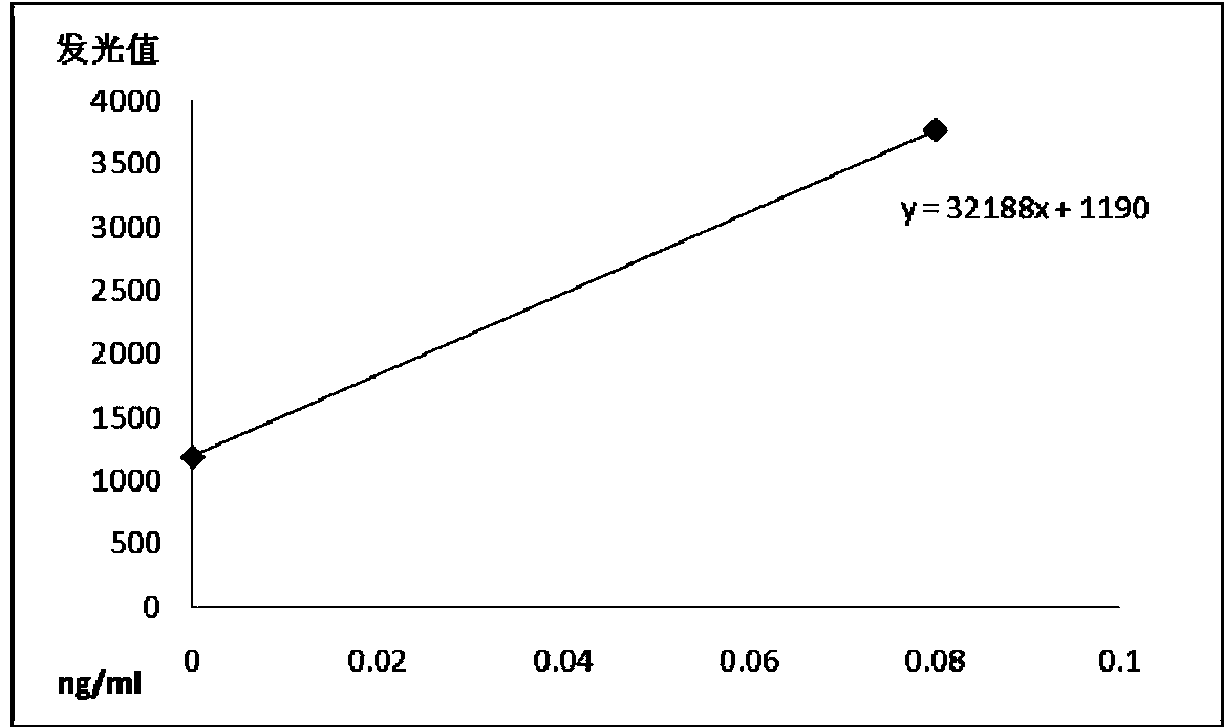
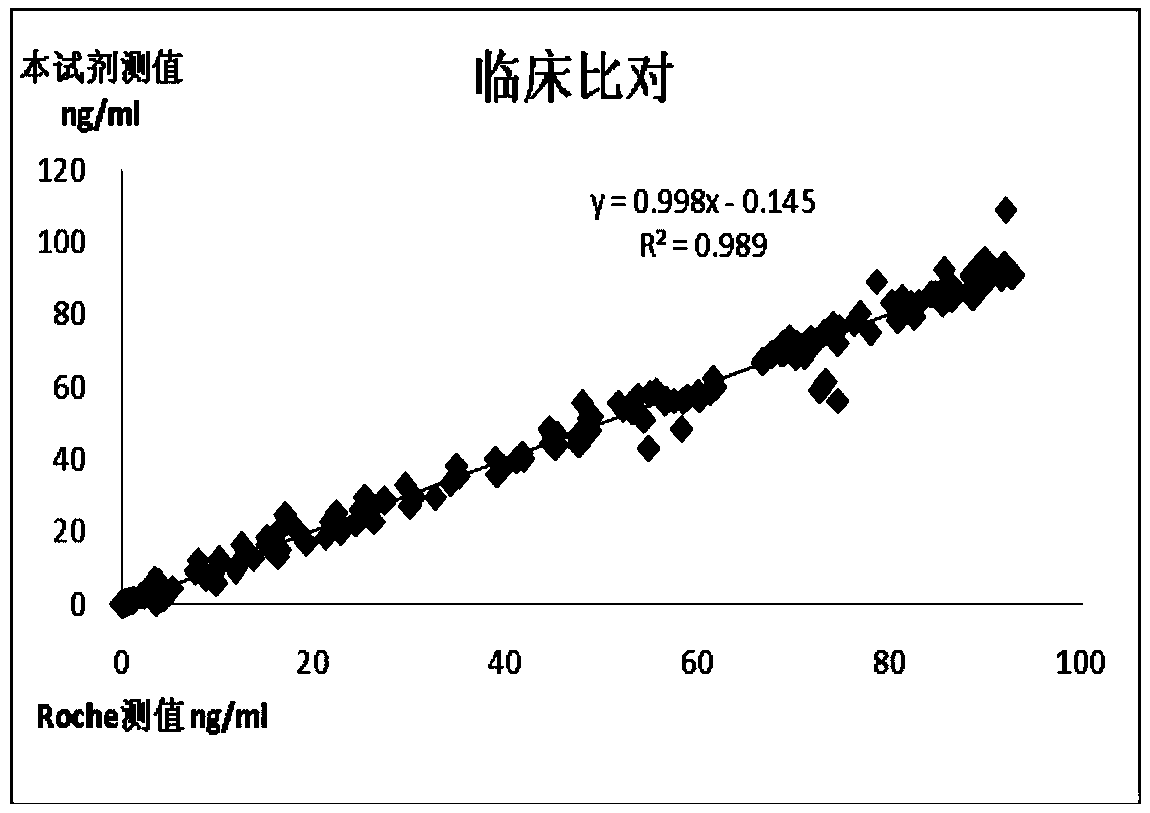
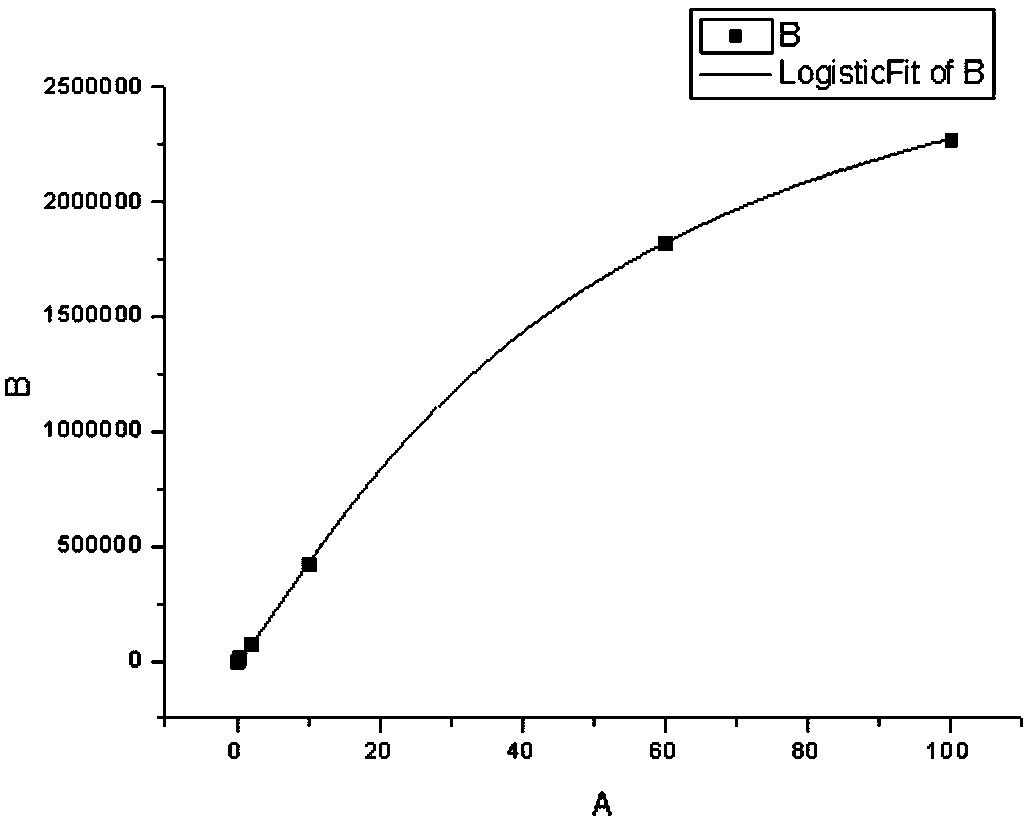
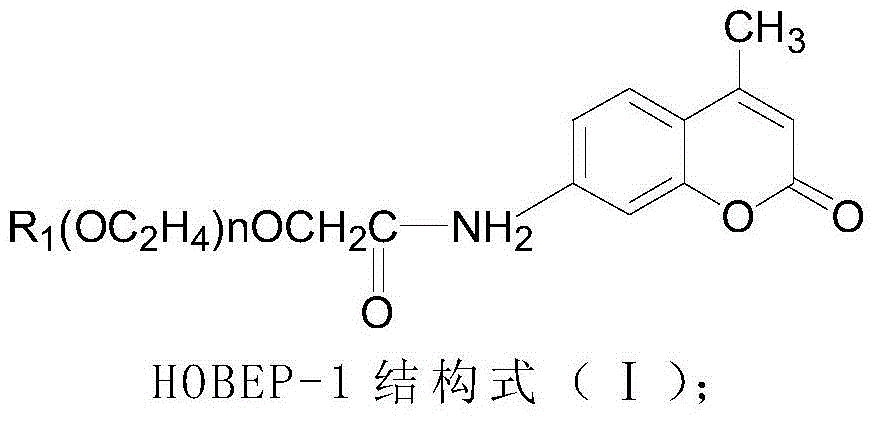
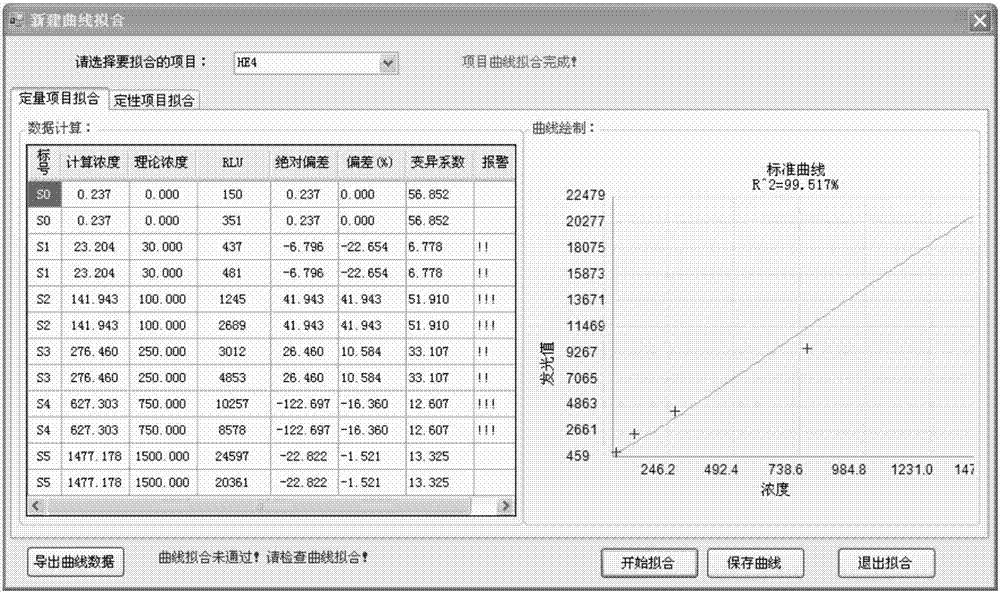
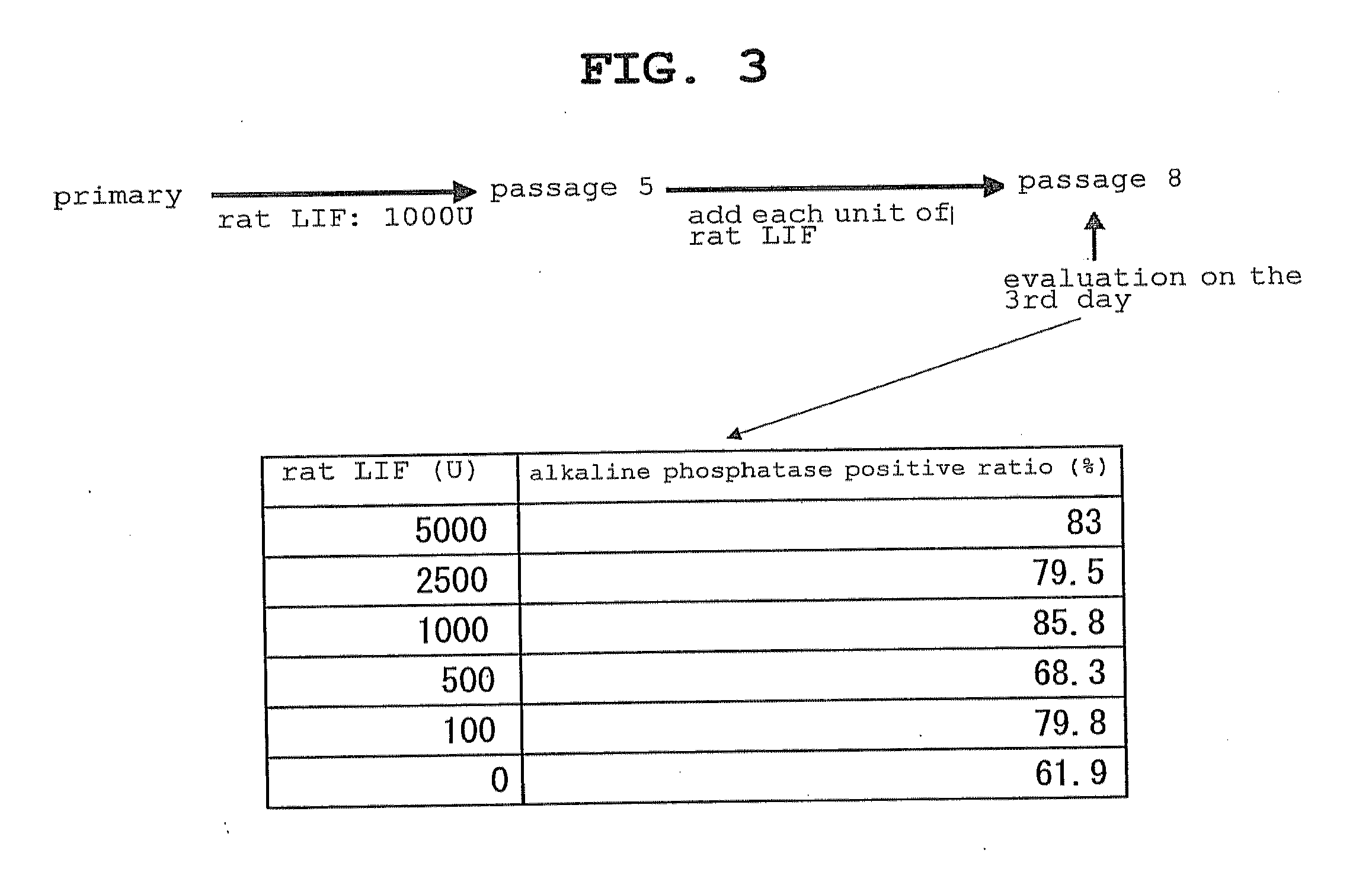
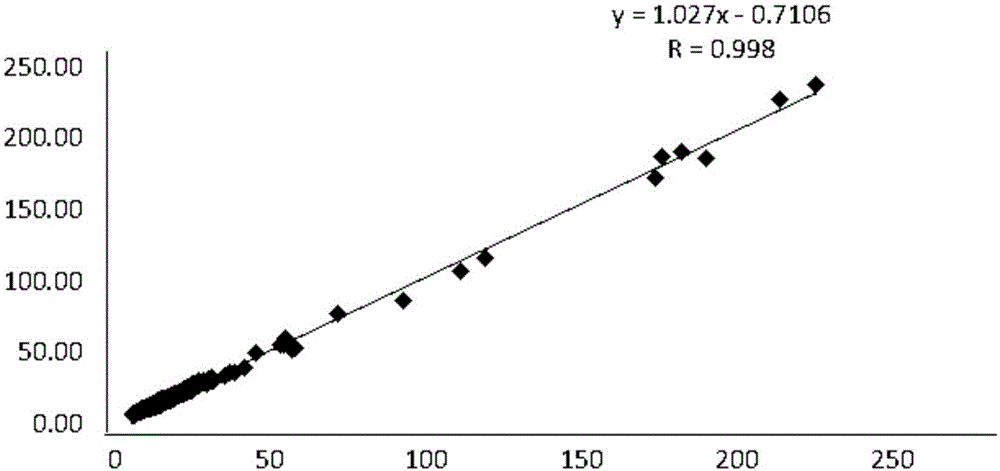
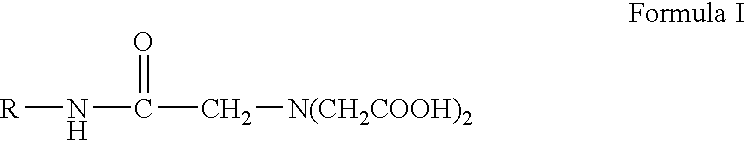Patents
Literature
1144 results about "Abnormal alkaline phosphatase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Additionally, abnormal levels of Alkaline phosphatase in the blood could indicate issues relating to the liver, gall bladder or bones. Kidney tumors, infections as well as malnutrition has also shown abnormal level of alkaline phosphatase in blood.
Tissue-nonspecific alkaline phosphatase (TNAP) activators and uses thereof
Disclosed herein are tissue-nonspecific alkaline phosphatase (TNAP) activators and uses thereof for promoting bone mineral deposition.
