Muc1-specific CAR-T cells capable of stably expressing PD-1 antibodies and usage thereof
A PD-1, cell technology, applied in the direction of targeting specific cell fusion, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, DNA/RNA fragments, etc., can solve the problem of expensive treatment, toxic Side effects, high cost of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
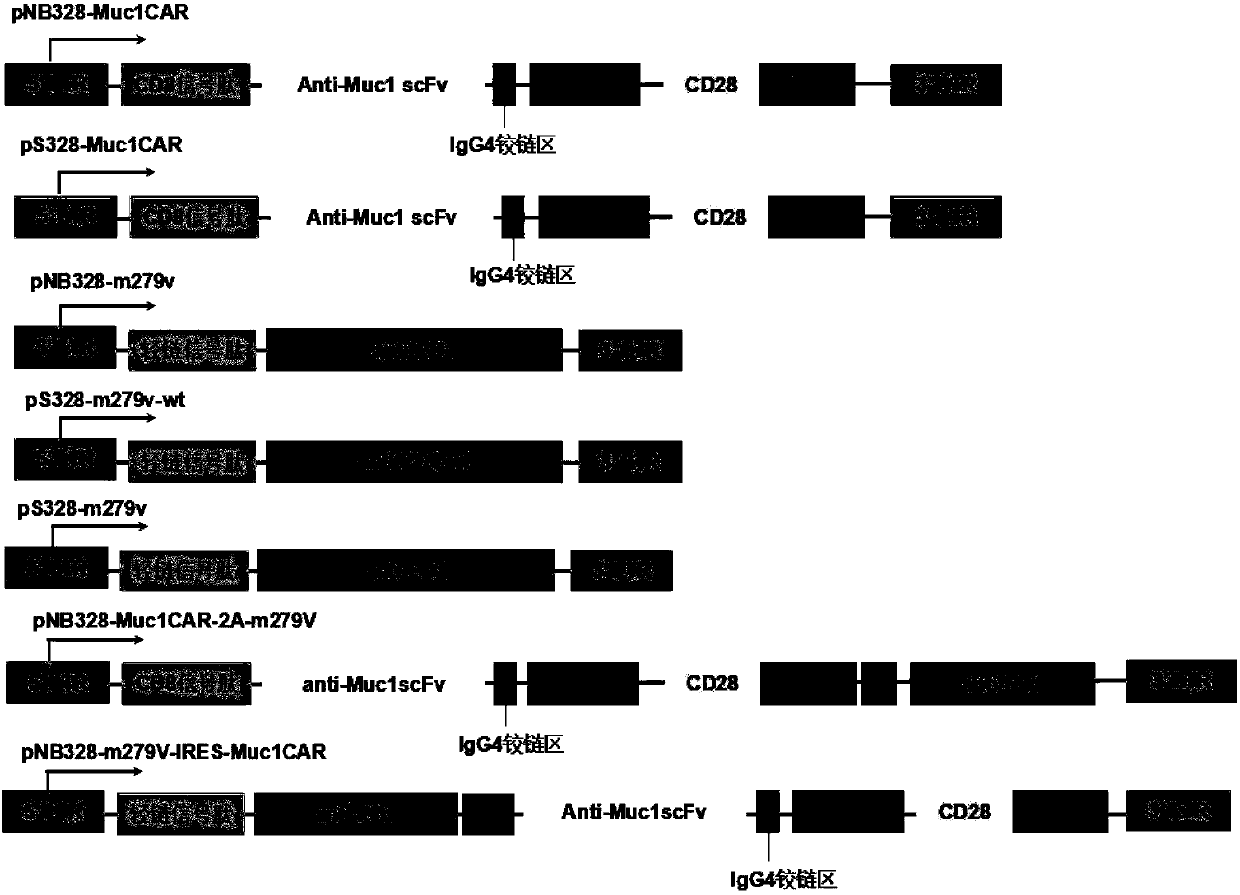
[0103] Example 1: Recombinant plasmids pNB328-Muc1CAR, pS328-Muc1CAR, pNB328-m279V, pS328- Construction of m279V-wt, pS328-m279V, pNB328-Muc1CAR-2A-m279V, pNB328-m279V-IRES-Muc1CAR and the acquisition of various types of pluripotent T cells.
[0104] 1. Construction of recombinant plasmids
[0105] Entrusted Shanghai Jierui Biological Company to synthesize Muc1CAR exogenous gene (including CD8 signal peptide, antigen recognition single-chain antibody, IgG4CH2CH3 hinge region, CD28 transmembrane region, CD28 intracellular domain and CD3ζ tyrosine activation motif, its nucleotide The sequence is shown in SEQ ID NO: 14, the encoded amino acid sequence is shown in SEQ ID NO: 13), and a multiple cloning restriction site (BglII-XbaI-EcoRI-BamHI) is introduced upstream, and the enzyme is inserted downstream Cutting site (SalI-NheI-HindIII-SpeI), which is loaded into the pNB328 vector or pS328 vector cut with EcoR1+SalI double enzymes (for the structure and sequence of pNB328, p...
