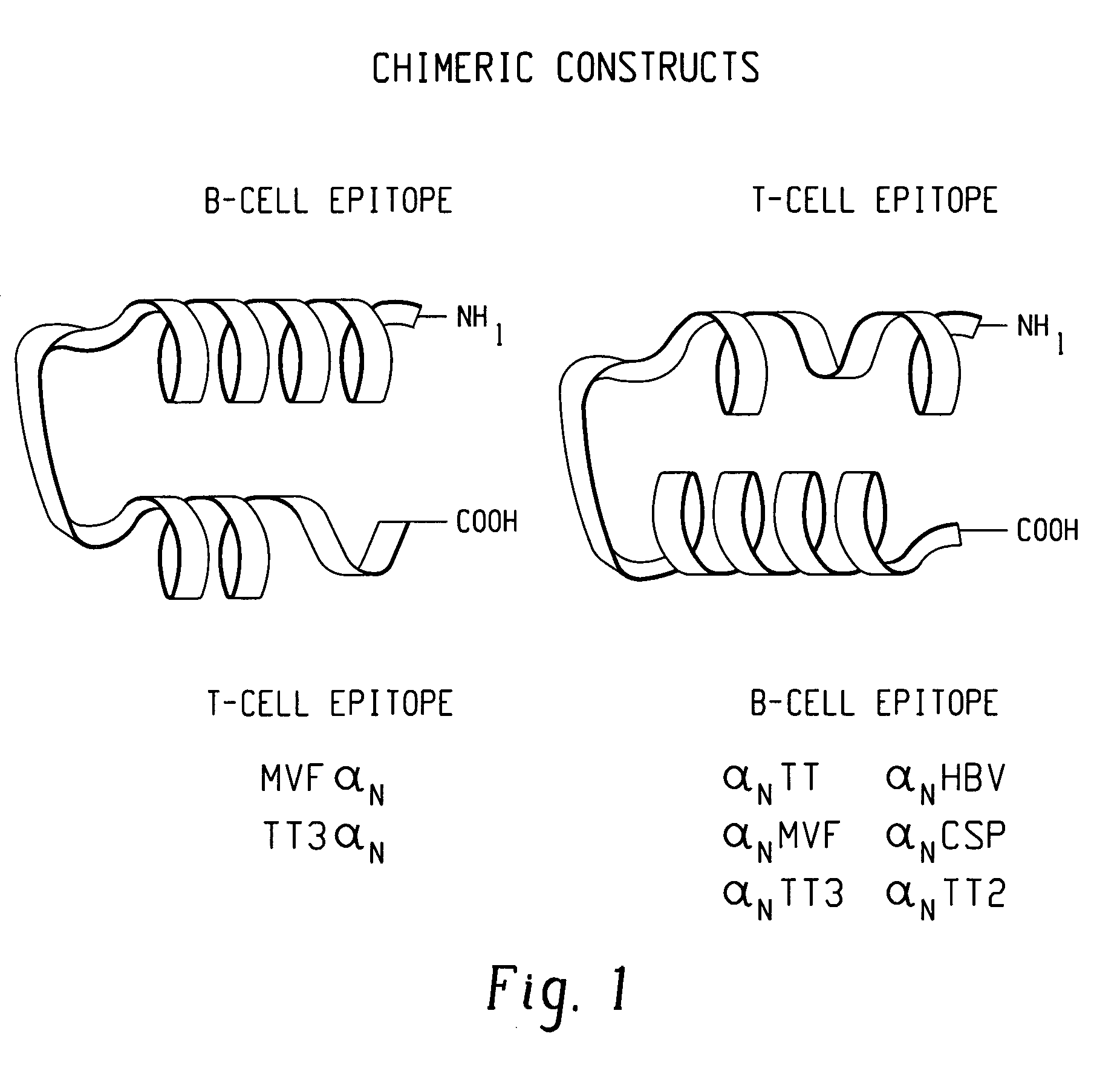
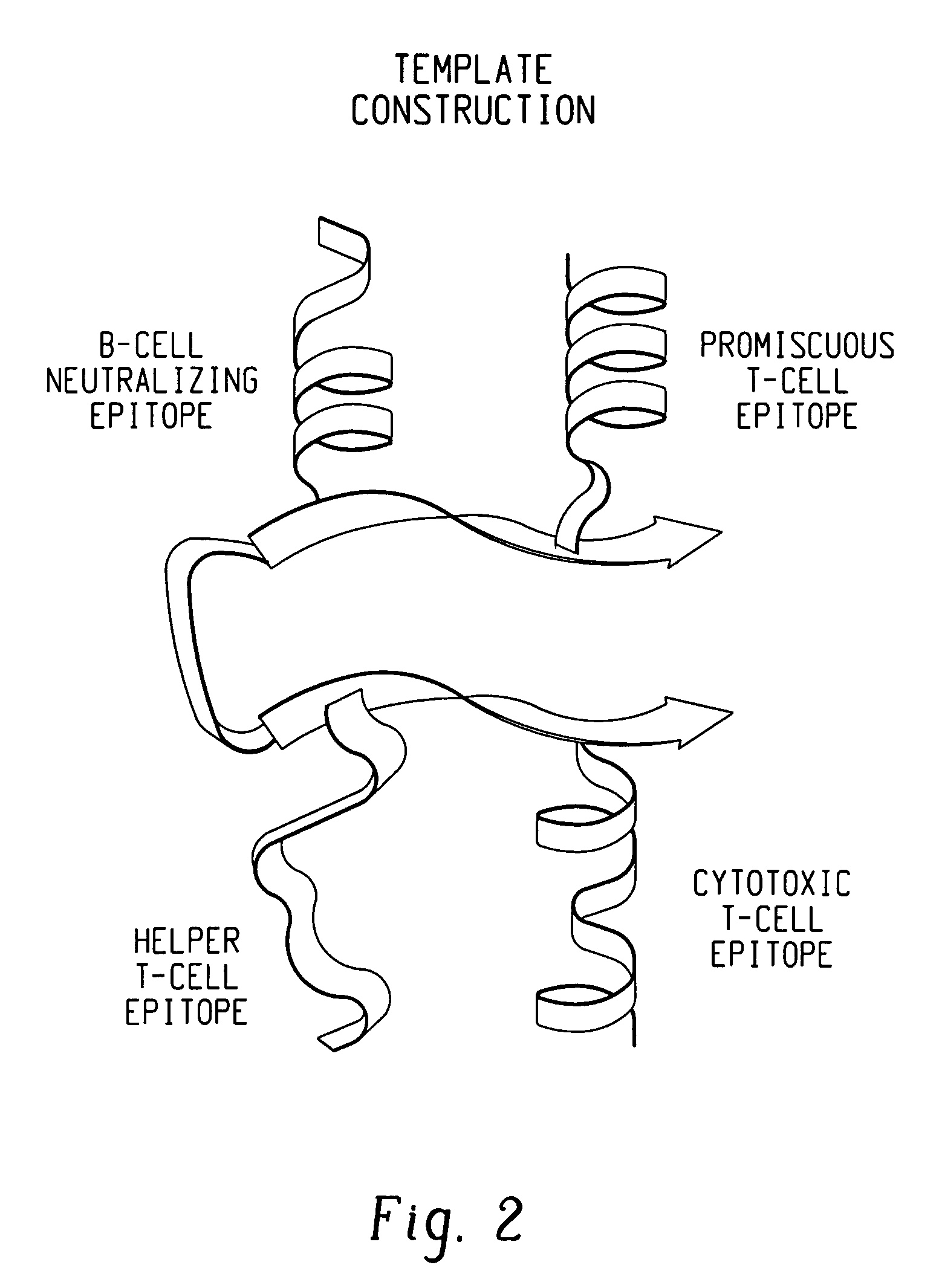
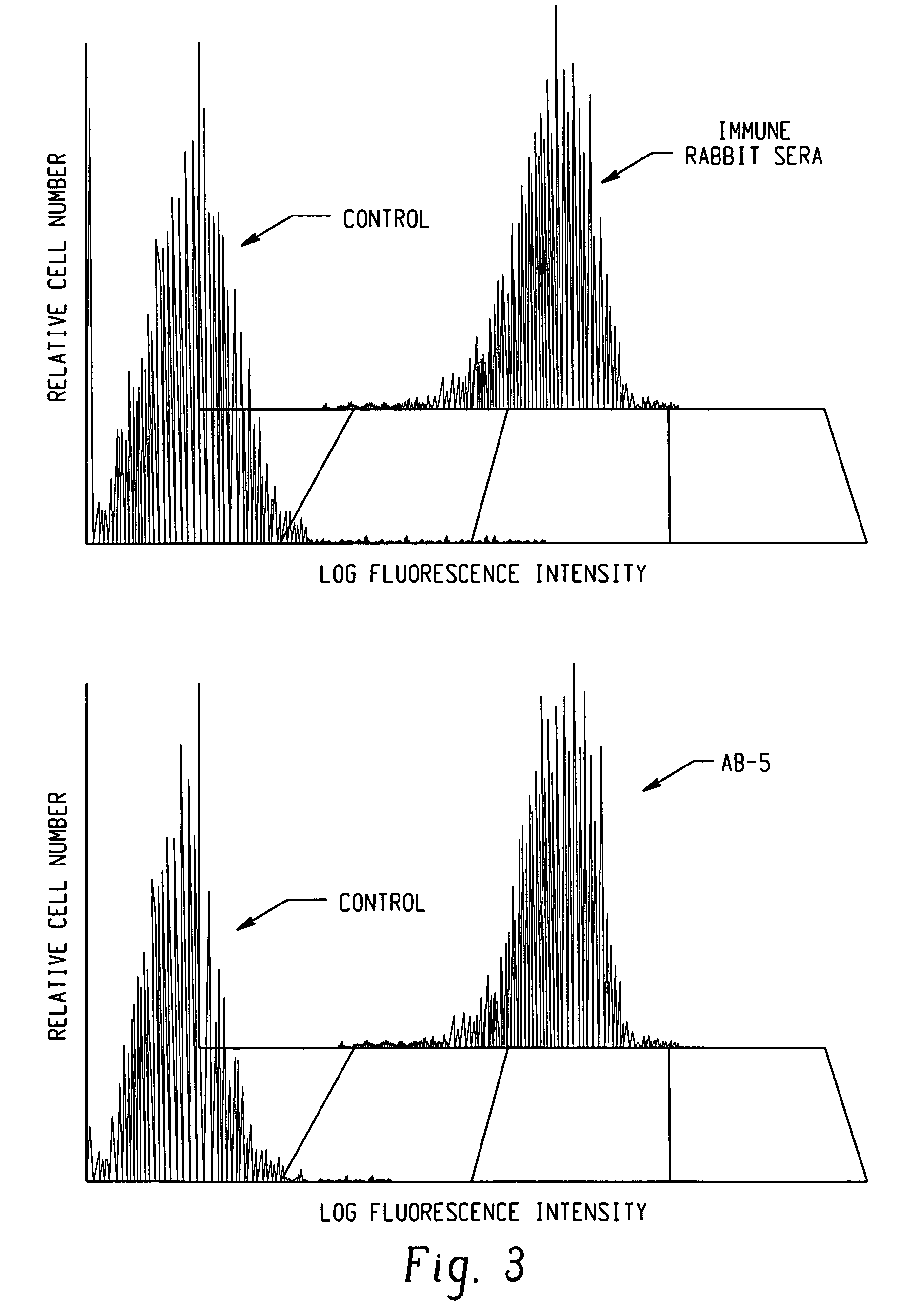
Polypeptides and polynucleotides for enhancing immune reactivity to HER-2 protein
a technology of polynucleotide and immune reactivity, which is applied in the field of polypeptides and polynucleotides for enhancing immune reactivity to her2 protein, can solve the problems of skin sensitivity or itchiness, interference with the immune system, and surgery alone cannot eliminate cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Peptide DWI MVF; HER-2 (376–395) MVF
[0092]The epitope, named DW1, comprises amino acids 376–291 of the HER-2 protein linked to the promiscuous Th cell epitope MVF. DW1 is predicted to be α-helical with a slight turn propensity.
[0093]Synthesis: The 20 amino acid HER-2 sequence was attached to the N-terminus of MVF 288–302 by the four amino acid linked sequence Gly-Pro-Ser-Leu SEQ ID NO. 20. The resulting peptide was named DW1MVF indicating DW1 placement at the N-terminus as opposed to MVFDW1 which would represent C-terminal position. The first amino acid was joined manually and completion monitored by Kaiser ninhydrin test. Subsequent couplings were performed on the