Owner:SANFORD BURNHAM MEDICAL RES INST
Method of detecting single gene copies in-situ
InactiveUS20020019001A1Bioreactor/fermenter combinationsBiological substance pretreatmentsNucleic acid sequencingNucleic acid sequence
A method for detecting single copies of a gene in-situ using brightfield microscopy is used in detection of nucleic acid sequences. Probes are directly or indirectly labeled with alkaline phosphatase with NBT / BCIP used as the chromogen.
Owner:VENTANA MEDICAL SYST INC
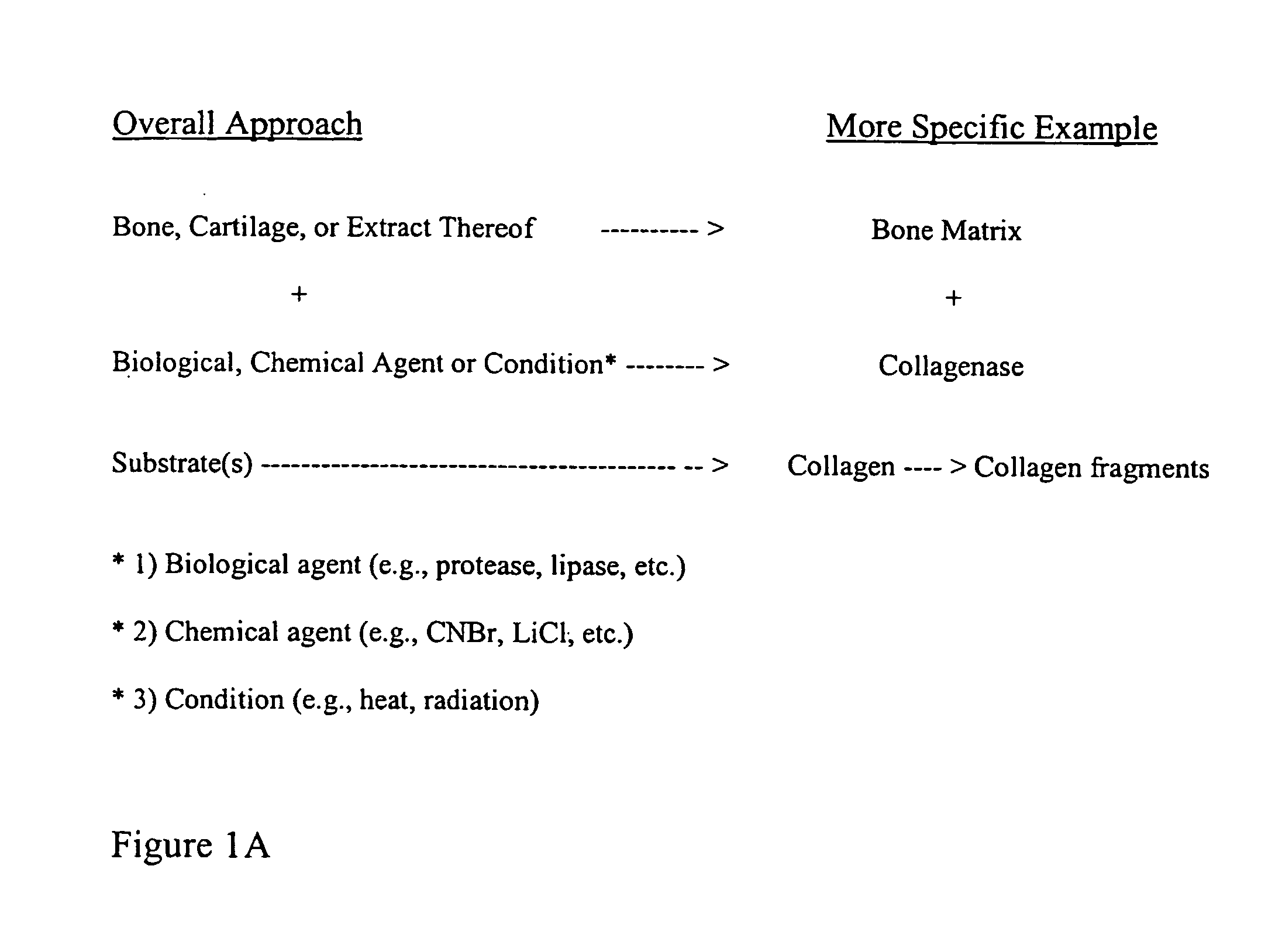
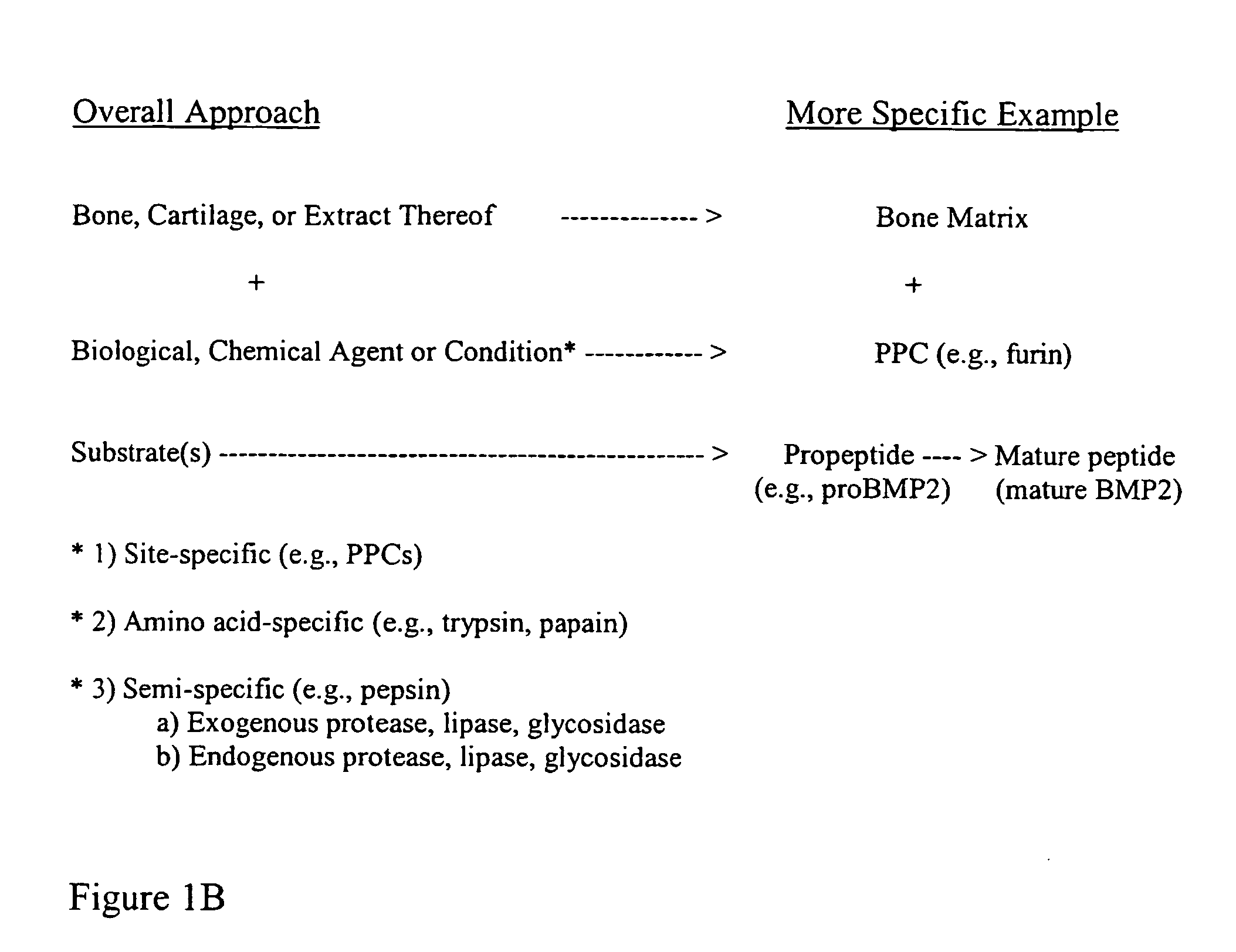
Bone matrix compositions and methods
ActiveUS20070154563A1Good osteoinductivityHigh activityHydrolysed protein ingredientsBone implantOsteoblastLine of therapy
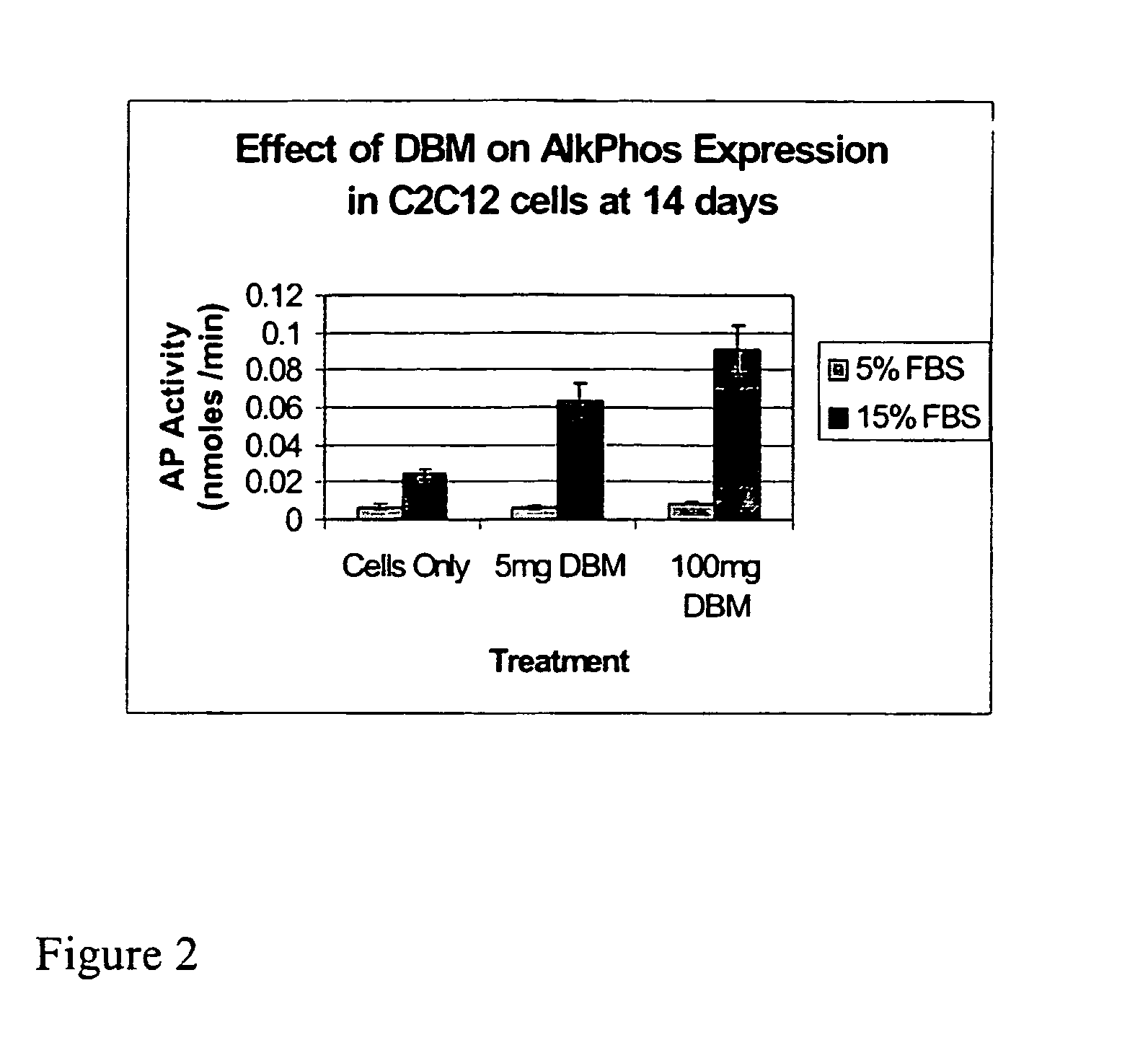
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. Ona assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
Sample collection and bioluminescent analysis system
ActiveUS20140004548A1Promote useReduce power consumptionBioreactor/fermenter combinationsBiological substance pretreatmentsPhoton detectionPhoton counter
Methods and apparatus for evaluating the quality of an environment or process by measuring light emitted from a bioluminescent sample containing ATP, ADP, or alkaline phosphatase. The apparatus comprises a sample collection and analysis system used to collect a sample, mix reagents, react the sample, and collect it in a measurement chamber. The system includes an instrument having a photon detection assembly for use with the sample testing device and one or more probe assemblies that optically cooperate with the instrument. The instrument includes a dark chamber with a reflective interior surface which may be concave or preferably spherical, and a photon detection sensor such as a multi-pixel photon counter sensor. A substantially transparent portion of the probe assembly, and liquid contained therein, focus bioluminescence toward the photon detection sensor.
Owner:BIOCONTROL SYST
Method of dephosphorylating an endotoxin in vivo with alkaline phosphatase
The invention relates to pharmaceutical compositions suitable for treating or curing clinical complications mediated by endotoxin, including sepsis. The compositions contain components suitable for detoxifying endotoxin rendering it less deleterious to mammals such as humans, in particular to patients with reduced host-defence resistance. The invention also relates to pharmaceutical compositions suitable for stimulating bone formation, e.g. for mending broken bone or for prophylaxis or therapy of metabolic bone diseases such as osteoporosis and osteomalacia and pharmaceutical compositions for decreasing or inhibiting undesired bone formation. The pharmaceutical compositions according to the invention are directed at modulating phosphatase activity in vivo.
Owner:UNIVERSITY OF GRONINGEN
Human stem cells originating from human amniotic mesenchymal cell layer
InactiveUS20060281178A1Stable supplyEffective drug deliveryNervous system cellsArtificial cell constructsCell layerOsteocyte
Owner:SAKURAGAWA NORIO +1
Automated hybridization/imaging device for fluorescent multiplex DNA sequencing
InactiveUS20020012910A1Easy to disassembleBioreactor/fermenter combinationsSludge treatmentHybridization probeSize fractionated
A method is disclosed for automated multiplex sequencing of DNA with an integrated automated imaging hybridization chamber system. This system comprises an hybridization chamber device for mounting a membrane containing size-fractionated multiplex sequencing reaction products, apparatus for fluid delivery to the chamber device, imaging apparatus for light delivery to the membrane and image recording of fluorescence emanating from the membrane while in the chamber device, and programmable controller apparatus for controlling operation of the system. The multiplex reaction products are hybridized with a probe, then an enzyme (such as alkaline phosphatase) is bound to a binding moiety on the probe, and a fluorogenic substrate (such as a benzothiazole derivative) is introduced into the chamber device by the fluid delivery apparatus. The enzyme converts the fluorogenic substrate into a fluorescent product which, when illuminated in the chamber device with a beam of light from the imaging apparatus, excites fluorescence of the fluorescent product to produce a pattern of hybridization. The pattern of hybridization is imaged by a CCD camera component of the imaging apparatus to obtain a series of digital signals. These signals are converted by the controller apparatus into a string of nucleotides corresponding to the nucleotide sequence an automated sequence reader. The method and apparatus are also applicable to other membrane-based applications such as colony and plaque hybridization and Southern, Northern, and Western blots.
