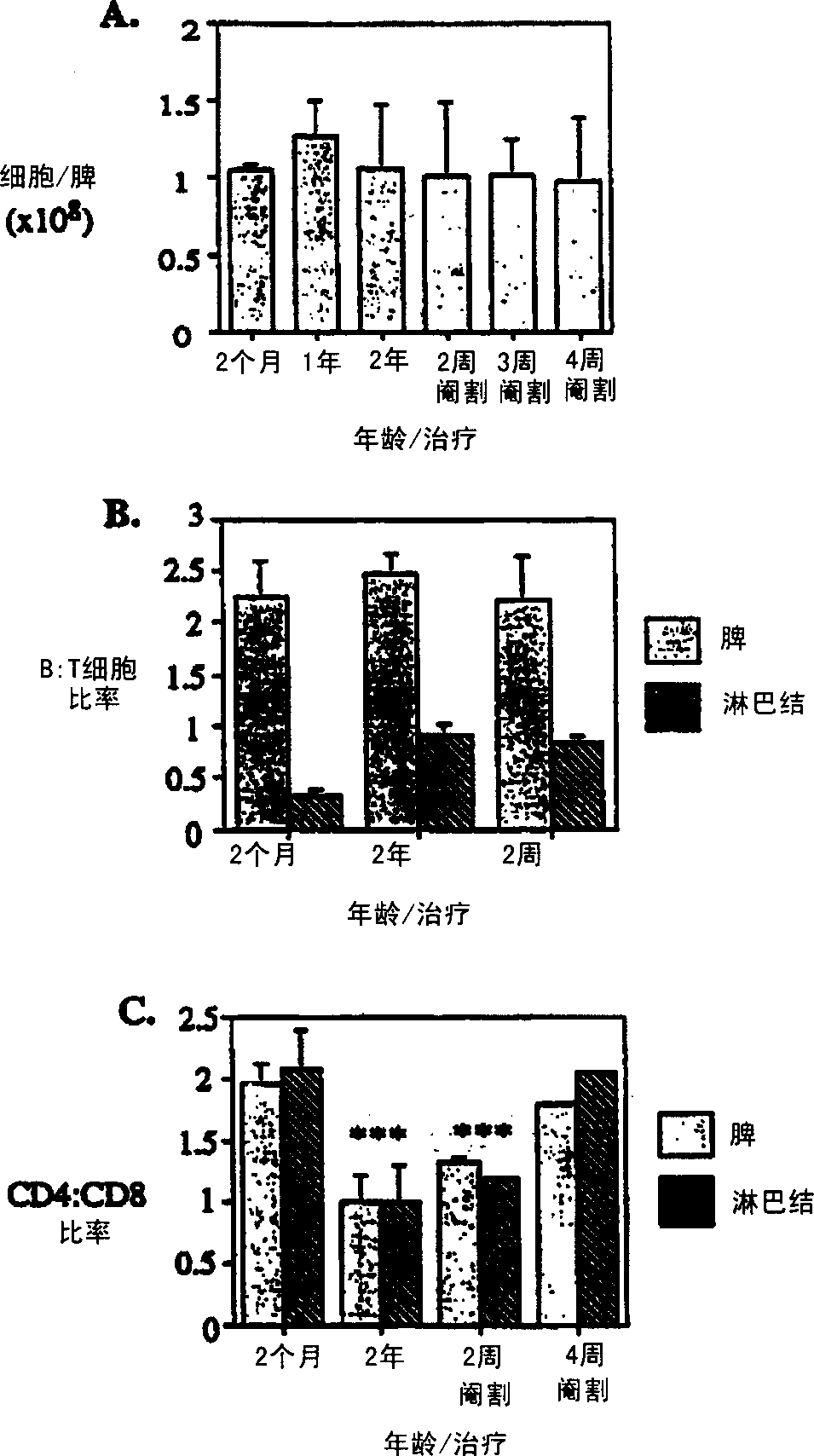
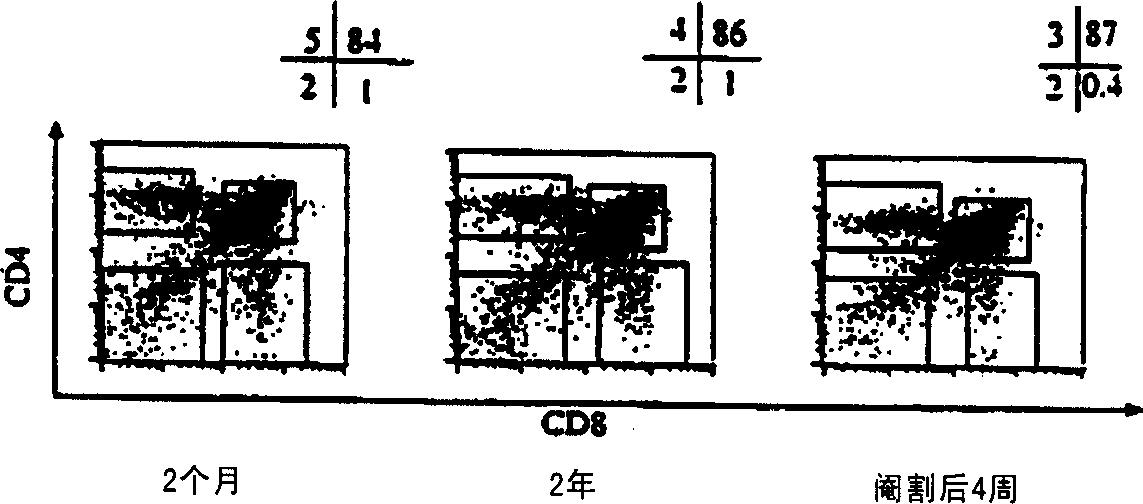
[0105] Approximately 1% of T cells migrate out of the thymus per day in juvenile mice (Scollay et al., 1980). We found that, although significantly (p≤0.001) less in number, migration occurred at the same rate as normal juvenile mice at 14 months and even 2 years of age ( Figure 5 ). The CD4:CD8 ratio of newly migrated thymic cells increased from about 3:1 at 2 months to about 7:1 at 26 months. By 1 week post-castration, the number of cells migrating to the periphery increased significantly, while the overall migration rate remained unchanged at 1-1.5%. Example 2: Reversal of Chemotherapy or Radiation Induced Thymic Atrophy
[0106] Following radiation or cyclophosphamide treatment, the rate of thymus regeneration was significantly increased in castrated mice (one week before or on the day of treatment).
[0107] In the thymus, the structure of the irradiated mouse thymus was severely disrupted, accompanied by a decrease in rapidly dividing cells. Cortical collapse, remin...
Embodiment 3
[0110] Figure 9 To illustrate the use of chemical castration in promoting T cell regeneration compared to surgical castration. The kinetics of chemical castration are much slower than surgical castration, that is, it takes about 3 more weeks for mice to reduce their circulating steroid levels. However, chemical castration is still effective in regenerating the thymus, as Figure 9 shown. Example 3: Thymus regeneration following sex steroid suppression leads to restoration of deficient peripheral T cell function
[0111] To determine whether castration can enhance the immune response, herpes simplex virus (HSV) immunity was tested, as it allows the study of disease progression and the role of CTL (cytotoxic) T cells. Castrated mice had qualitatively and quantitatively improved reactivity to the virus. Mouse footpads were immunized and popliteal (draining) lymph nodes were analyzed on day 5 post immunization. Additionally, footpads were taken and homogenized to determine v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com