Adjuvant combinations of liposomes and mycobacteriaial lipids for immunization compositions and vaccines
a technology of liposomes and mycobacteria, which is applied in the direction of antibacterial agents, antibacterial antigen ingredients, antibody medical ingredients, etc., can solve the problems of not always providing satisfactory resistance to human tb in every population, inducing a sufficient immune response to confer protection, and many highly purified substances are not very immunogenic. , to achieve the effect of synergistic adjuvant effect of dda and increased immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
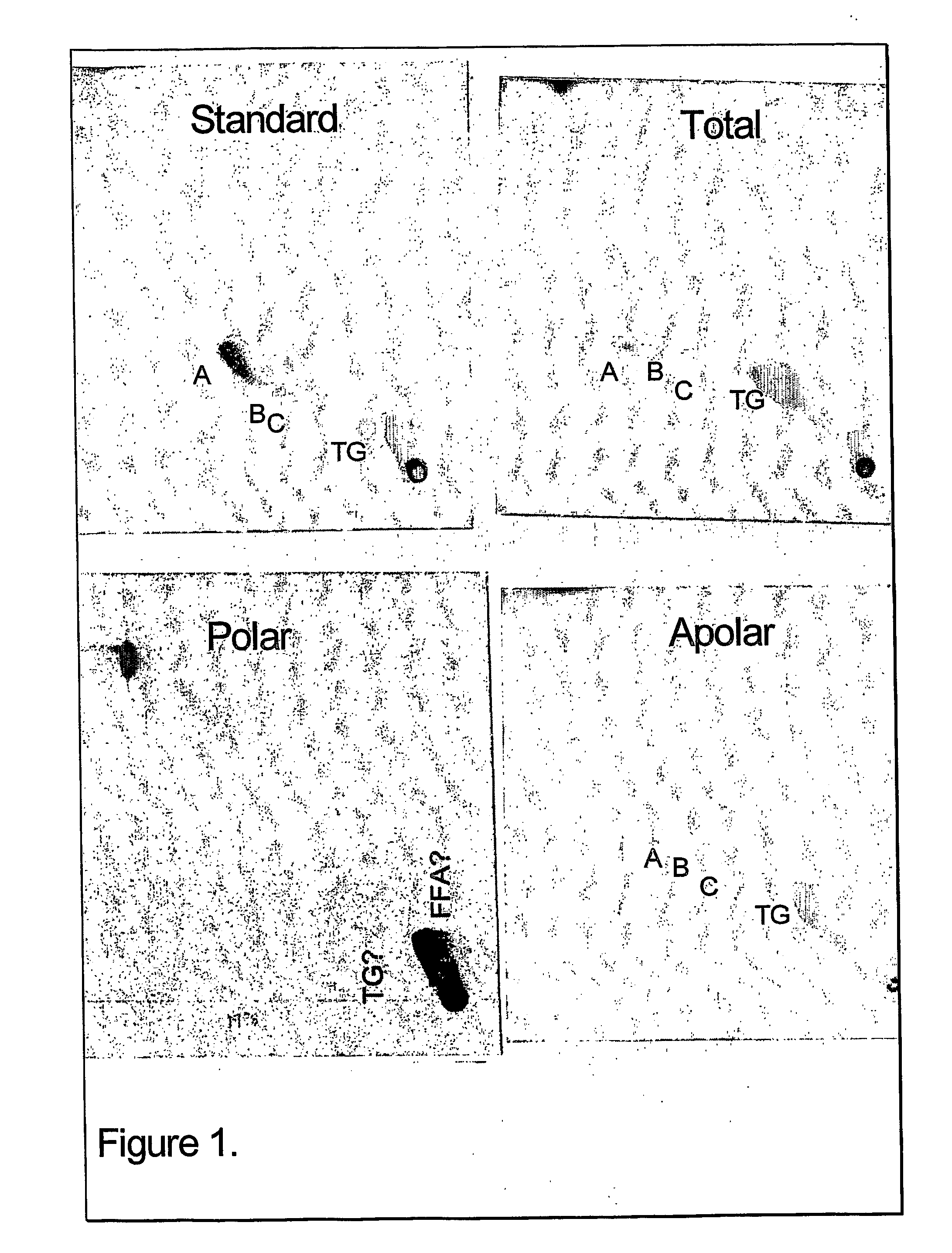
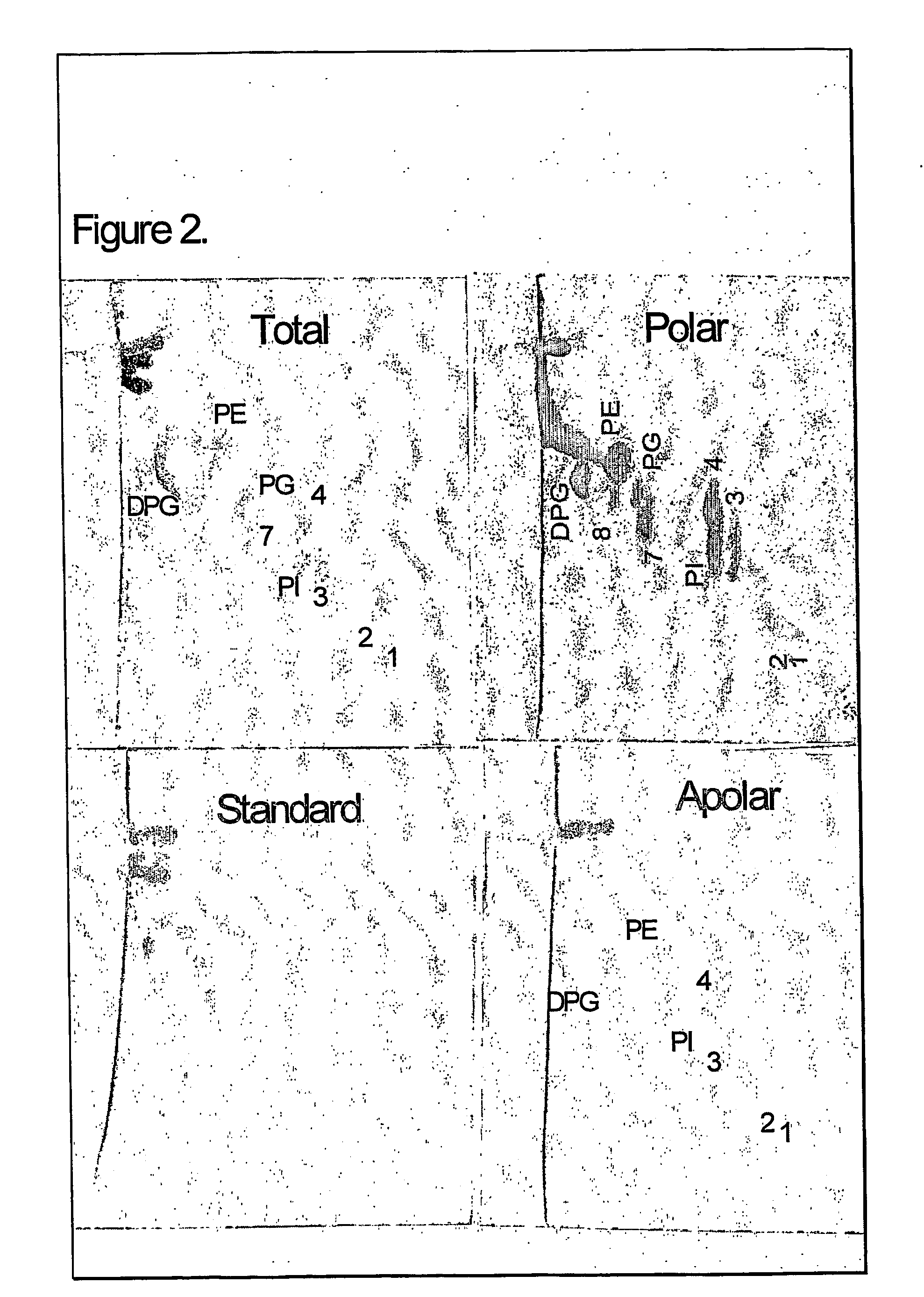
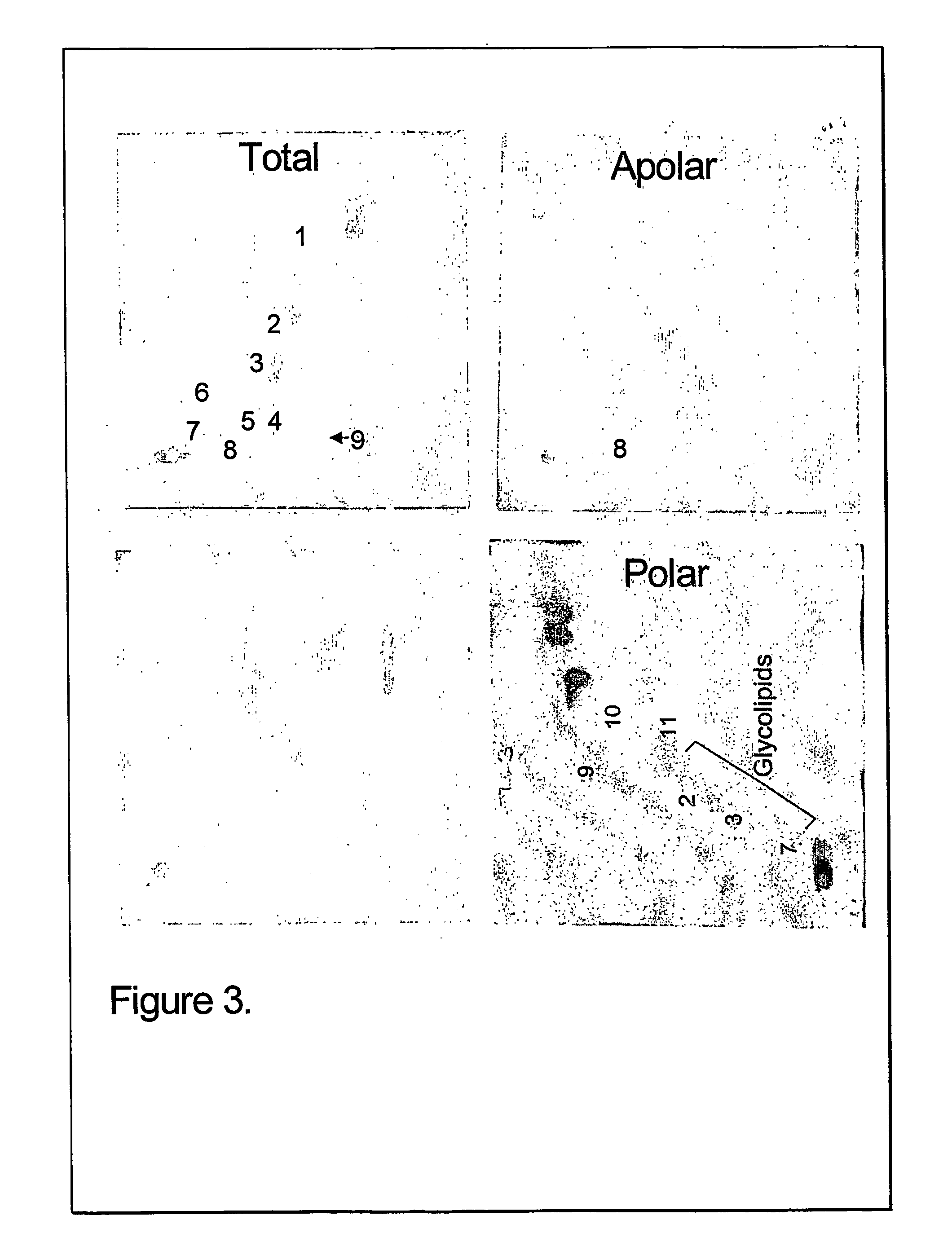
TLC of Total, Polar and Apolar Lipids from M. bovis BCG
[0092] The apolar lipids identified in the total extract were phthiocerol A dimycocerosate (A), phthiocerol C dimycocerosate (C), and a small amount of