Owner:WEISS ROBERT B +6
CRISPR/Cas9 system-containing targeted knockout vector and adenovirus and applications thereof
InactiveCN104004778AHigh knockout rateGenetic material ingredientsViruses/bacteriophagesBiotechnologyEnzyme digestion
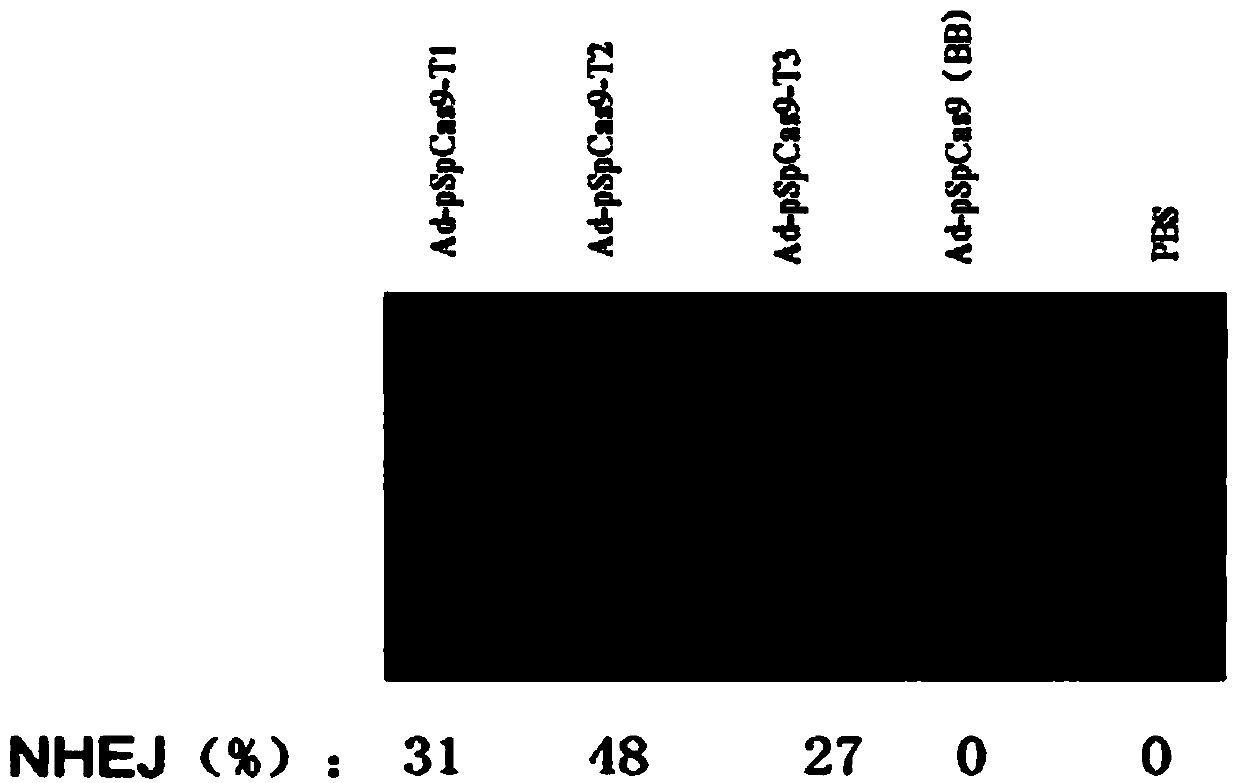
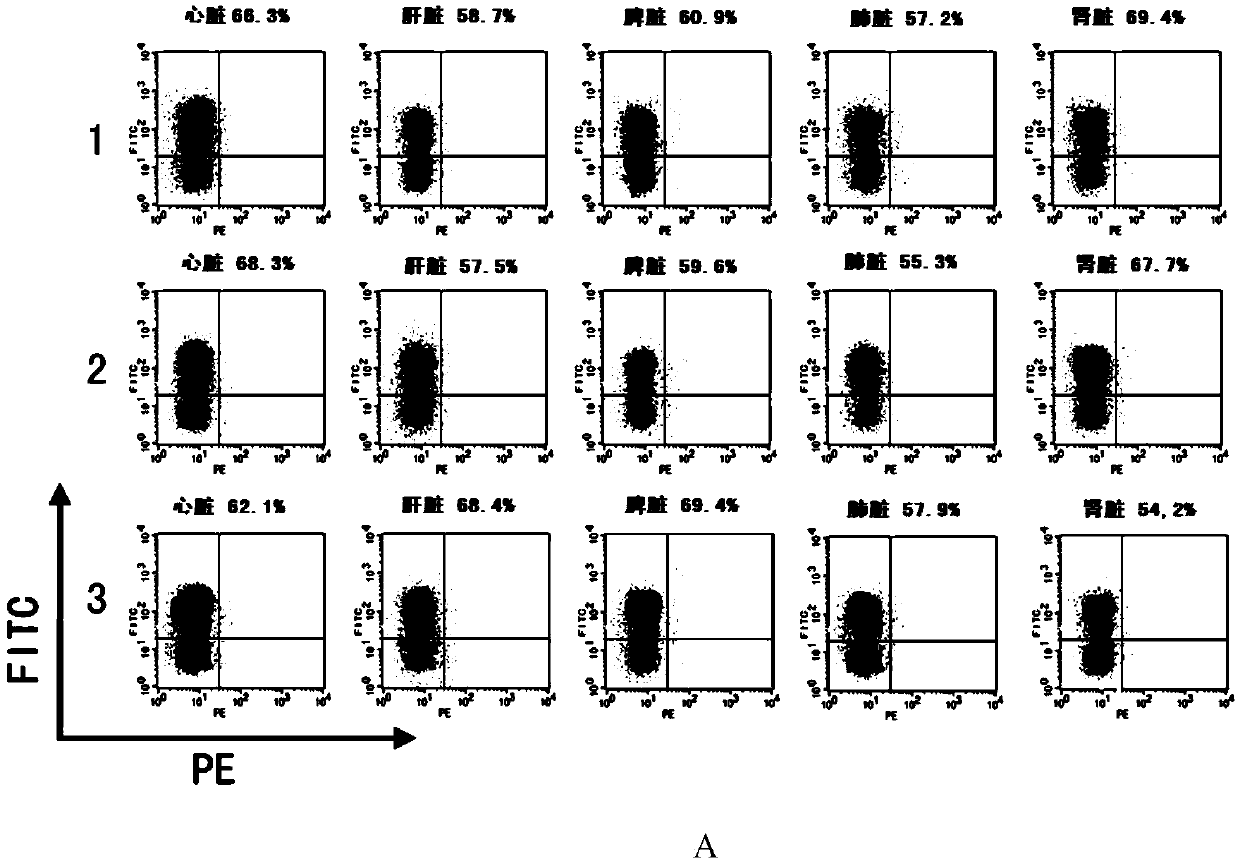
The invention discloses a CRISPR / Cas9 system-containing targeted knockout vector and adenovirus and applications thereof. The targeted knockout vector is prepared through the following steps: after a pX330 U6-Chimeric_BB-CBh-hSpCas9 plasmid is subjected to enzyme digestion and filling-in by using EcoRI and SacII, connecting the pX330 U6-Chimeric_BB-CBh-hSpCas9 plasmid with a pAdTrack-CMV plasmid subjected to enzyme digestion and filling-in by using BstXI; after the obtained product is linearized by using BbsI, connecting the obtained product to a specific target sequence of a desired gene; and after the obtained object is linearized by using PmeI and dephosphorylated by using CIAP alkaline phosphatase, recombining the obtained product with a pAdEasy-1 plasmid. The targeted knockout vector can mutate gene sequences in target sequence areas, and the mutation rate is high, up to 30.6-45.8%, therefore, the targeted knockout vector can be used for gene site-directed mutation, and lays a foundation for gene therapy.
Owner:重庆高圣生物医药有限责任公司
Isolation of adult multipotential cells by tissue non-specific alkaline phosphatase
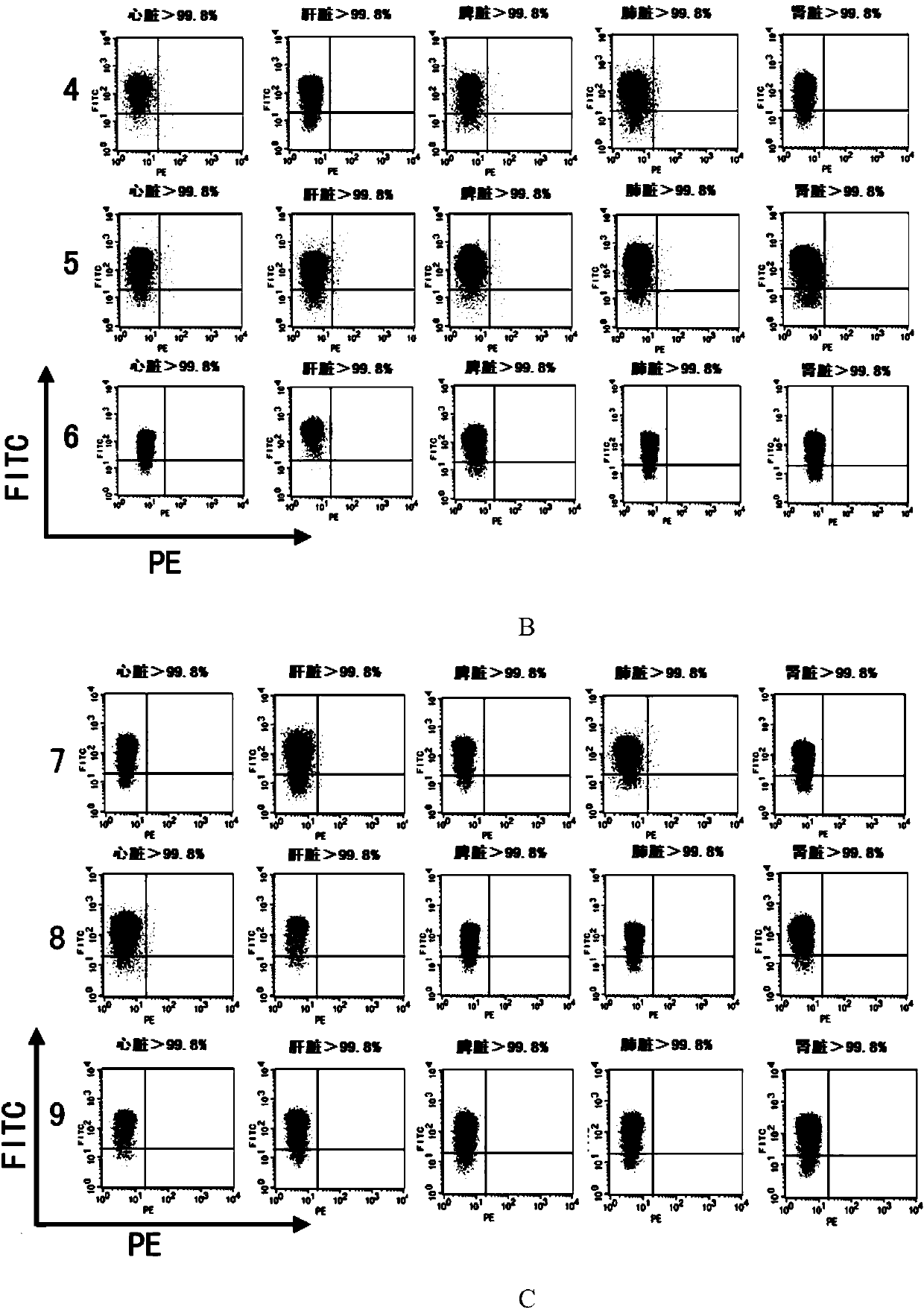
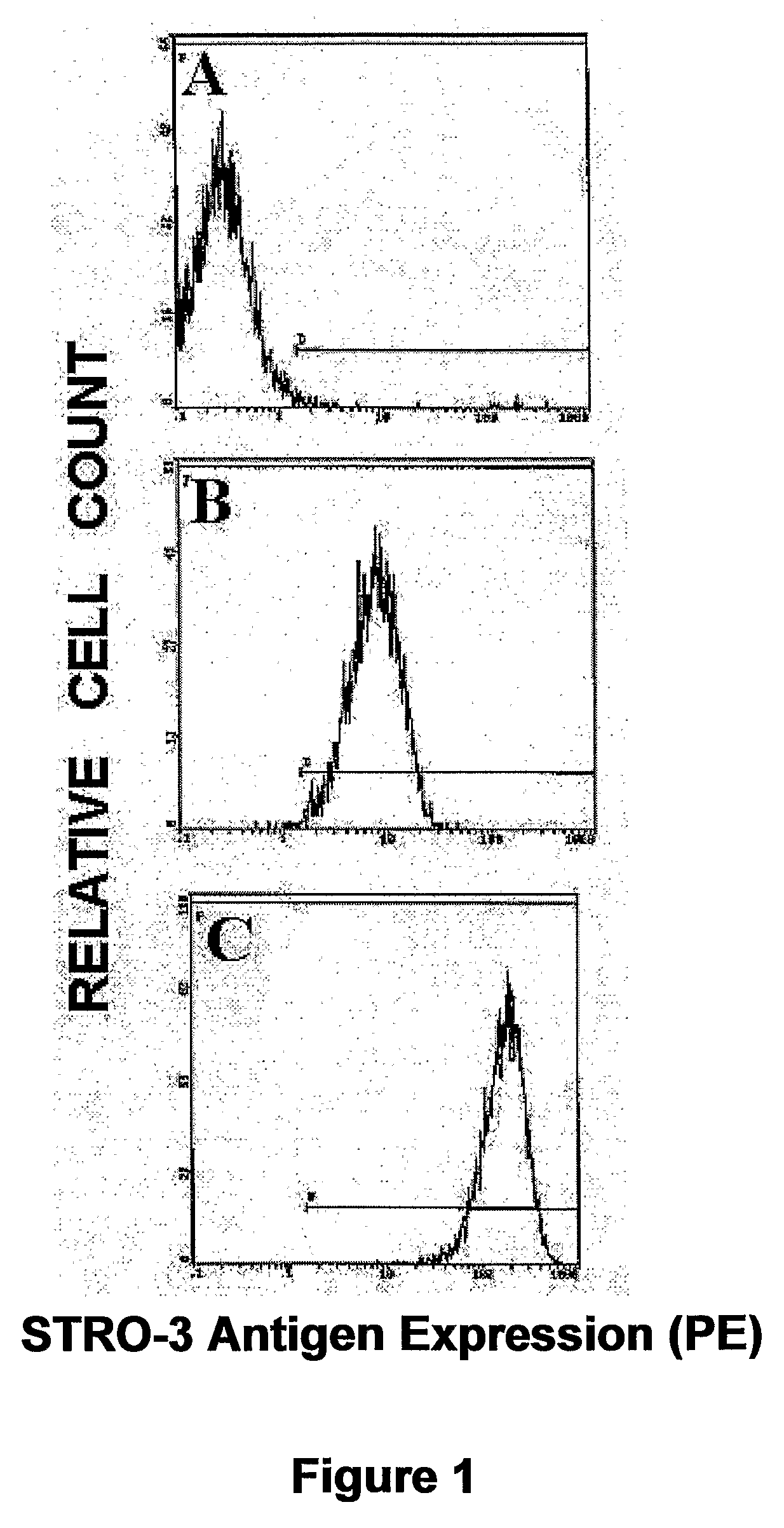
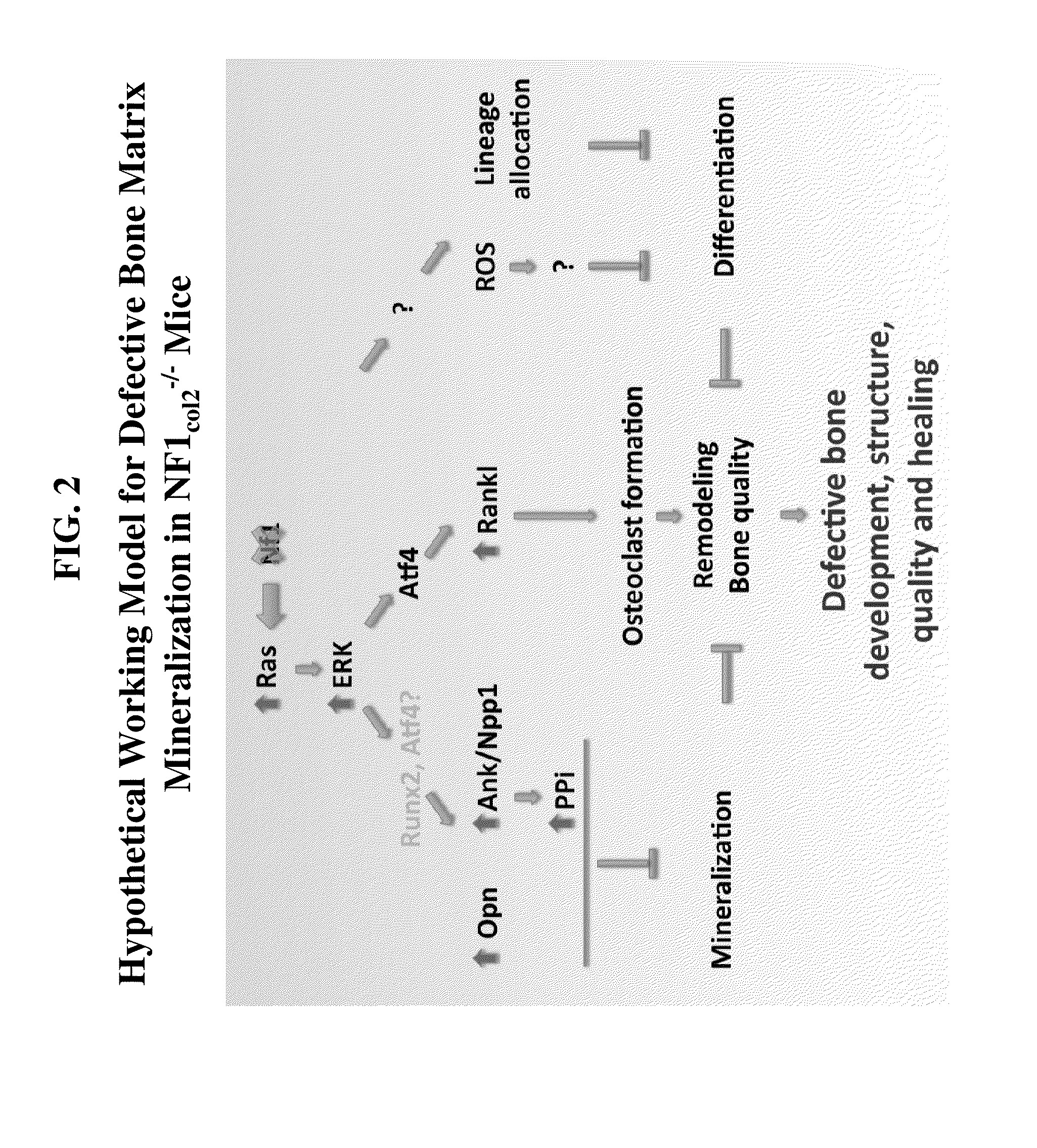
ActiveUS20090074728A1Increase ratingsEasy to transplantAnimal cellsHydrolasesCell biologyAbnormal alkaline phosphatase
The present invention relates to the use of tissue non-specific alkaline phosphatase (TNAP) as a marker for identifying and / or isolating adult multipotential cells. The present invention also relates to cell populations enriched by methods of the present invention and therapeutic uses of these cells.
Owner:MESOBLAST
Alkaline phosphatase labeled antigen-antibody diluent
The invention relates to a medical reagent, and particularly relates to an alkaline phosphatase labeled antigen-antibody diluent. The diluent is characterized by consisting of the following substances: a phosphate buffer, polyhydroxy organic compounds, amino acid and protein compounds, metal ions, a nonionic surface active agent, and a preservative. Compared with the prior art, the diluent provided in the invention can stabilize the activity of an alkaline phosphatase labeled antigen-antibody, and the alkaline phosphatase labeled antigen-antibody is prepared into a lyophilized product by using the freeze-drying method by adding the diluent into the alkaline phosphatase labeled antigen-antibody, so that the activity of the alkaline phosphatase labeled antigen-antibody can be stabilized in a long term.
