Compositions and Methods
a technology of immune response and composition, applied in the field of composition and method, can solve the problems of inability to generate protective cross reactivity from strain to strain, approach would not be expected to work, and the vaccine designer is constantly struggling to keep up, etc., to achieve excellent cross reactivity, assess cross reactivity or the breadth of coverage, the effect of easy characterisation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
T Cell Inducing Vaccines for Flu
[0142]Rationale:
Nucleoprotein (NP) identity
92% between H3N2 and H1N1 strains
91% between H3N2 and H5N1 strains
M1 (matrix) identity
95% between H3N2 and H1N1 strains
93% between H3N2 and H5N1 strains
Vaccine Design: MVA-Flu
Antigen: NP:M1 Fusion Protein
[0143]H3N2 sequence used
Flexible linker between NP and M1
total coding sequence of 758 amino acids
Inserted at TK locus of MVA
No marker gene
[0144]Most adults have been exposed to influenza and have some T cell memory against NP and M1. Immunisation with recombinant MVA expressing NP and M1 boosts these to potentially protective levels.
[0145]The vaccine is GMP manufactured.
[0146]Phase I trial uses healthy volunteers, tests T cell responses before and after immunisation with MVA.
[0147]Dose comparison 5×107 pfu i.d. (n=12) and 2.5×108 pfu i.d. (n=12)
Phase IIa Study
[0148]Choose immunisation dose according to above.
[0149]Immunise 12 people with low antibody titres