phthiocerol B dimycocerosate (B). Large amounts of triacylglycerol (TG) were also detected. These lipids were also detected in the apolar extract as expected. In the polar extract, only a trace of TG and a spot probably representing free fatty acids (FFA) were detected (FIG. 1).
[0093] The total and polar extract contained the following polar lipids: Phosphatidyinositol mannosides, probably triacylated (1) and tetracylated (2) pentamannosides, and triacylated (3) and tetracylated (4) dimannosides. Phosphatidylinositol (PI), phosphatidylethanolamine (PE) and diphosphatidylglycerol (cardiolipid, DPG) were major phospholipids. Small amounts of L-alpha-Phosphatidyl-DL-glycerol sodium salt (PG) and another phospholipid (7) were also present A glycolipid (8) was also observed in the ...
example 2
Use of Total Lipids from M. bovis BCG for Immunization of Mice
[0096] In this example studying the adjuvant activity of M. bovis BCG total lipids, mice were immunized with experimental vaccines consisting of 1 microgram of fusion protein Ag85B-ESAT6 emulsified in DDA adjuvanted with 100 μg of total lipids. Groups of mice receiving the Ag85B-ESAT6 alone, the Ag85B-ESAT6 and DDA alone, the Ag85B-ESAT6 in combination with the total lipids and a group of naïve mice was included as negative controls, while Ag85B-ESAT6 / DDA / TDB and a standard BCG vaccine were included as positive controls. This experiment was carried out using Th2 prone Balb / C mice (Peftoniemi et al, 2002), as only remarkably potent Th1-inducing adjuvants are capable of switching the immune response of this mouse strain in favour of a response characterized by production of Th1 cytokines such as IFN-gamma.
[0097] Five weeks after the first immunization, the IFN-gamma release was evaluated after in vitro re-stimulation of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Polarity | aaaaa | aaaaa |
| Antigenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com