Owner:AILEX TECH GRP CO LTD +1
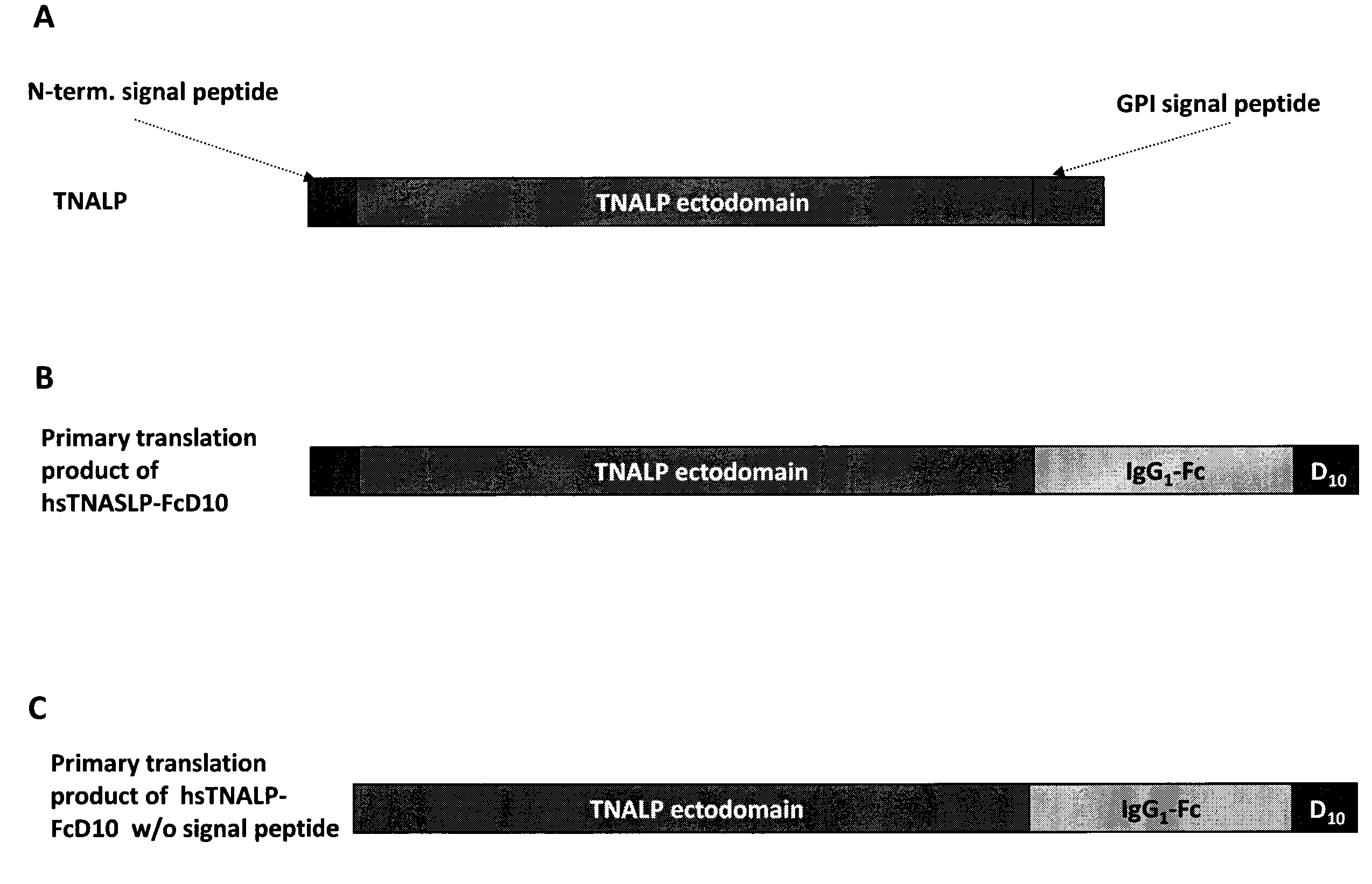
Bone targeted alkaline phosphatase, kits and methods of use thereof
InactiveUS20100297119A1Efficient foldingReduce forceVirusesSugar derivativesPolyaspartic acidExtracellular Structure
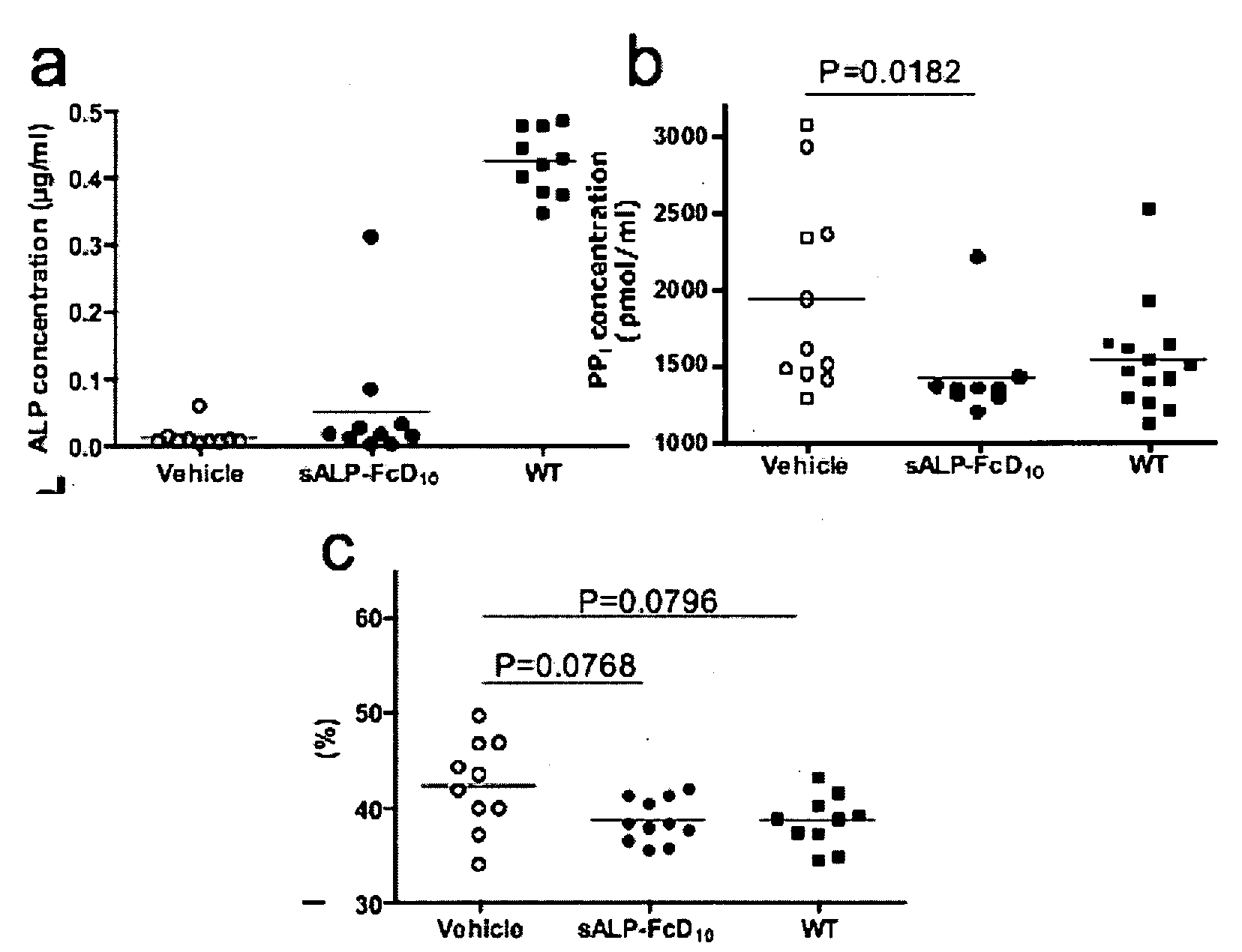
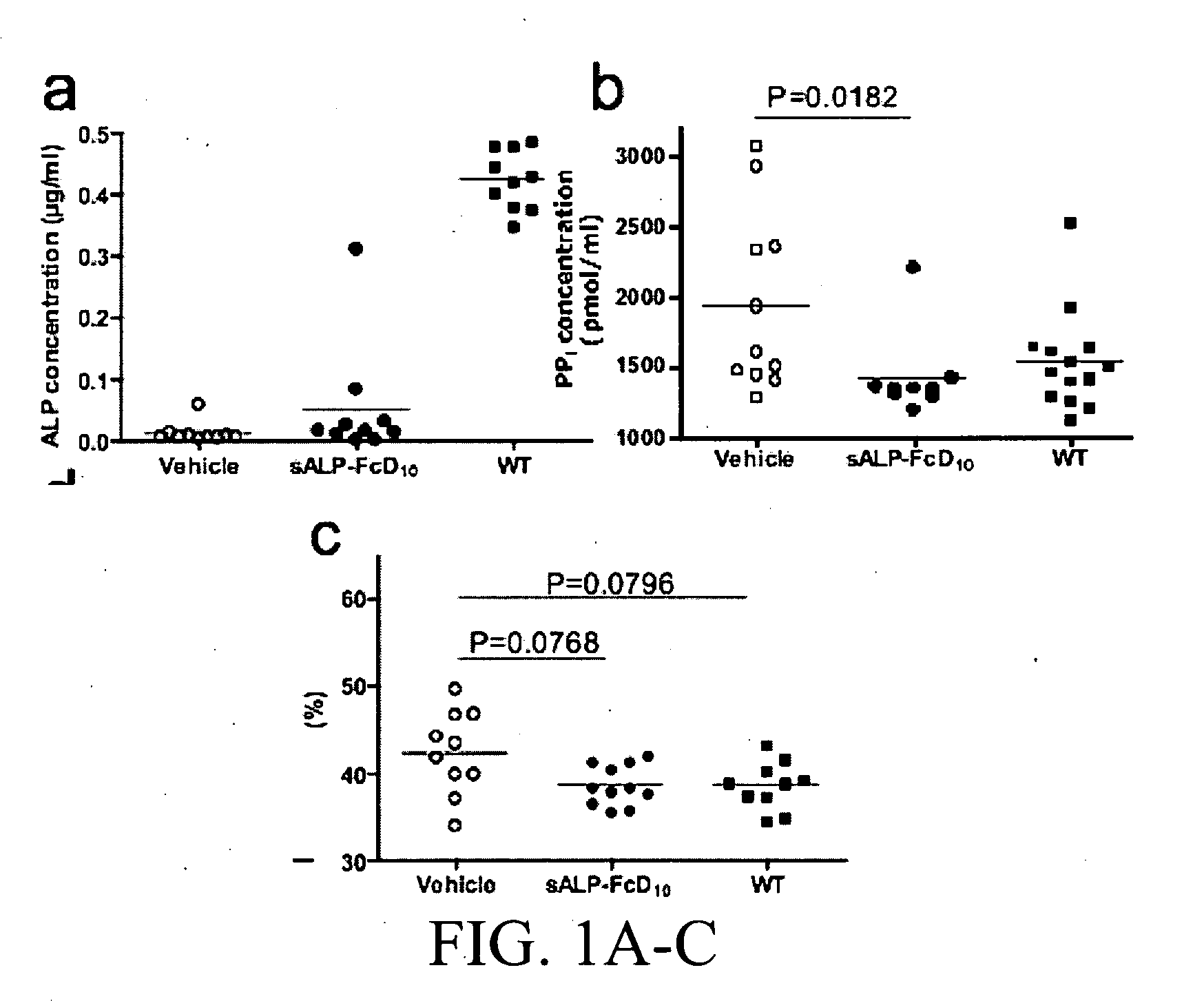
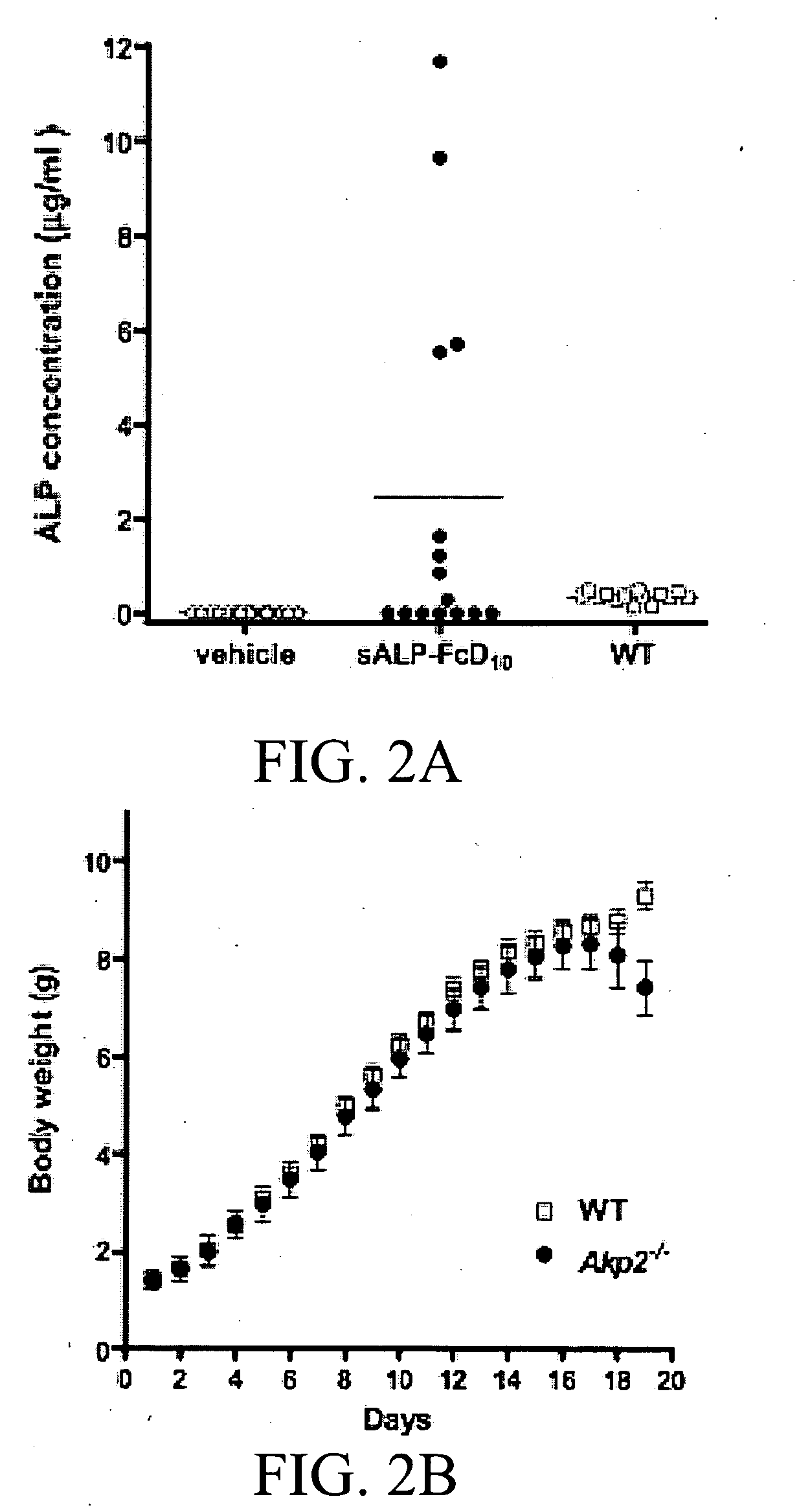
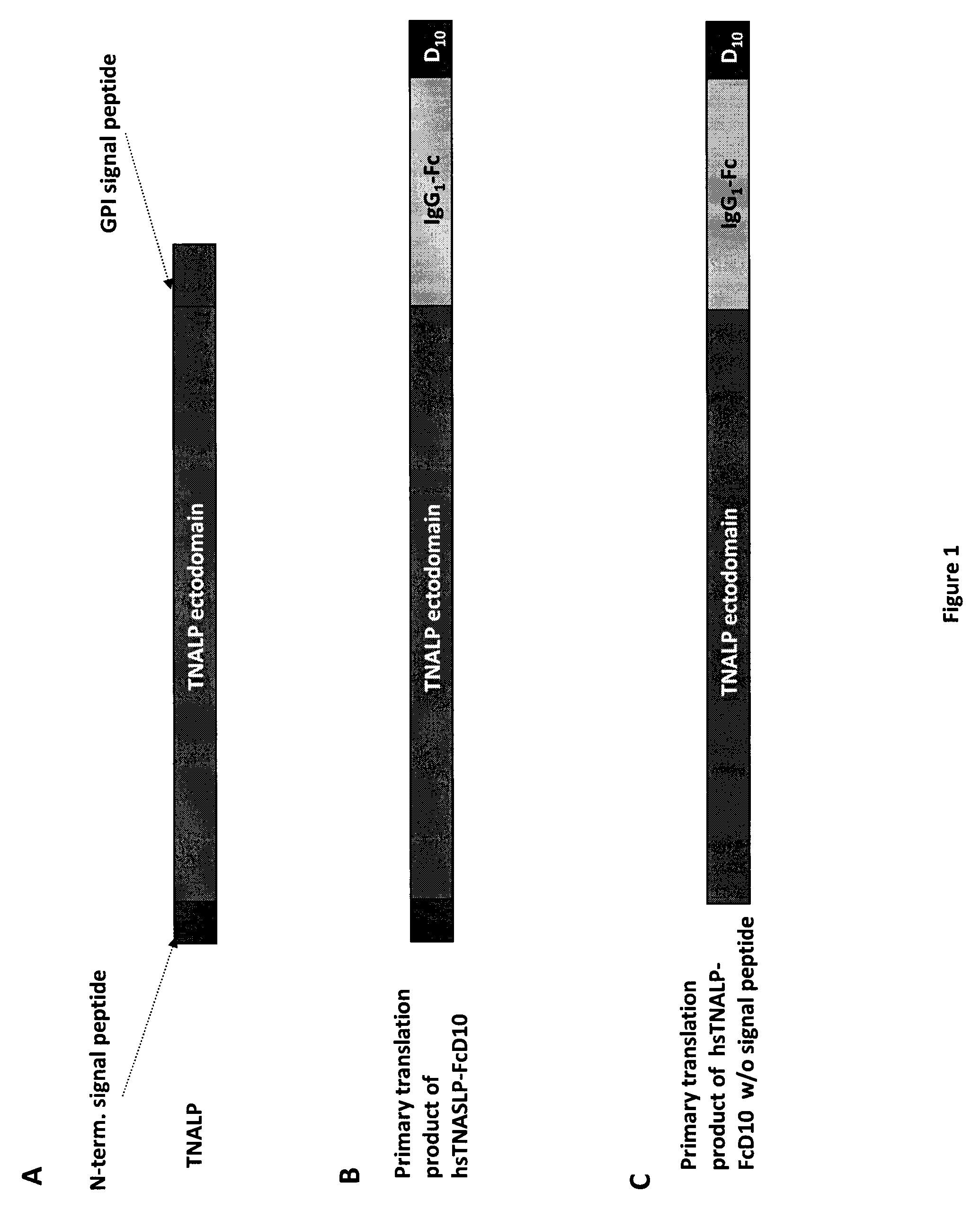
A bone targeted alkaline phosphatase comprising a polypeptide having the structure: Z-sALP-Y-spacer-X-Wn-V, wherein sALP is the extracellular domain of the alkaline phosphatase; wherein V is absent or is an amino acid sequence of at least one amino acid; X is absent or is an amino acid sequence of at least one amino acid; Y is absent or is an amino acid sequence of at least one amino acid; Z is absent or is an amino acid sequence of at least one amino acid; and Wn is a polyaspartate or a polyglutamate wherein n=10 to 16. Kits and methods of use thereof.
