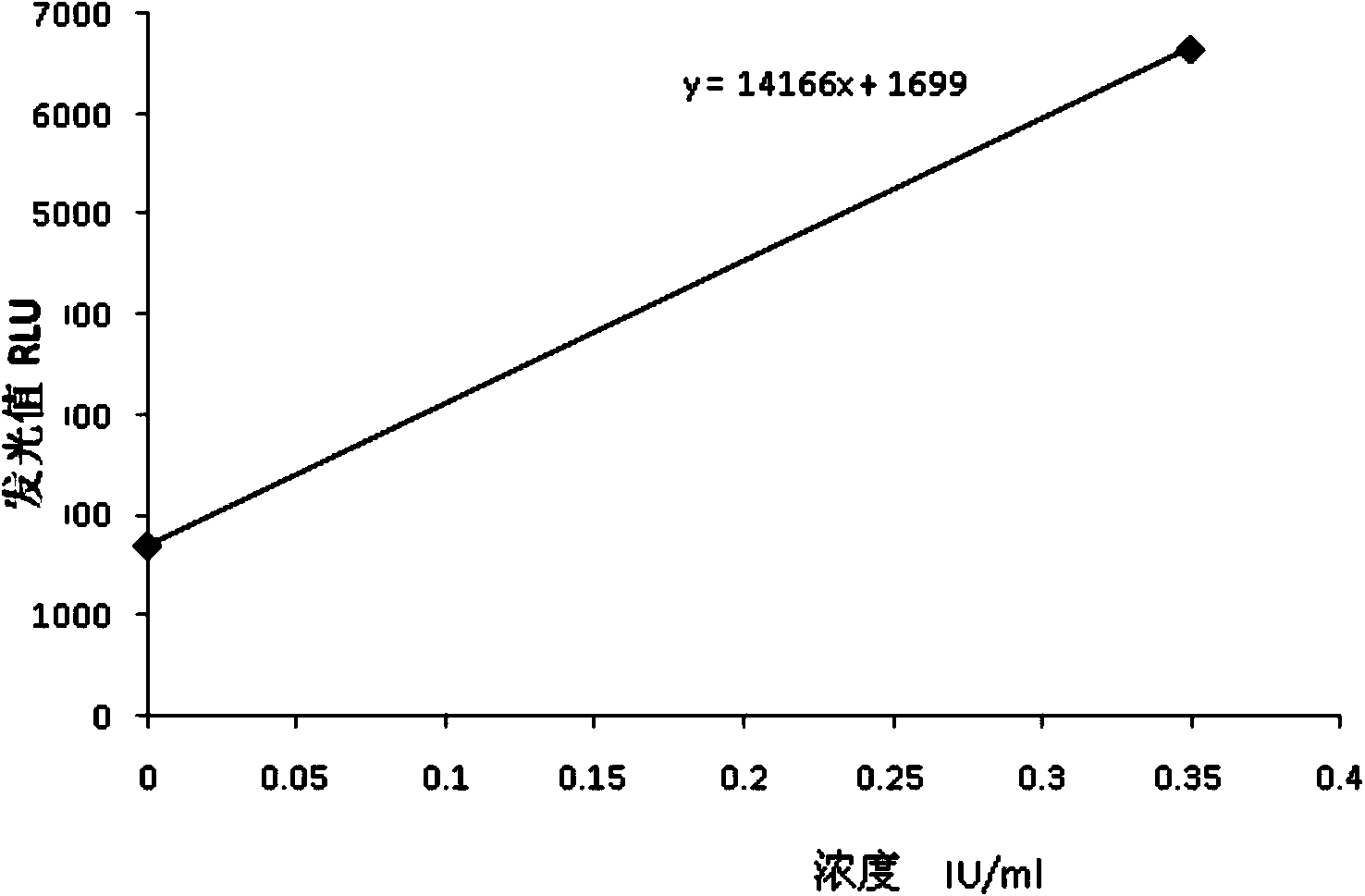
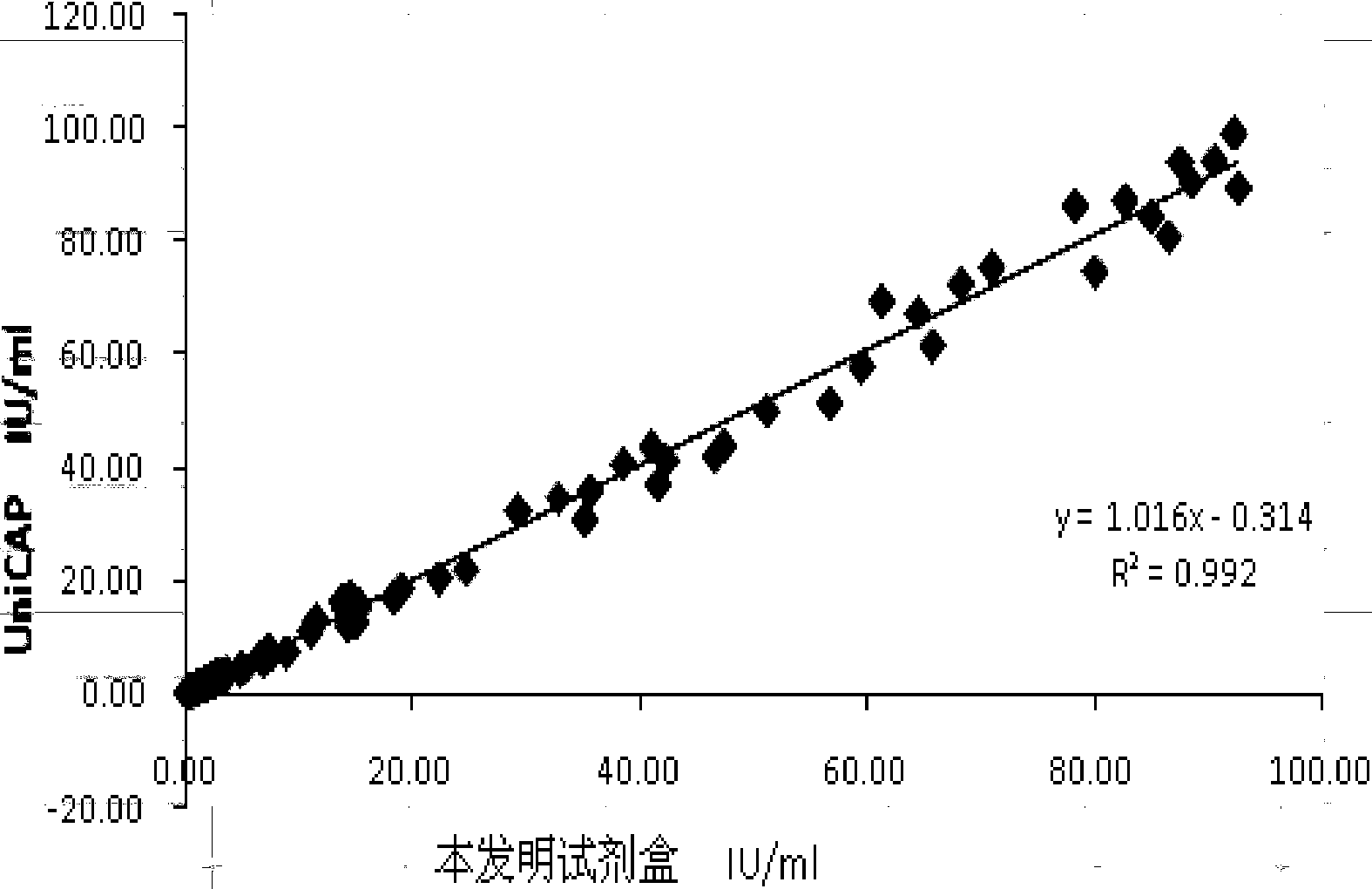
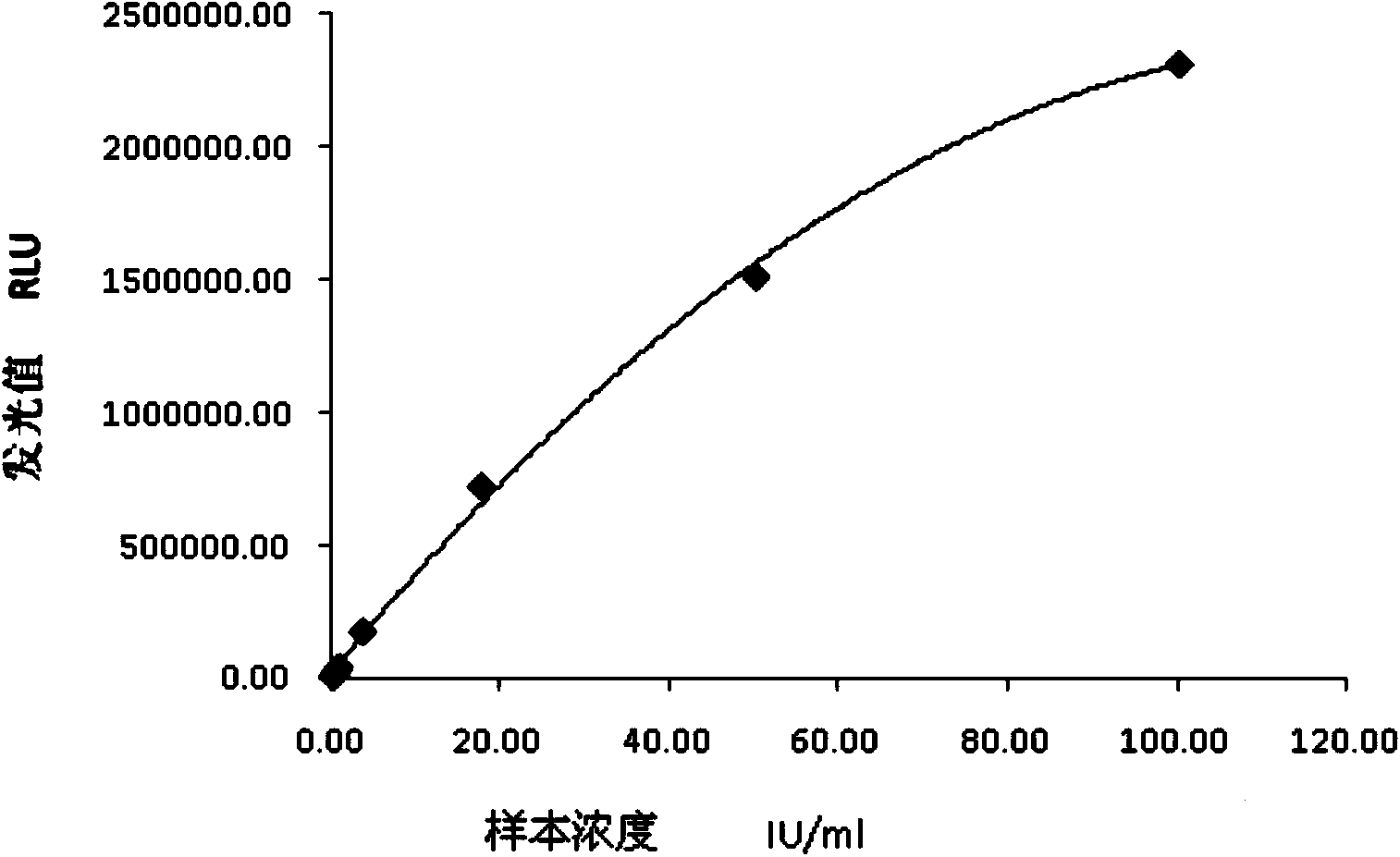
Chemiluminescence quantitative detection kit for inhaled allergens, and preparation method and detection method thereof
A Quantitative Detection, Chemiluminescence Technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of the kit
[0034] (1) Preparation of magnetic separation reagent:
[0035] Materials and instruments:
[0036] 1. Magnetic particles: containing carboxyl group (COOH) active group, the content of carboxyl group per gram (g) of magnetic particles (dry weight) is 1 millimoles (mmol), with superparamagnetism, diameter of 1μm.
[0037] 2. Inhalation of allergens: Dissolve 10 mg of the lyophilized dust mite allergen in 2 ml of phosphate buffer (pH 7.2, 0.02M) to obtain an allergen solution with a concentration of 5 mg / ml. Put the obtained solution into a dialysis bag and tighten it. After the dialysis bag, perform dialysis with 500ml of phosphate buffer solution at 4°C for 4-8 hours, change the fluid 3-4 times, and collect the dialysis product. The purity of allergen protein is 90%.
[0038] 3. 2-Morpholine ethanesulfonic acid (MES), carbodiimide (EDC), tris (TRIS) and other reagents should be chemically pure.
[0039] Steps:
[0040] 1. Take 100 mg of magneti...
Embodiment 2
[0055] Example 2: Implementation of testing and evaluation of testing results:
[0056] (1) Implementation of testing
[0057] Materials and instruments:
[0058] 1. Magnetic separation reagent and enzyme-labeled reagent were prepared in Example 1.
[0059] 2. IgE calibrators, quality control products, luminescent substrates, cleaning solutions, and specific IgE determination kits (fluorescence method) are produced by Pharmacia.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com