[0150]Challenge these 12 plus 1...
example 2
Preferred Epitope Construct
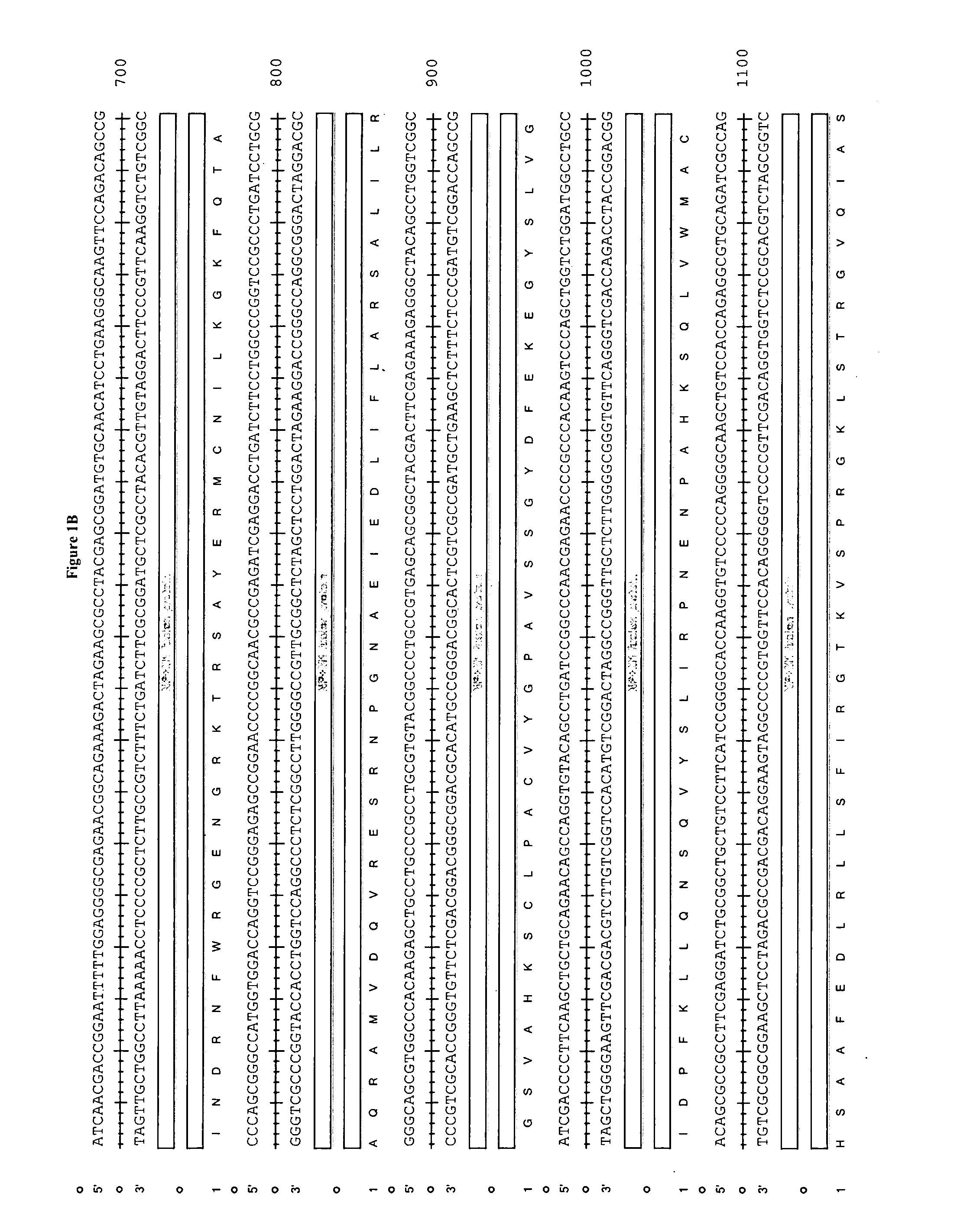
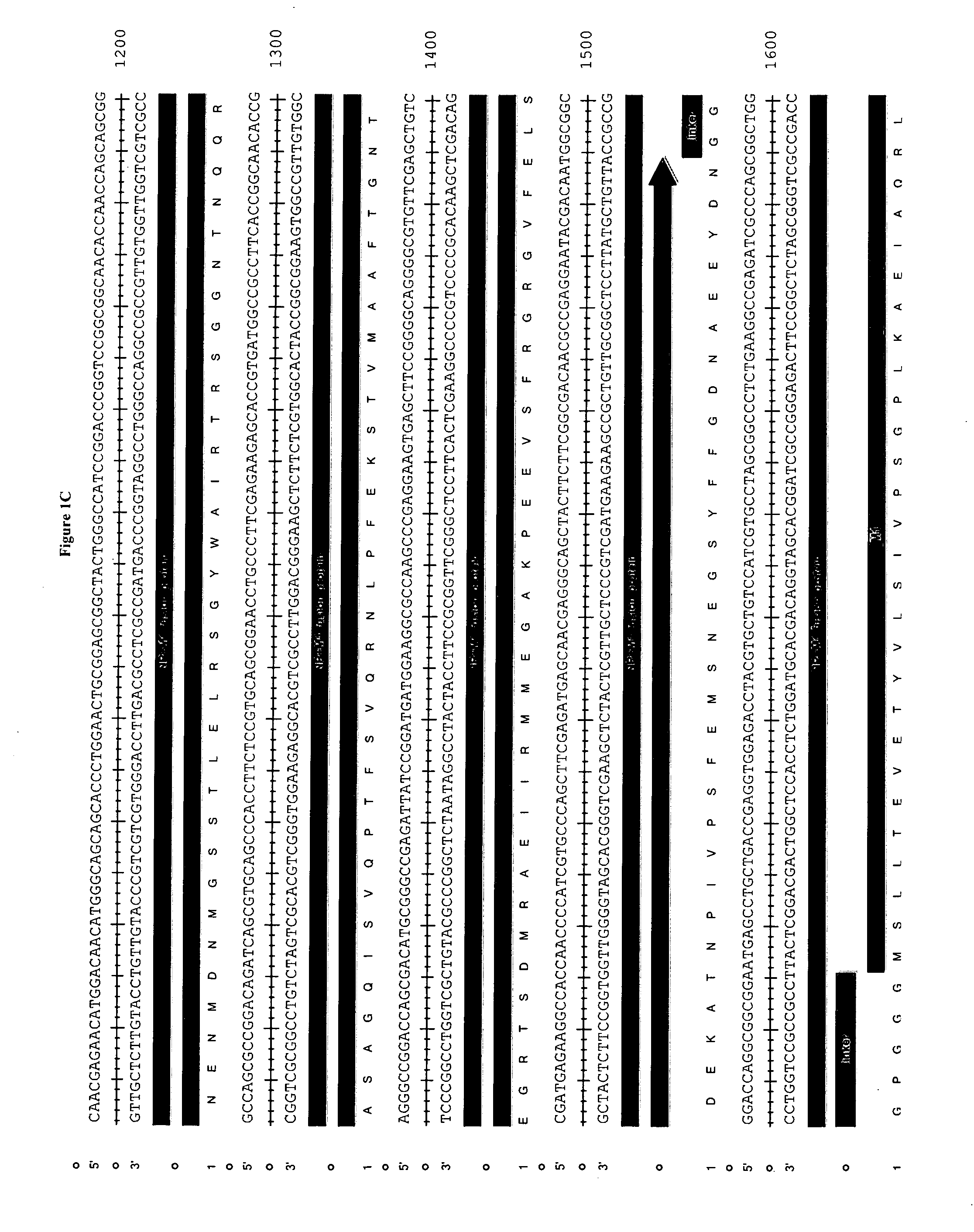
[0152]The epitope construct is prepared according to the sequence shown in FIG. 1. In this example the nucleic acid is synthesised in vitro and is then ready for cloning into a delivery system such as a viral vector eg. MVA.
example 3
Immunogenicity of MVA-NP+M1 in Mice
[0153]Groups of four mice BALB / c mice were immunized with either 1×106 pfu MVA-NP+M1 intradermally, or 50 μg of a DNA vaccine expressing the same NP+M1 insert intramuscularly followed by 1×106 pfu MVA-NP+M1 intradermally two weeks later. Two weeks after the MVA immunisation the spleens were tested for T cell responses to the immunodominant peptide TYQRTRALV (NP amino acid residues 147-155) in an interferon-gamma ELISPOT assay as described in Schneider, J., et al., (1998) Nat Med 4, 397-402.
[0154]Results are plotted in FIG. 2 as the mean of four mice, and the response generated by vaccination to peptide TYQRTRALV (flu peptide) are shown compared to the non-specific background response (no peptide). In these mice which have not been previously exposed to influenza, MVA is able to prime a T cell response to NP, as shown by the MVA alone group. In mice which are first primed using a DNA vaccine, the response is boosted to a higher level by MVA-NP+M1.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com