Owner:ALEXION PHARMA INC
Patient-specific stem cell lines derived from human parthenogenetic blastocysts
InactiveUS20080299091A1Increase alkaline phosphatase activityHigh levelBiocideSenses disorderTelomeraseOocyte donor
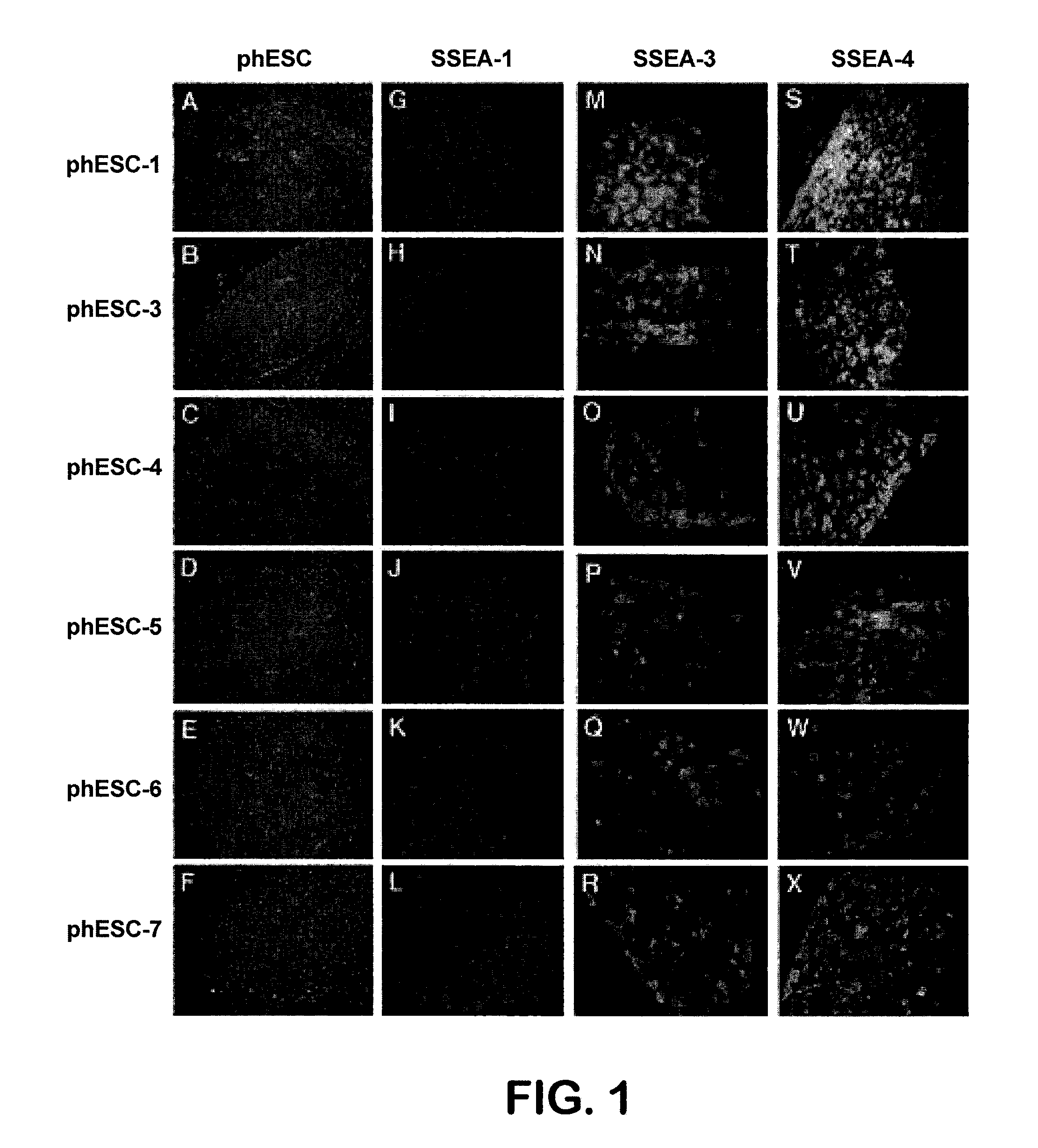
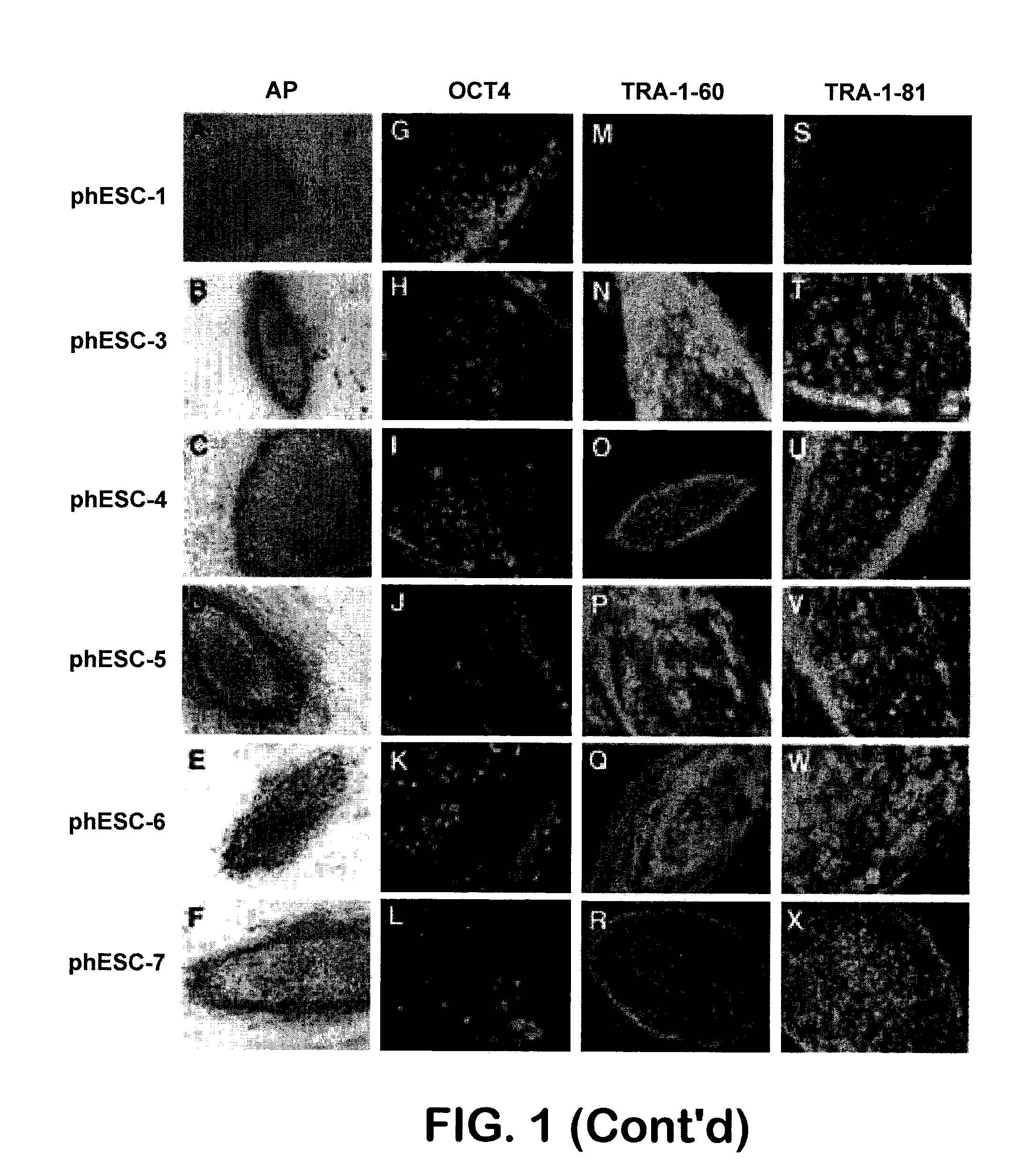
Methods are disclosed for generating HLA homozygous parthenogenetic human stem cell (hpSC-Hhom) lines from both HLA homozygous and HLA heterozygous donors. These hpSC-Hhom lines demonstrate typical human embryonic stem cell morphology, expressing appropriate stem cell markers and possessing high levels of alkaline phosphatase and telomerase activity. Additionally, injection of these cell lines into immunodeficient animals leads to teratoma formation. Furthermore, in the case of HLA heterozygous donors, the hpSC-Hhom lines inherit the haplotype from only one of the donor's parents. SNP data analysis suggests that hpSC-Hhom lines derived from HLA heterozygous oocyte donors are homozygous throughout the genome as assessed by single-nucleotide polymorphism (SNP) analysis. The protocol as disclosed minimizes the use of animal-derived components, which makes the stem cells more practical for clinical application.
Owner:INT STEM CELL CORP
Chemiluminescence quantitative detection kit for procalcitonin, and preparation method and detection method thereof
ActiveCN103901203ALow cross-reactivityImprove accuracyChemiluminescene/bioluminescenceBiotin-streptavidin complexN-Hydroxysuccinimide
The invention relates to a chemiluminescence quantitative detection kit for procalcitonin, and a preparation method and detection method thereof. The kit comprises a procalcitonin series standard substance, a magnetic separation reagent (magnetic particle suspension coupled with streptavidin), a first reagent (anti-procalcitonin monoclonal antibody solution containing biotin N-hydroxysuccinimide ester label) and a second reagent (anti-procalcitonin monoclonal antibody solution containing alkaline phosphatase label). The sensitivity of the kit prepared from the magnetic particle suspension coupled with streptavidin, anti-procalcitonin monoclonal antibody solution containing biotin N-hydroxysuccinimide ester label and anti-procalcitonin monoclonal antibody solution containing alkaline phosphatase label is up to 0.008ng / ml; and the kit has the advantages of high accuracy, high precision, no need of prediluting the sample, and wide detection range, and is simple and time-saving to operate.
Owner:SUZHOU HAOOUBO BIOPHARML
Preparing monomeric metal ion chelator containing diacetyl glycine group linked to proteinaceous molecule
A precursor for the construction of chelated metal conjugates which demonstrate improved assay performance and utility in minimizing non-specific binding while maintaining specificity for target molecules is disclosed. The precursor has tridentate functionality towards multivalent ions such as iron and nickel and contains a diacetyl glycine group covalently linked via an amide to a molecule such as a proteinaceous molecule providing a primary amide group for amide bond formation. The precursor is preferably prepared in monomeric form by reacting nitrilotriacetic acid or a salt thereof in an aqueous medium at an alkaline pH of at least 8 with a proteinaceous molecule containing a primary amine group in the presence of a carbodiimide. The proteinaceous molecule may be bovine serum albumin or an enzyme such as alkaline phosphatase or horseradish peroxidase.
Owner:PIERCE BIOTECHNOLOGY
Compositions comprising alkaline phosphatase and/or natriuretic peptide and methods of use thereof
ActiveUS20130323244A1Reduced dose-dependent side effectPeptide/protein ingredientsHydrolasesDiseaseNeurofibromatosis type I
The present invention provides methods, compositions, and kits for the treatment of neurocutaneous syndromes, such as neurofibromatosis type I; disorders associated with overactivation of FGFR3, such as achondroplasia; bone or cartilage disorders; or vascular smooth muscle disorders; or for the elongation of bone. In some embodiments, the present invention provides polypeptides having an alkaline phosphatase peptide fused to an Fc domain of an immunoglobulin or a natriuretic peptide fused to an Fc domain of an immunoglobulin. Such polypeptides can be administered to subjects, e.g., subcutaneously, to treat a neurocutaneous syndrome, a disorder associated with overactivation of FGFR3, a bone or cartilage disorder, or a vascular smooth muscle disorder, or to elongate bone. The invention also features nucleic acid molecules encoding such polypeptides and the use of the nucleic acid molecules for treating neurocutaneous syndromes, disorders associated with overactivation of FGFR3, bone or cartilage disorders, or vascular smooth muscle disorders, or for elongating bone.