Milligen / Biosearch 9600 peptide synthesizer. After final deblocking the peptide was cleaved from the resin with Reagent PU. An extended cleavage time is necessary since the peptide contains Arg-2,2,5,7,8-pentamethylchroman-6-sulfonyl(PMC) and His. (A yellow cleavage solution is observed when histidine is present).
[0094]Pu...
example 2
Peptide MVFDW4: HER-2 (628–647) MVF
[0096]MVFDW4 comprises an altered sequence of the peptide extending from amino acid 628 through 647 of the HER-2 protein. The native sequence contains 3 cysteine residues whose disulfide bonding pairs are unknown. Since the cysteines at position 634 and 642 had the potential to form a bridge, Cys 630 was substituted with Gly. Substituting glycine for cysteine is one way to preserve the relative size of the R group at that position. Synthesis proceeded by first making the DW4 (628–647) peptide attached to the linker then extending the sequence N-terminally by addition of the NWF (288–302) T helper cell sequence. This produced the MVFDW4 peptide.
[0097]In order to create the disulfide bond, the tBut protecting group was cleaved giving the free thiol form. The mercuric acetate / 2-mercaptoethanol procedure reduces production of disulfide bonded multimers. Analytical HPLCs of the crude product and samples were compared. In the crude sample, two sharp peak...
example 3
Peptide DW5MVF; HER-2 (115–136) MVF
[0100]Synthesis: MVF 288–302 plus the four residue amino acid linker was connected to the resin as described above in example 1 and the sequence continued with amino acids 115–136 of the HER-2 protein. This produced the peptide DW5MVF. The sequence is predicted to be a β-turn with high aggregation potential. This necessitated double coupling critical residues A115, V116, T127, V129 and S133.
[0101]Purification and characterization: DW5MVF was cleaved and extracted with ether and water. Extraction was quite difficult as the peptide formed dense, sticky aggregates which were only minimally soluble by addition of acetic acid. Analytical HPLC of the crude sample showed one predominant peak with a minor doublet. Semipreparative HPLC was used to separate the doublet. The lyophilized sample was readily dissolved in dilute acetic acid for analytical HPLC. A sample was subjected to time of flight mass spectrometry and yielded a molecule of the correct molecu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| path length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com