Embodiment 2
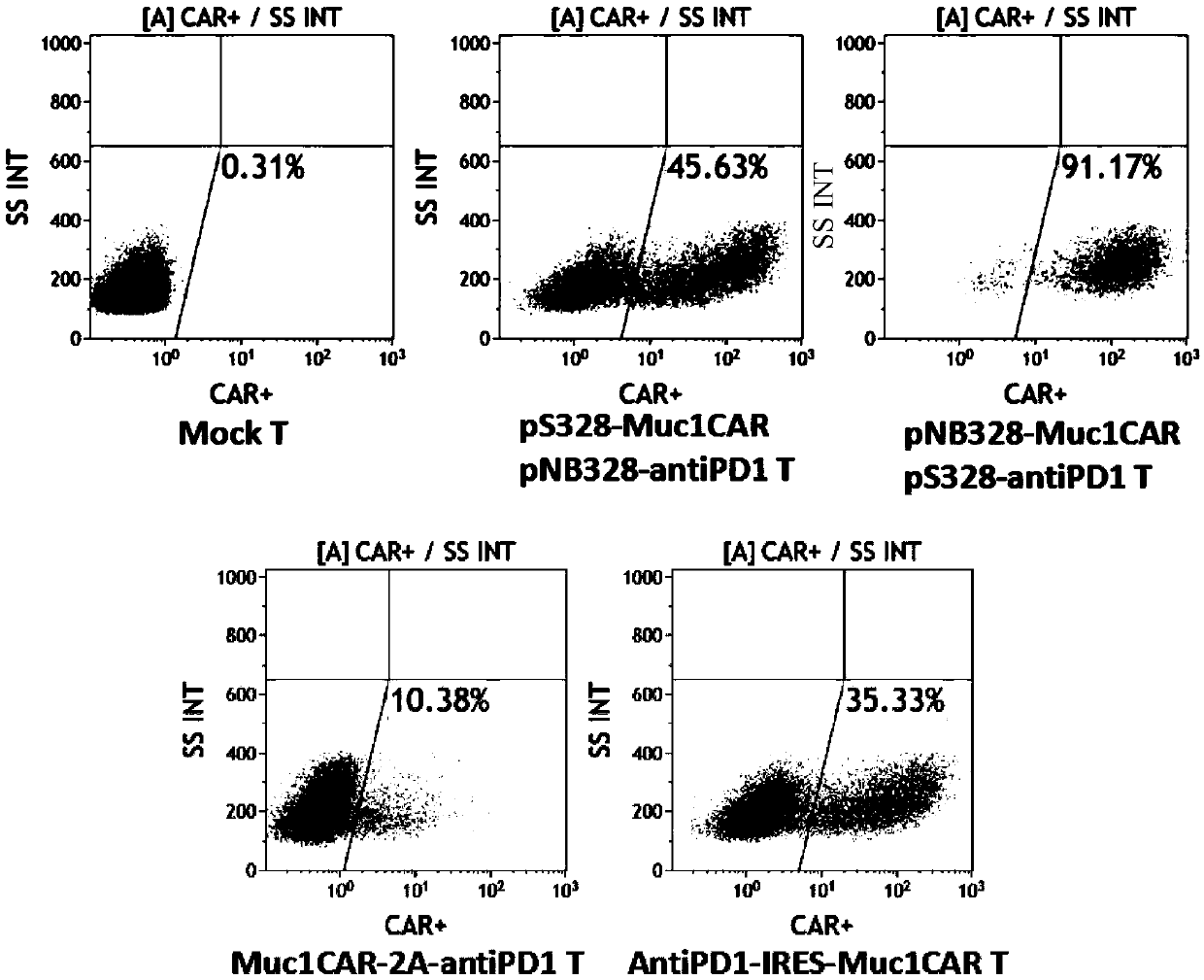
[0113] Example 2: Expression of PBMCs modified and activated by different combinations of Muc1CAR gene and PD-1 antibody gene The positive rate of Muc1CAR gene and the expression of PD-1 antibody were determined.
[0114] The activated T cells of different types and origins from different patients constructed above were cultured on the 12th day after electroporation according to 1×10 6 Cell pellet, wash cell pellet 2 times with PBS, add 2.5ul Biotin-Muc1 antibody, incubate at 4°C for 30min, wash cell pellet 2 times with PBS, add 2ul PE-streptomycin secondary antibody, wash cell pellet 2 times with PBS, add 400ul PBS was used to transfer the cells to flow tubes for detection on the machine. Similarly, collect 2×10 6 Cell number and press 2×10 6 Cells / well were spread in a 6-well plate with 3ml of AIM-V culture medium, cultured in a 5% CO2 incubator at 37°C, and the cell supernatant was collected after 24 hours of culture, and stored at -20°C for future use. Double-antibo...
Embodiment 3
[0117] Example 3: Expression of PBMCs modified with different mass ratios of pNB328-Muc1CAR and pS328-m279V plasmids Determination of Muc1CAR gene positive rate and PD-1 antibody expression
[0118]As described in Example 1, the recombinant plasmids pNB328-Muc1CAR and pS328-m279V were electrotransfected into PBMCs cells at different mass ratios (1:1, 3:5, 1:3, 1:7), and multiple simultaneous expression of Muc1CAR gene and PD -1 antibody T cells. On the 12th day after electroporation, these T cells were divided into 2×10 6 Cell number The cells were collected and divided into 2×10 6 Cells / well were spread in a 6-well plate with 3ml of AIM-V culture medium, cultured in a 5% CO2 incubator at 37°C, and the cell supernatant was collected after 24 hours of culture, and stored at -20°C for future use. Double-antibody sandwich ELISA method (use PD-1 recombinant protein of human origin to coat the microtiter plate, detect with HRP-labeled mouse anti-human IgG4 mAb, use commercial...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com