Owner:VANDERBILT UNIV +1
Reagent and method for stabilizing alkaline phosphatase or marker of alkaline phosphatase
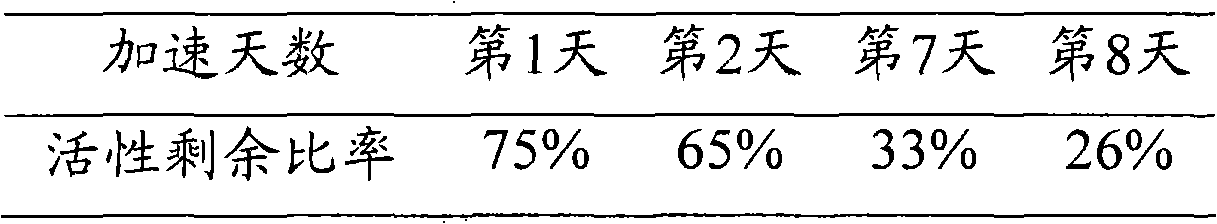
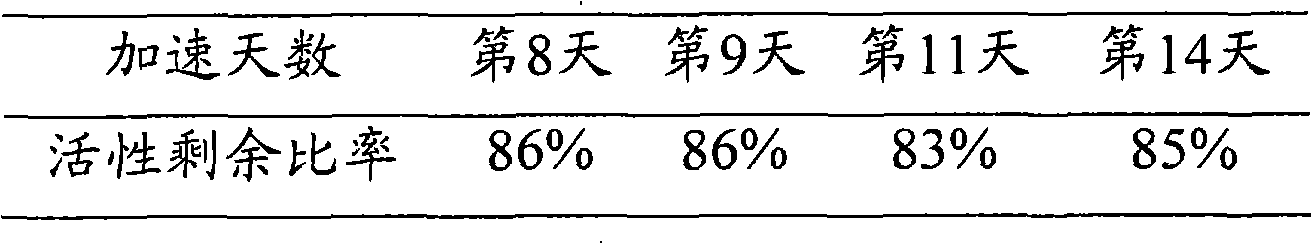
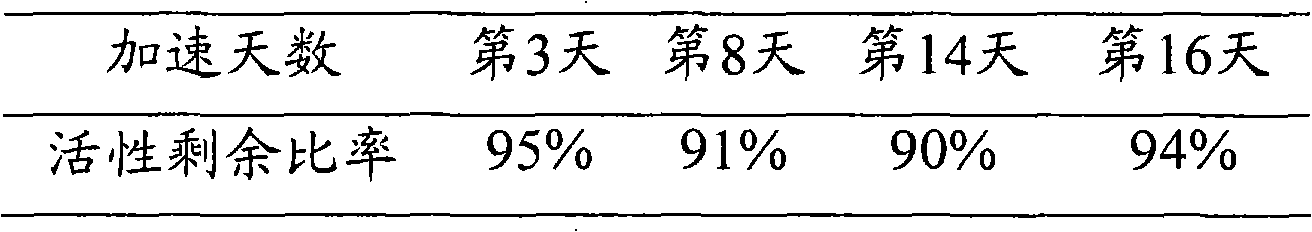
ActiveCN102115737AStable storageStabilizer can be stored stably for a long timeHydrolasesEnzyme stabilisationAbnormal alkaline phosphataseReagent
The invention discloses and relates to a stabilizing agent of alkaline phosphatase or a marker of the alkaline phosphatase, a preparation method of the stabilizing agent and a method for stabilizing the alkaline phosphatase or the marker of the alkaline phosphatase by using the stabilizing agent. The invention also relates to the alkaline phosphatase or a marker reagent of the alkaline phosphatase and a preparation method thereof. The invention also relates to a kit which comprises the stabilizing agent and the alkaline phosphatase or the marker of the alkaline phosphatase disclosed by the invention. The stabilizing agent disclosed by the invention can stably preserve the alkaline phosphatase or the marker of the alkaline phosphatase for a long time, so that the retention period of the alkaline phosphatase or a marker solution of the alkaline phosphatase is prolonged.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD
Enzymatic fluorimetric assay of camp and adenylate cyclase
InactiveUS6762026B1Easy to operateReaction period can be extremely shortenedMicrobiological testing/measurementMaterial analysisRadioactive agentFluorescence
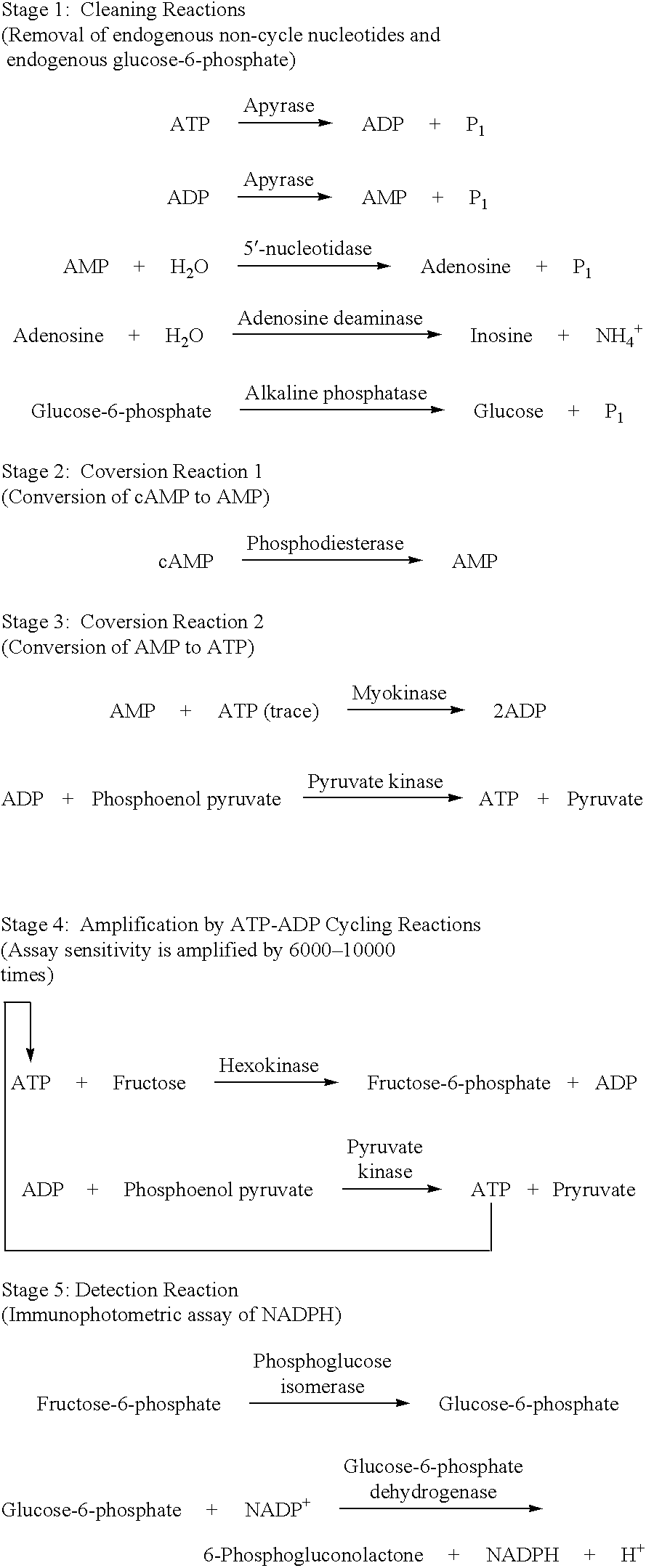
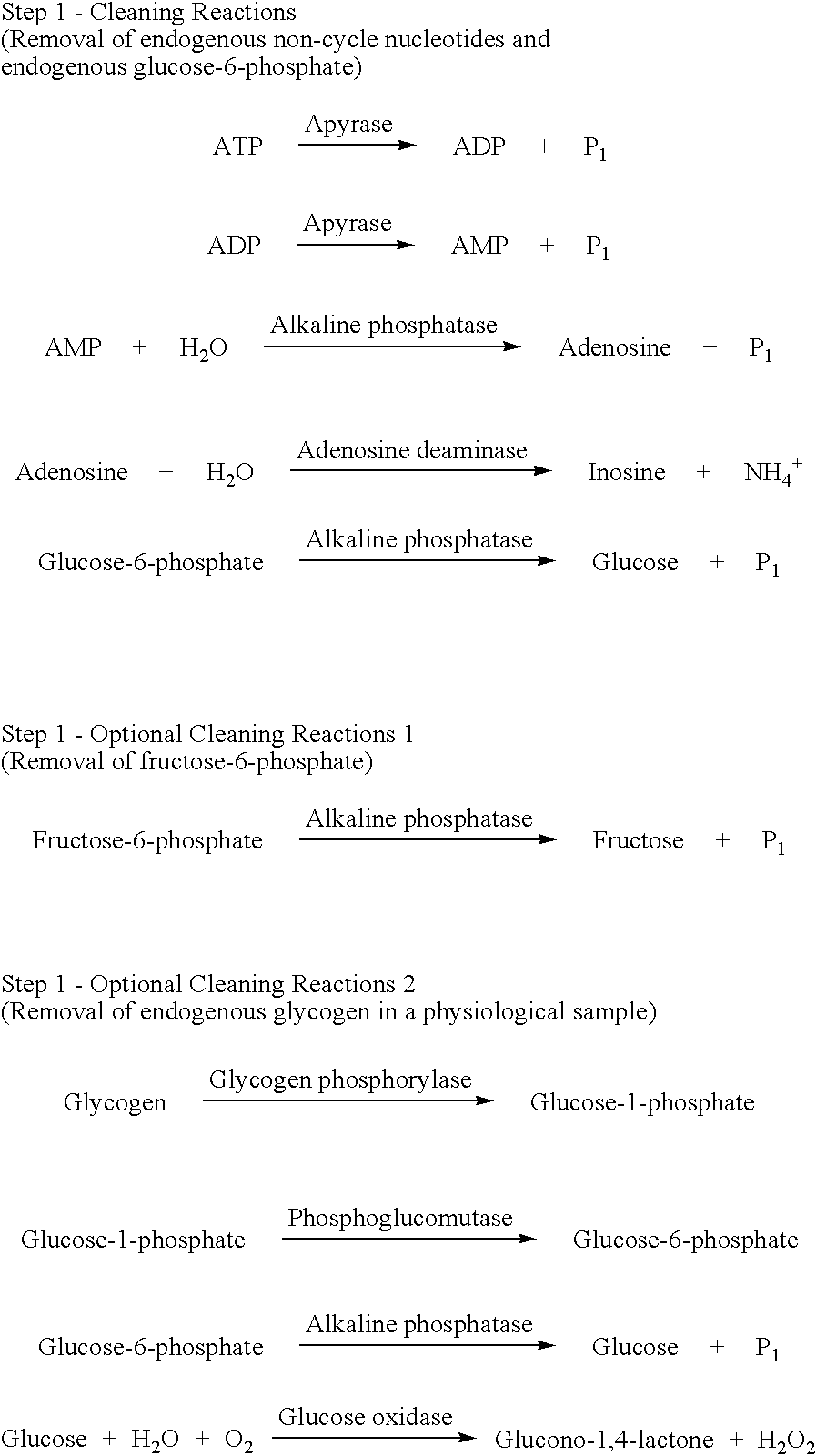
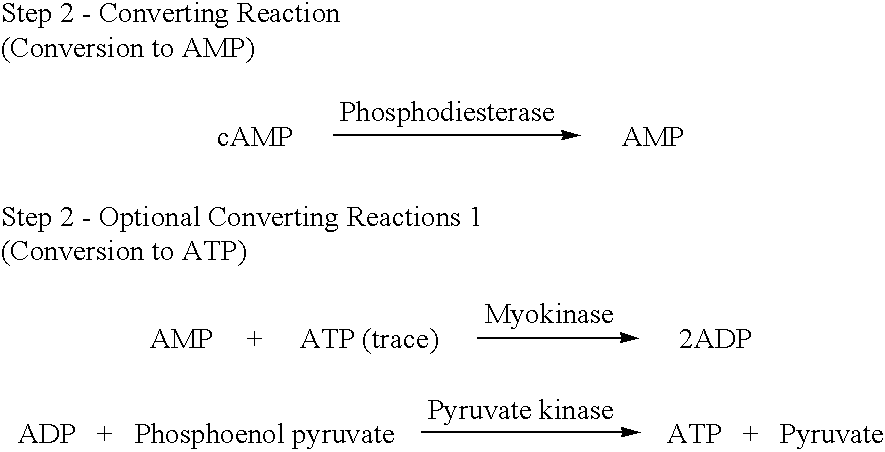
The present invention relates to a method for quickly determining cAMP content or an adenylate cyclase activity in a biological sample containing non-cyclic adenine nucleotides without the use of radioactive agents.Particularly, the present invention provides a method of determining cAMP content or an adenylate cyclase activity in a biological sample containing non-cyclic adenine nucleotides selected from the group consisting of cAMP produced by endogenous adenylate cyclase, and AMP, ATP, ADP and a mixture thereof, which comprises (1) combining a biological sample with effective amounts of apyrase, adenosine deaminase and alkaline phosphatase to enzymatically remove non-cyclic adenine nucleotides other than cAMP, and glucose-6-phosphate in the sample; (2) enzymatically converting cAMP into AMP; (3) determining an amount of AMP without the use of radioactive agents, and a kit to carry out the method.
Owner:FUSO PHARMA INDS
Method of detecting single gene copies in-situ
InactiveUS7087379B2Bioreactor/fermenter combinationsBiological substance pretreatmentsNucleic acid sequencingBioinformatics
Owner:VENTANA MEDICAL SYST INC
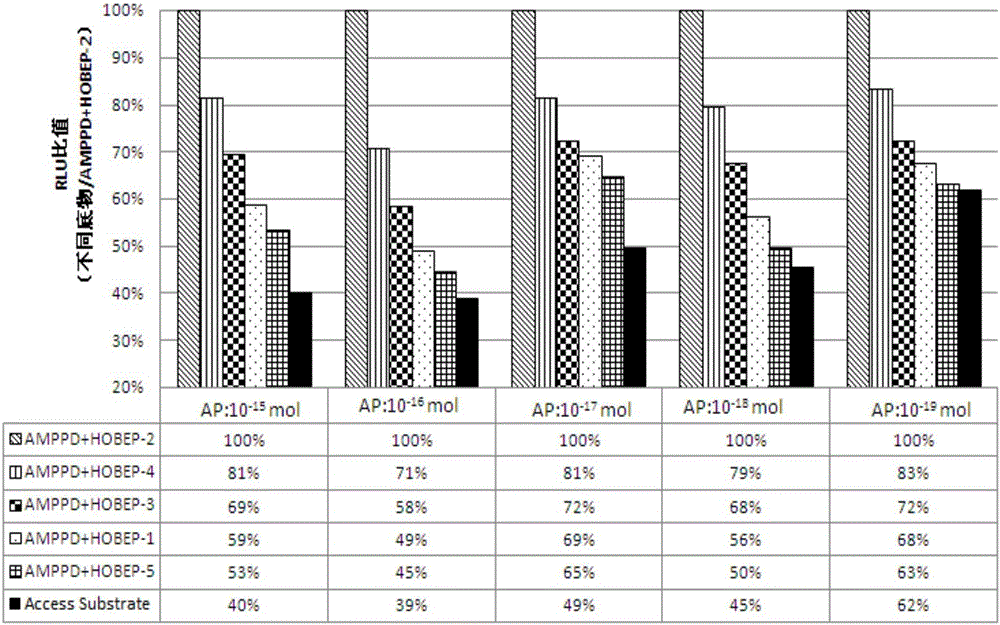
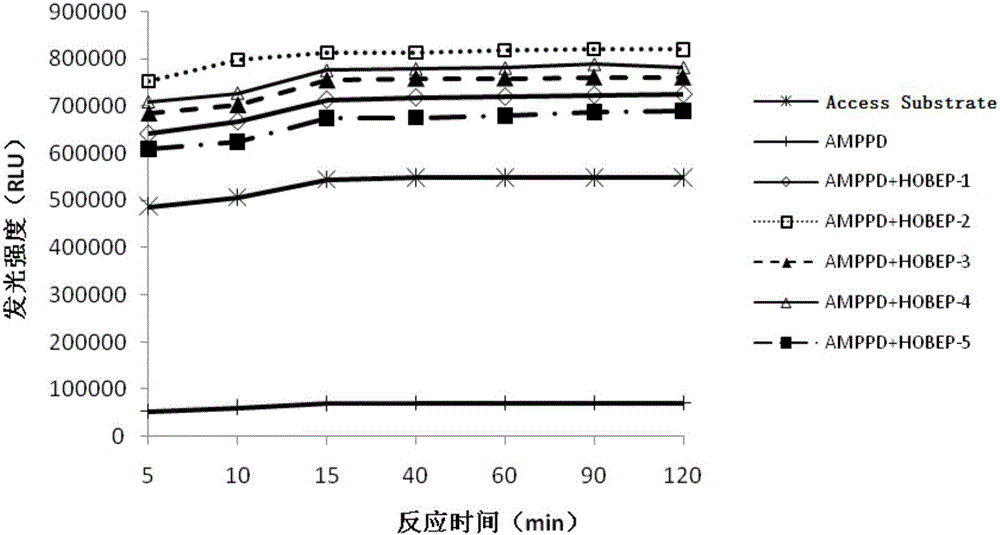
Enzyme-promoting chemiluminiscence substrate using alkaline phosphatase
ActiveCN104990912AHigh strengthLow costChemiluminescene/bioluminescenceBiological testingFluorescencePerformance index
The invention relates to an enzyme-promoting chemiluminiscence substrate using alkaline phosphatase. Using water as solvent, the enzyme-promoting chemiluminiscence substrate comprises 2-amino-2-methyl-1-propyl alcohol, AMPPD, and a luminescence enhancer link-coupled by sodium fatty alcohol polyoxyethylene ether carboxylate and fluorescent compounds. According to the enzyme-promoting chemiluminiscence substrate using alkaline phosphatase, the luminescence enhancer can achieve the effect of co-surfactant, the compounds can be better combined into a chemiluminiscence buffer system, and thus the chemiluminiscence efficiency is greatly improved. The enzyme-promoting chemiluminiscence substrate using alkaline phosphatase has the advantages of being high in strength, high in sensitivity, long in duration, good in stability and the like. Clinical testing requirements can be met completely, the main performance index has already reached the level of foreign products, and the cost of the chemiluminiscence substrate is greatly lowered.
Owner:SUZHOU HAOOUBO BIOPHARML
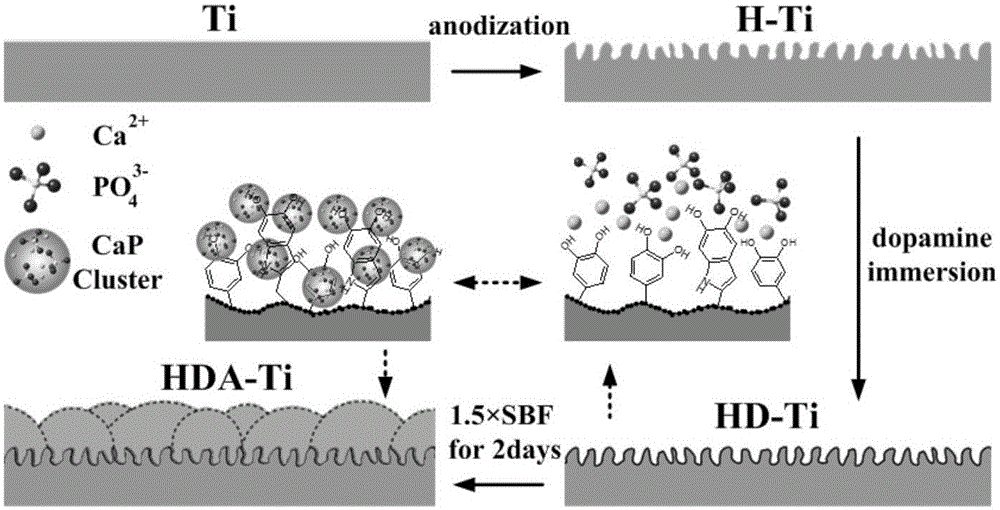
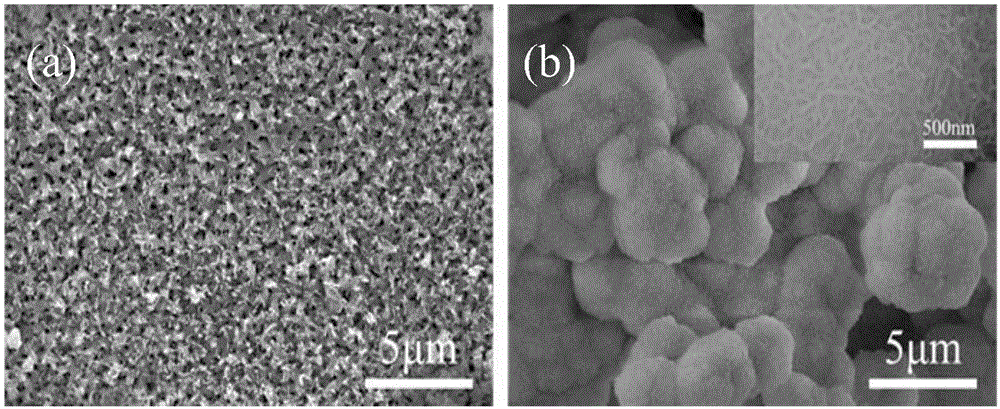
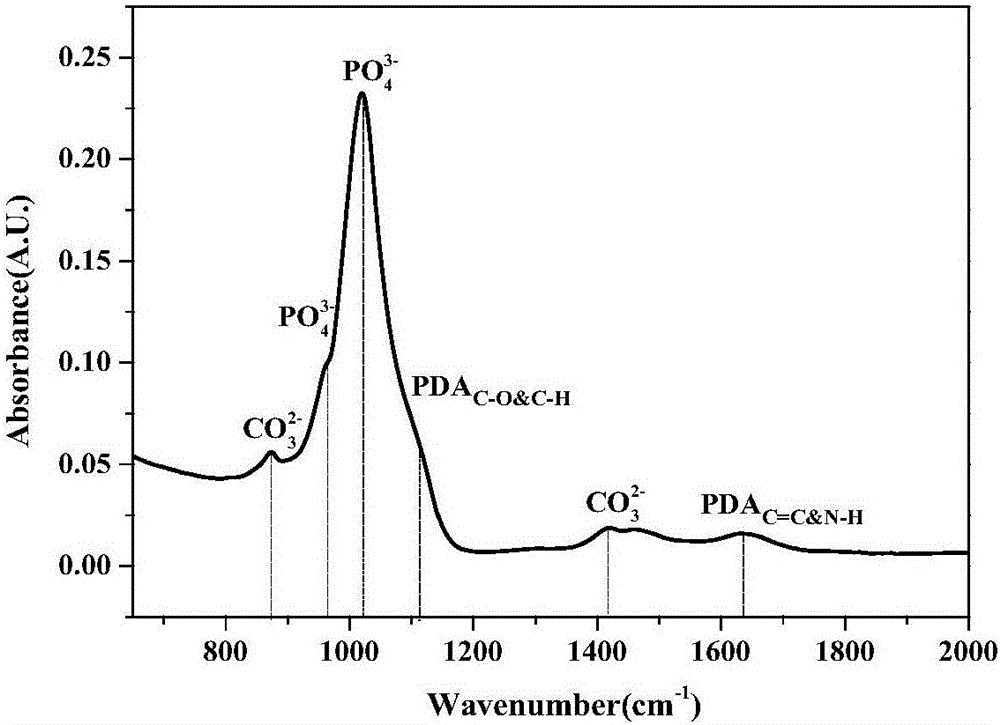
Preparation method of dental implant and composite surface thereof
InactiveCN105903076AImprove corrosion resistanceImprove surface hydrophilicityPharmaceutical delivery mechanismTissue regenerationOsteoblastNa k atpase activity
The invention relates to a preparation method of a dental implant and a composite surface thereof. In the dental implant, medical titanium or titanium alloy is used as a substrate, and porous morphology is prepared on the surface while polydopamine and hydroxyapatite are deposited to obtain a dental implant composite surface with good bioactivity. In the invention, a uniform multi-layer pore oxidation film is quickly formed on the surface of the titanium substrate to obtain nano porous morphology; and by enabling a thin layer of protein analogue (polydopamine) to tightly adhere to the surface, the surface hydrophilicity and the corrosion resistance of the dental implant can be improved. Finally, the hydroxyapatite deposition is induced on the surface to obtain a three-layer composite surface; moreover, the nano porous composite surface can effectively reinforce the adhesion and spreading of osteoblast on the implant surface as well as the alkaline phosphatase activity, early bone integration of the implant is accelerated, and firm combination of the material and the organism interface is realized so as to improve the stability and success rate of the implant.
Owner:UNIV OF SCI & TECH BEIJING
Reporter gene system for use in cell-based assessment of inhibitors of the Hepatitis C virus protease
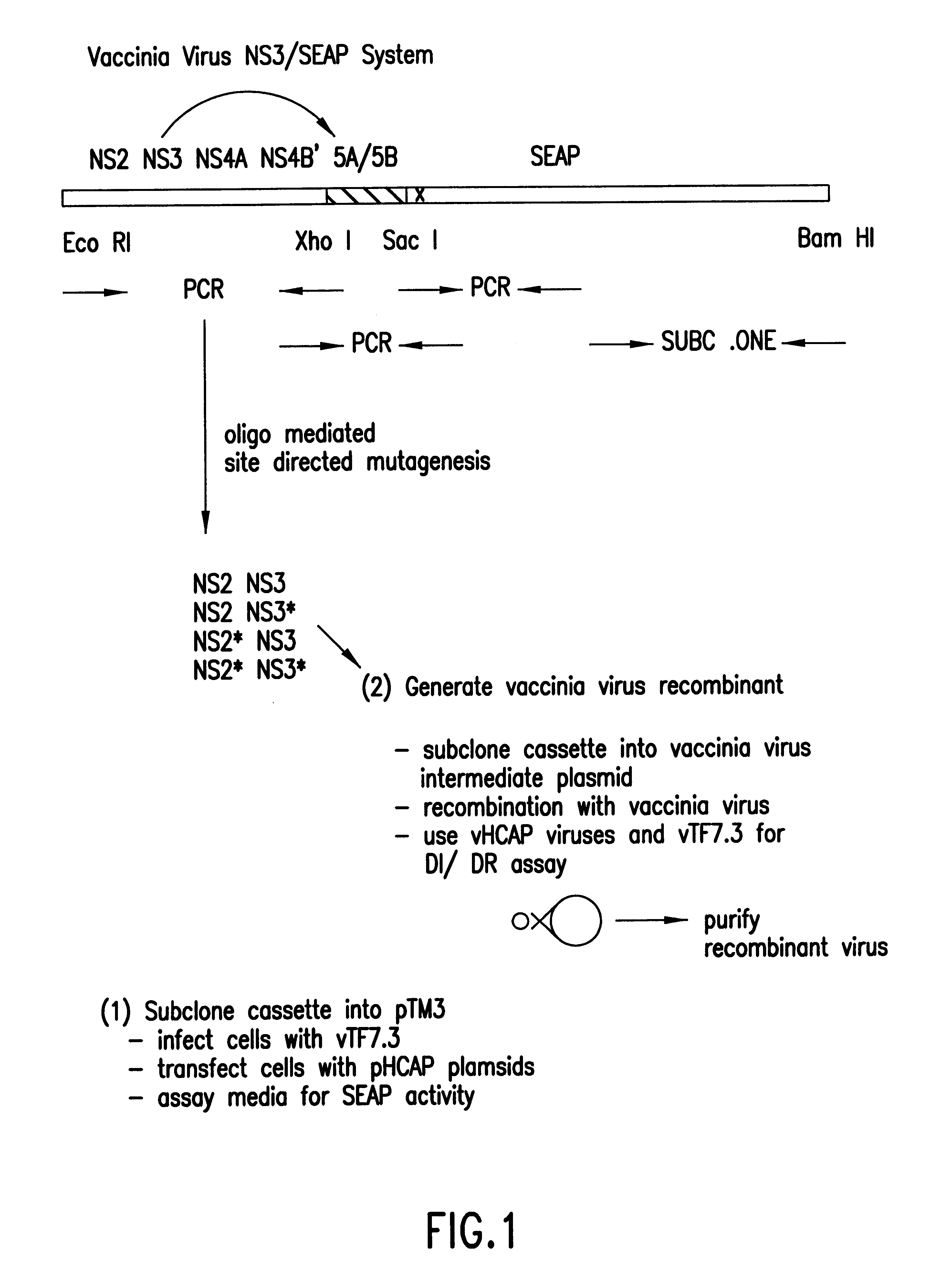
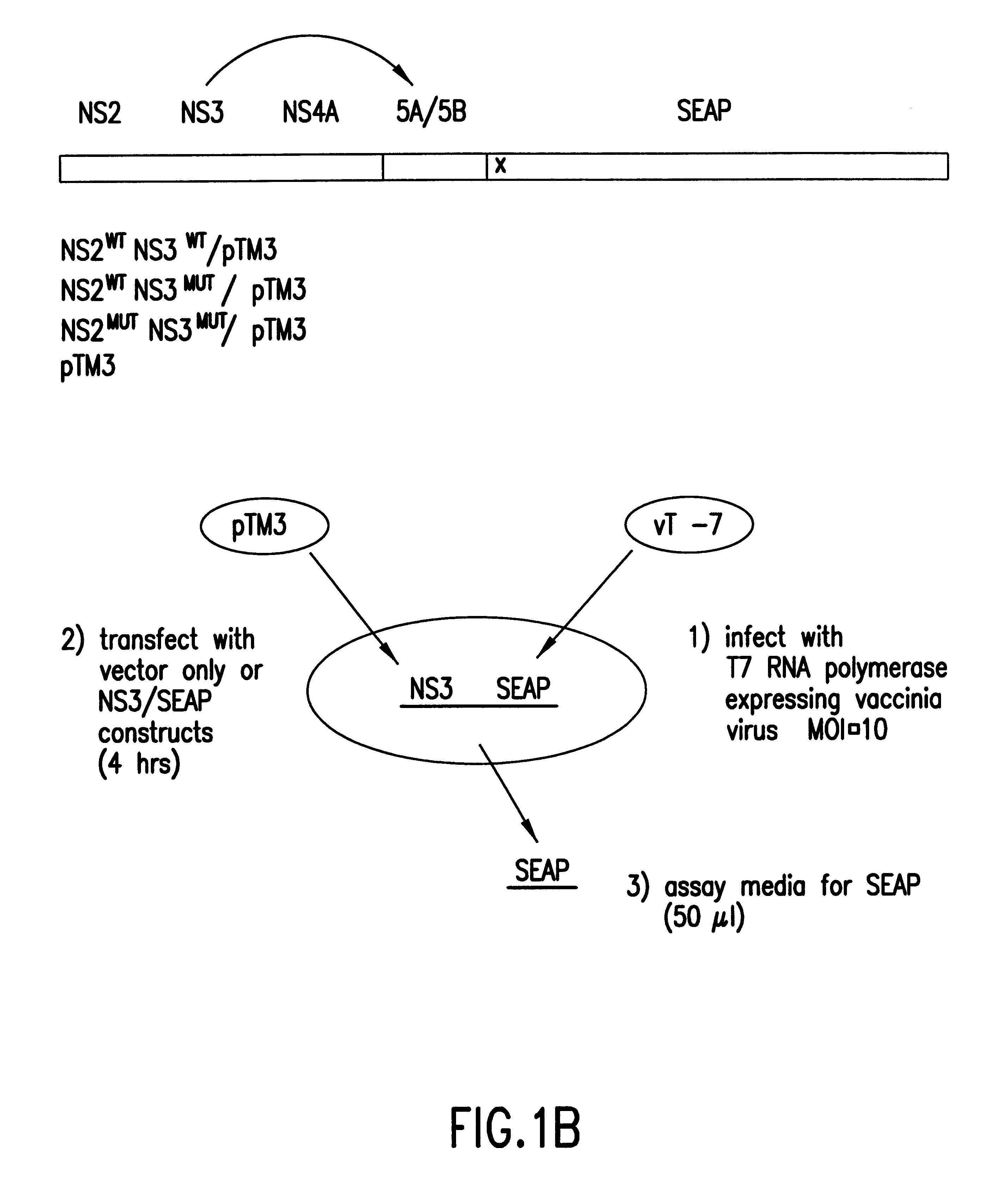
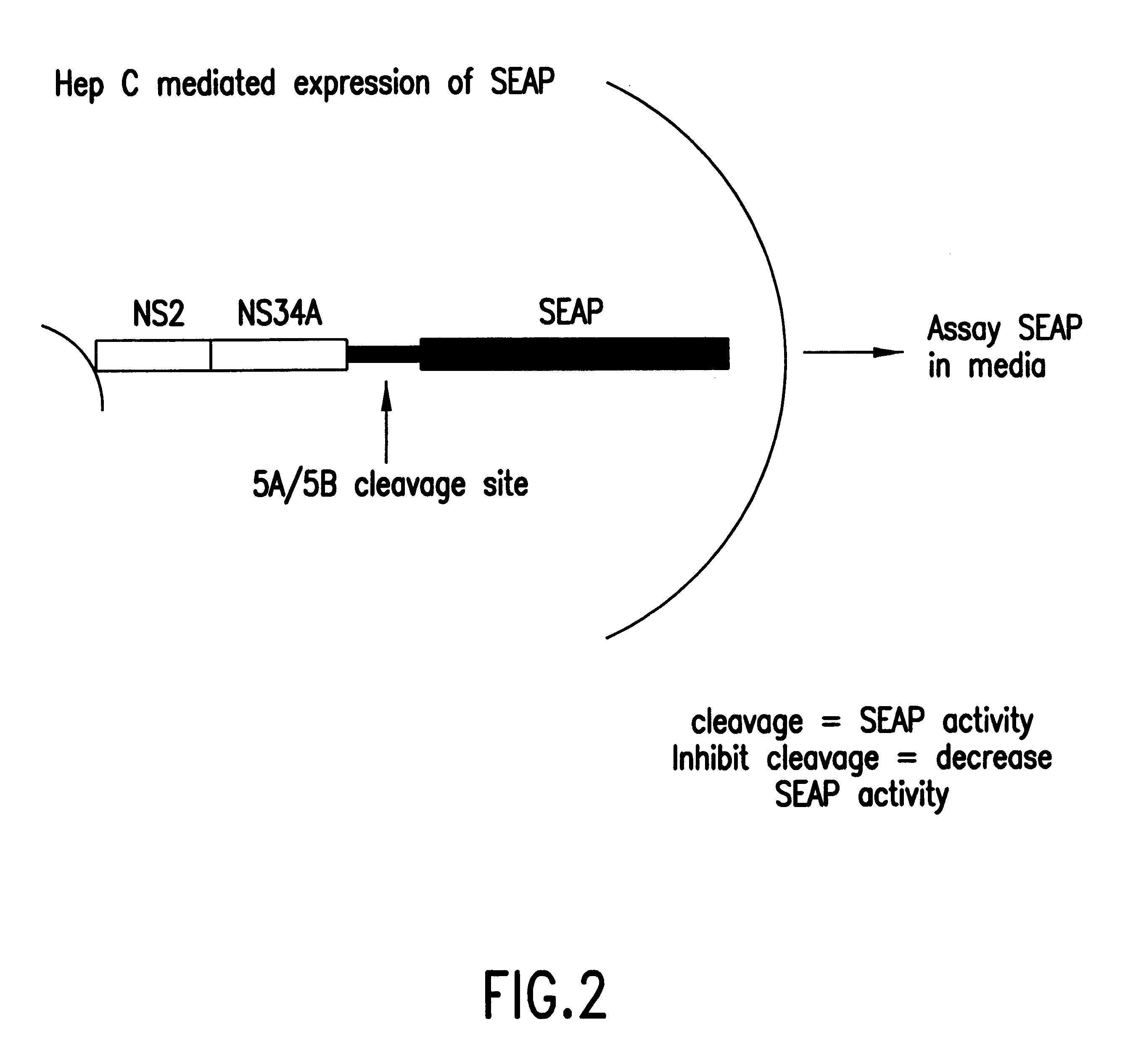
InactiveUS6280940B1High sensitivityDecrease intraSsRNA viruses positive-senseMicrobiological testing/measurementMammalCell culture media
A cell-based assay system in which the detection of the reporter gene activity, or secreted alkaline phosphatase (SEAP), is dependent upon the protease activity of the Hepatitis C virus NS3 gene product. This system can be used to assess the activity of candidate protease inhibitors in a mammalian cell-based assay system. The assay system is simpler than previously described assays due to the use of SEAP which allows the reporter gene activity to be quantified by measuring the amount of secreted gene product in the cell media by monitoring the conversion of luminescent or calorimetric alkaline phosphatase substrate.
Owner:AGOURON PHARMA INC
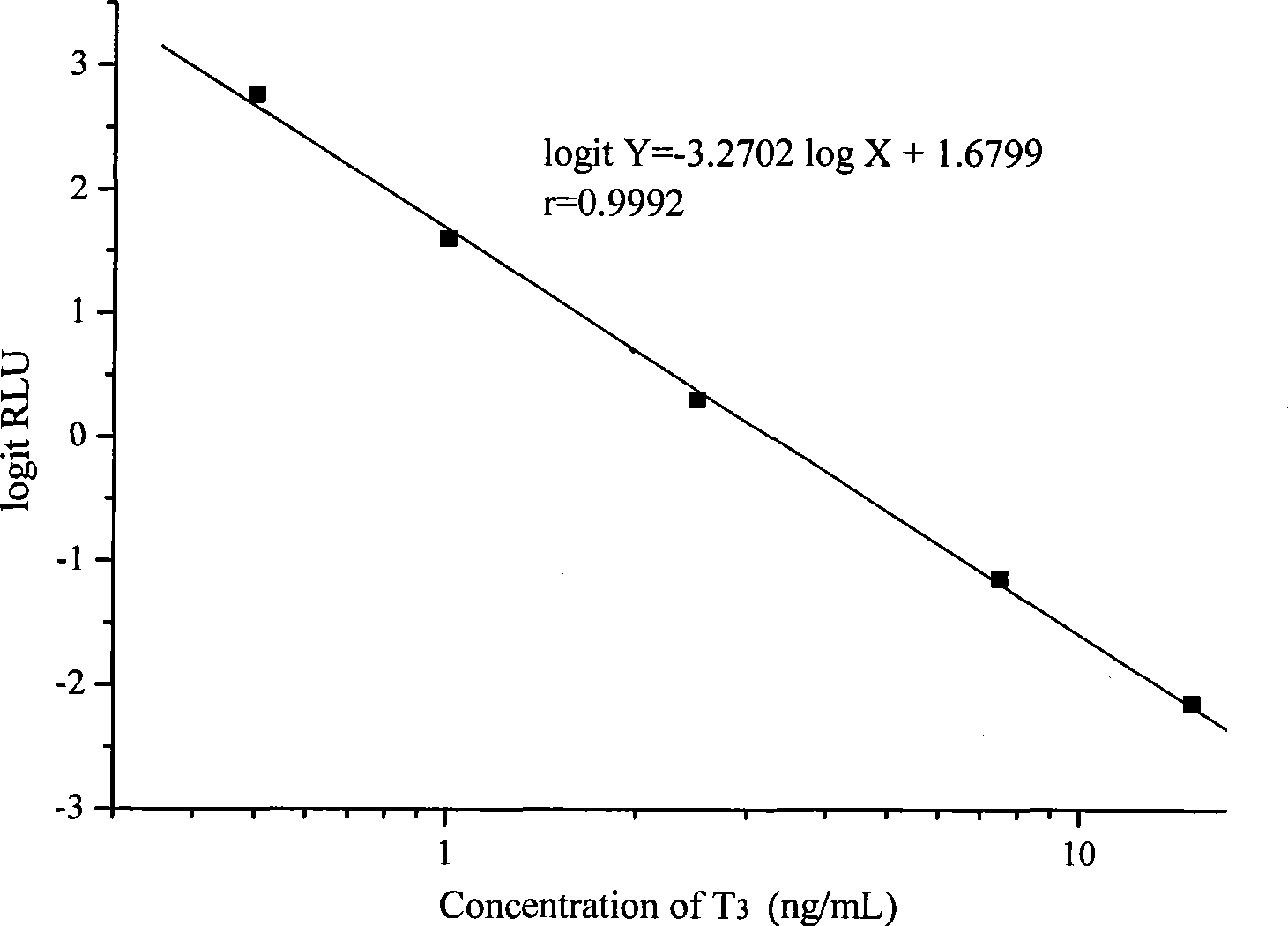
Chemoluminescence immunoassay measuring kit and preparation method thereof for triiodothyronine magnetic particles
InactiveCN101545913AEasy to measureStability determinationChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexAntigen
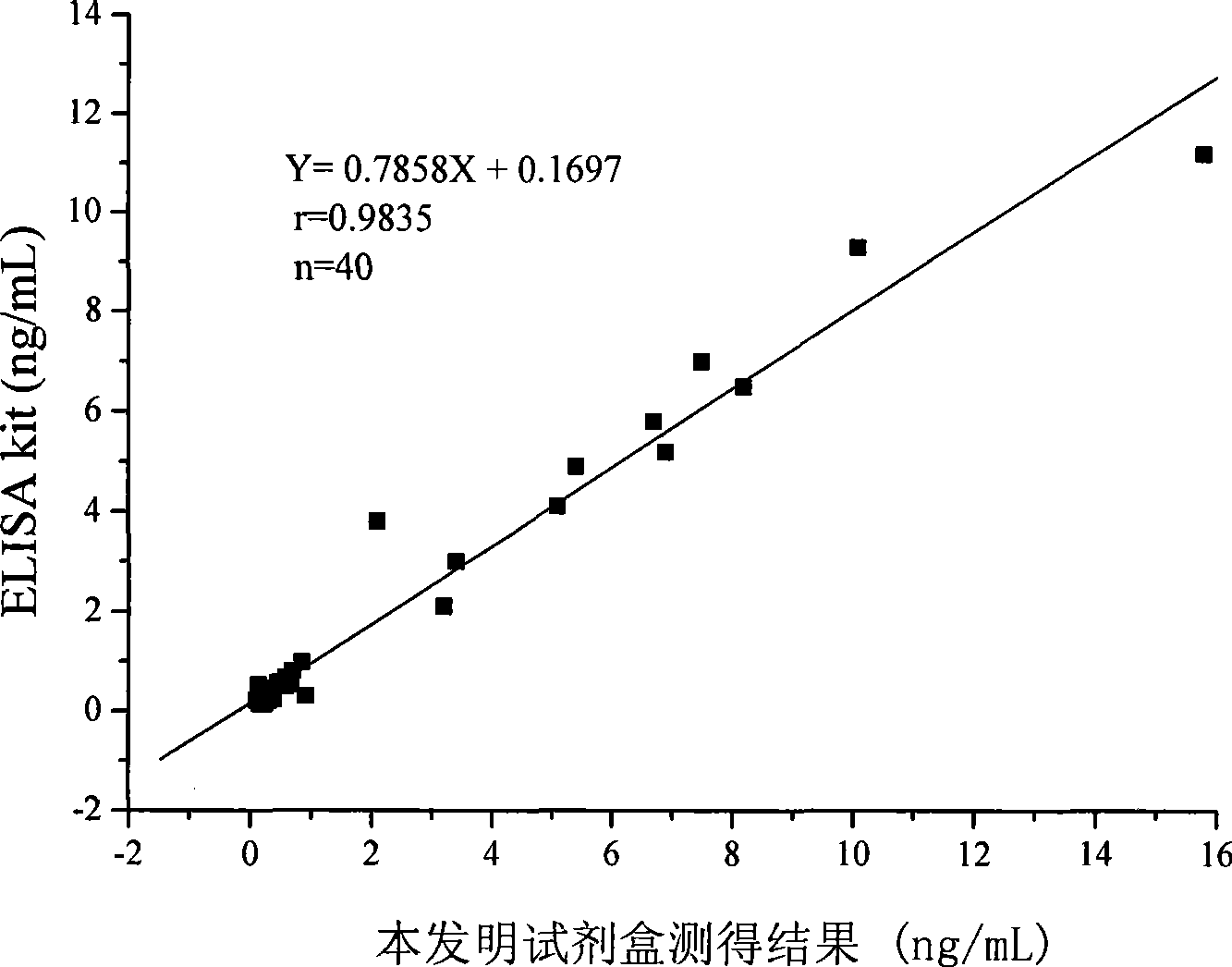
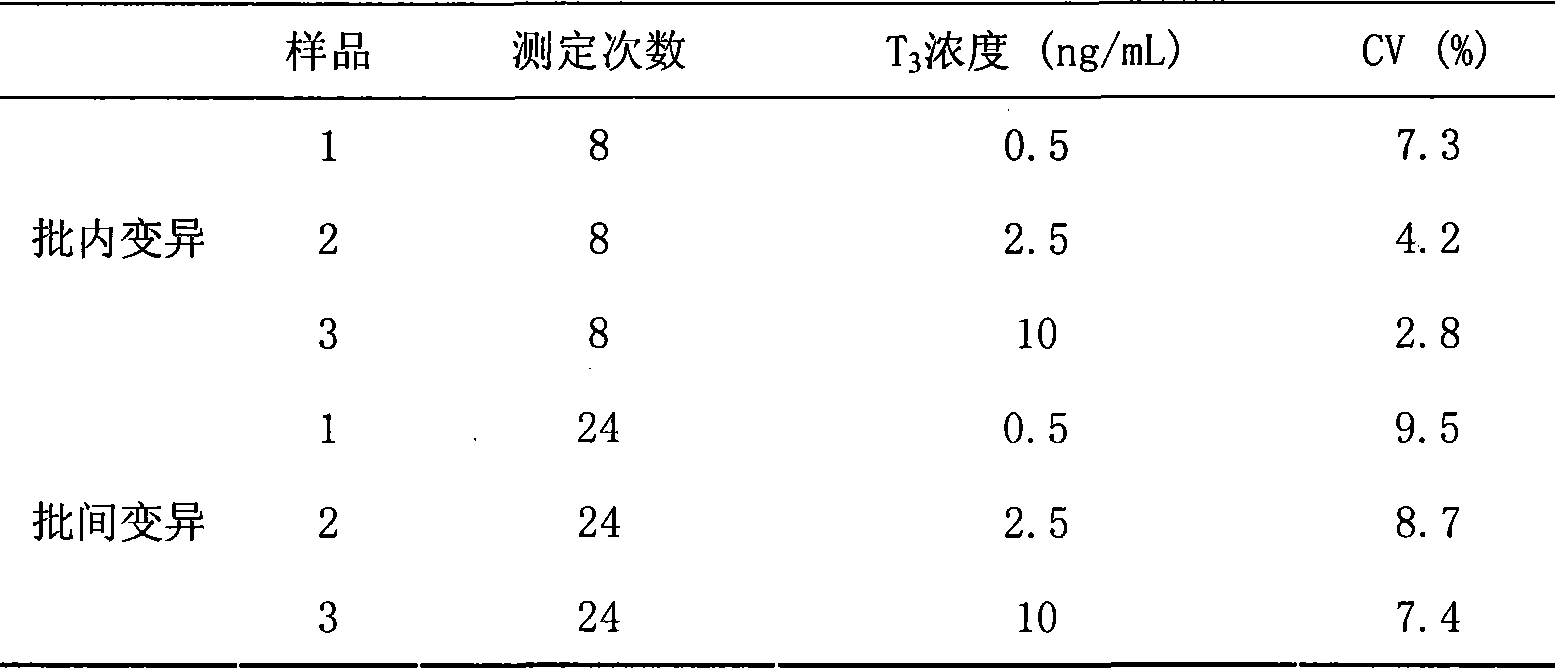
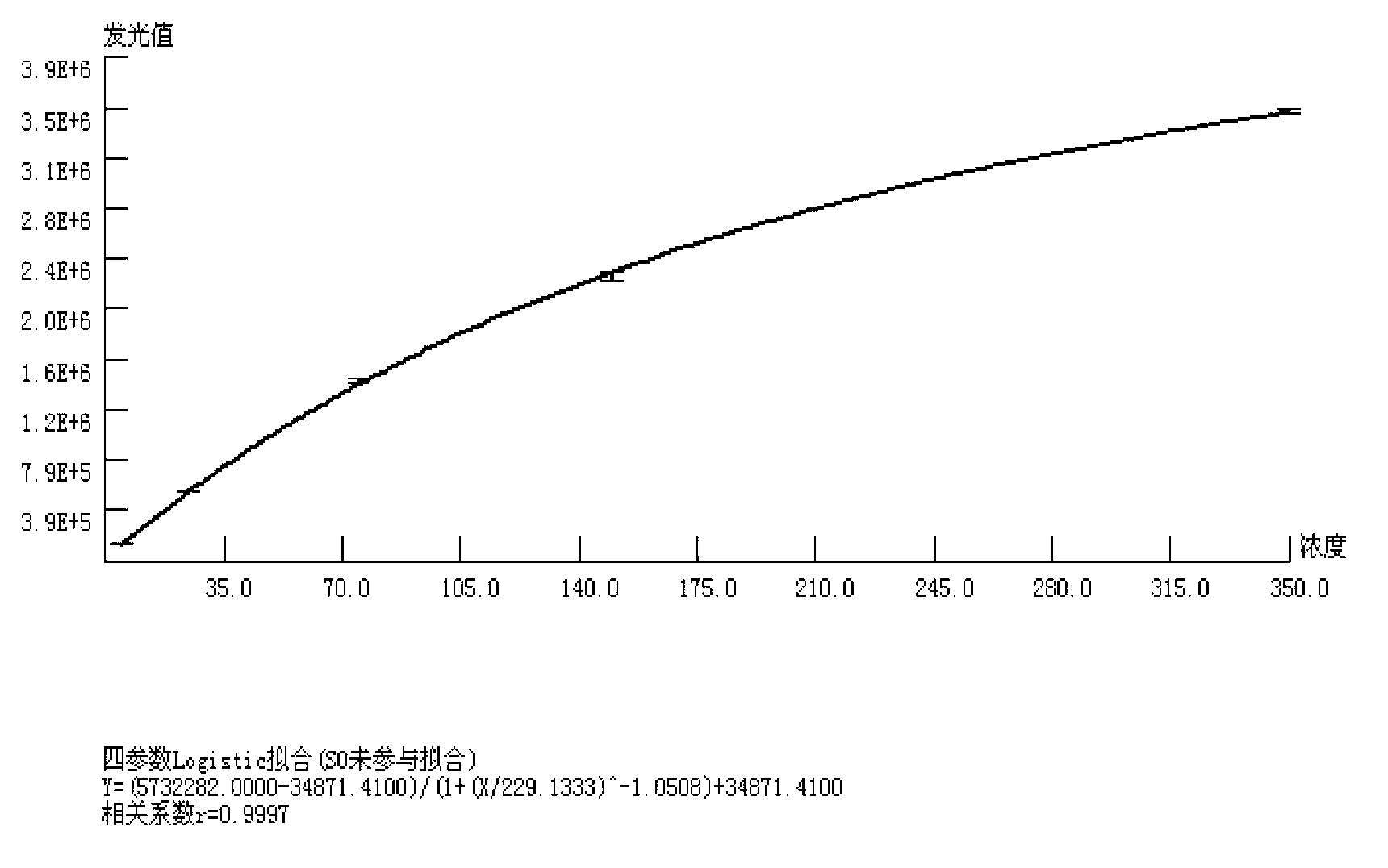
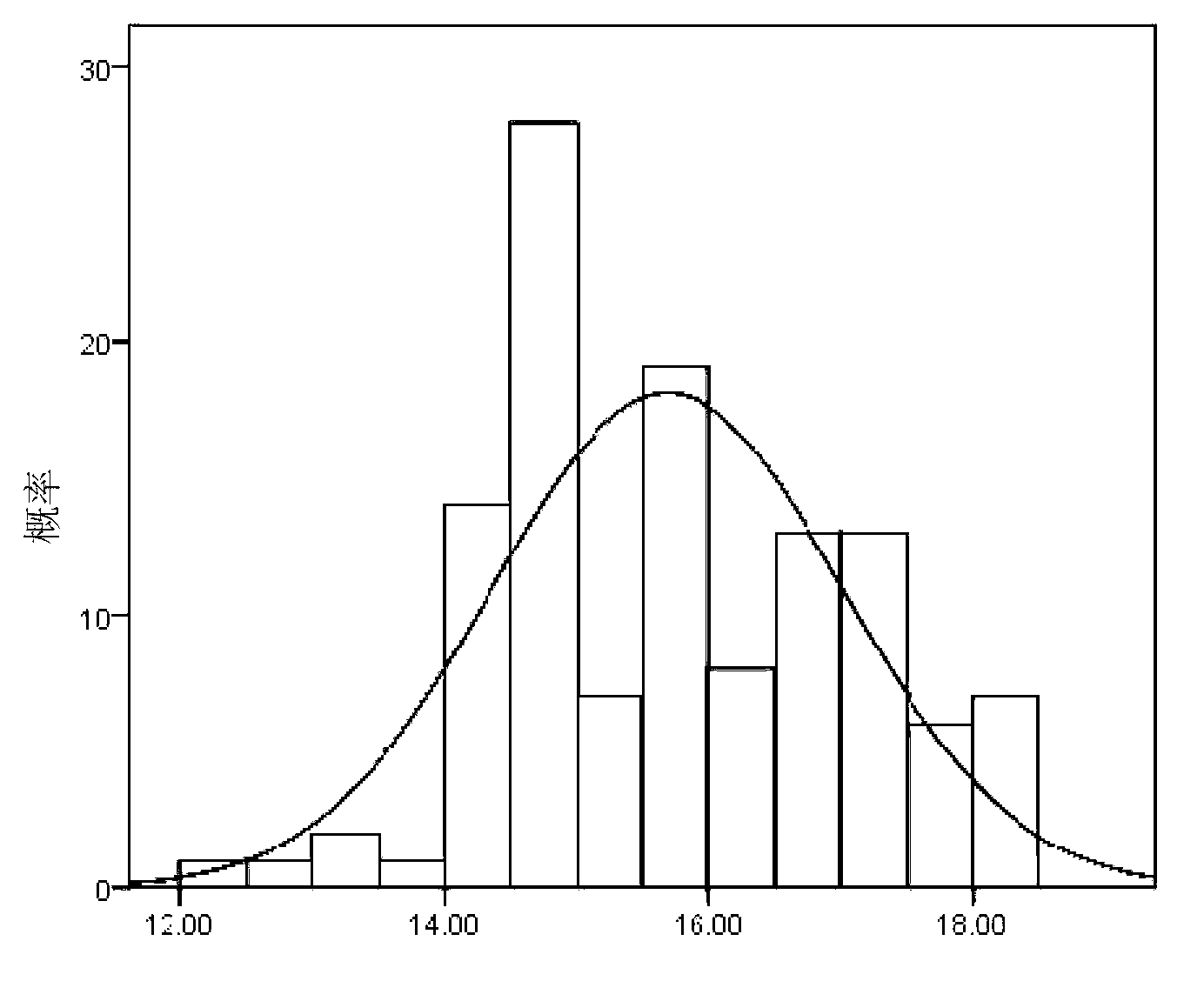
The invention provides a chemoluminescence immunoassay measuring kit and a preparation method thereof for quantificationally detect triiodothyronine (T3) magnetic particles. The kit mainly comprises a triiodothyronine serial calibration sample, magnetic particle solution coated by an anti-fluorescein isothiocyanate (FITC) monoclonal antibody, a T3 antigen marked by biotin, T3 monoclonal antibody marked by FITC, streptavidin marked by alkaline phosphatase, chemoluminescence substrate solution and 20-time concentrated washing solution. The invention adopts a competitive-method reaction mode, effectively utilizes the chemoluminescence technology combined with magnetic particles and biotin-avidin immunity magnifying technology principle to quantificationally detect the content of T3 in blood serum and blood plasma samples of human bodies and ensure the sensitivity of the detection. The kit is simple, convenient, fast, sensitive and stable to use, and provides a very valuable detection method for clinic diagnosis and scientific research works.
Owner:北京科美东雅生物技术有限公司
Quantitative detection kit for neuronspecific enolase (NSE) and preparation method and application thereof
InactiveCN102914650AImprove stabilityEasy to operate manuallyMaterial analysisBiotin-streptavidin complexAntigen
The invention relates to a quantitative detection kit for NSE and a preparation method and application of the quantitative detection kit. The kit comprises a calibrator, a magnetic separation reagent, an enzyme reactant, a stable reinforcing agent and a chemiluminiscent substrate, wherein the calibrator is obtained by treating NSE antigen through a reducing agent solution and diluting the NSE antigen to a buffer solution containing a nonionic surfactant; the magnetic separation reagent is obtained by immunofixation of a biotinylation antibody and streptavidin magnetic particles; the enzyme reactant comprises a NSE tracing antibody marked by alkaline phosphatase; and the stable reinforcing agent comprises a multicomponent immune compound interfered by an anti-heterophilic antibody. The invention further relates to a preparation method of the kit and a method of applying the kit to quantitatively detect a tumor marker NSE. The kit is reliable in performance, high in flexibility, and wide in linear range, and matched up with an automatic instrument for use. At present, the kit has already obtained a third registration certificate of a diagnostic reagent in SFDA (State Food and Drug Administration).
Owner:BEIJING DIACHA BIO ENG
Chemiluminescence immunity analysis detecting myocardium calcium protein T hypersensitization method for acridine ester and alkaline phosphatase
InactiveCN101226200AChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexCalcium protein
The invention relates to a chemical illumination immunity analysis method for checking human cTnT, which uses acridiniumester and / or alkaline phosphatase as label. The immunity reaction uses two-site immunoassay and / or competition law. The immunity reaction can use streptavidin-biotin two-site immunoassay to improve sensitivity. The invention uses NaOH and H2O2 as acridiniumester illumination initiating agent, uses 1, 2-dioxo cyclohexane derivative (adamantine derivative) as the illumination substrate of alkaline phosphatase, and uses fluorescein derivative and surface activator as illumination renforcing agent. The solid carrier is orifice plate, macromolecule polymer tube (ball) and ferriferrous oxide magnetic particles or the like.
Owner:天津天美生物技术有限公司
Ionic liquid-graphene nanocomposite, preparation method and electrochemical immunodetection method thereof
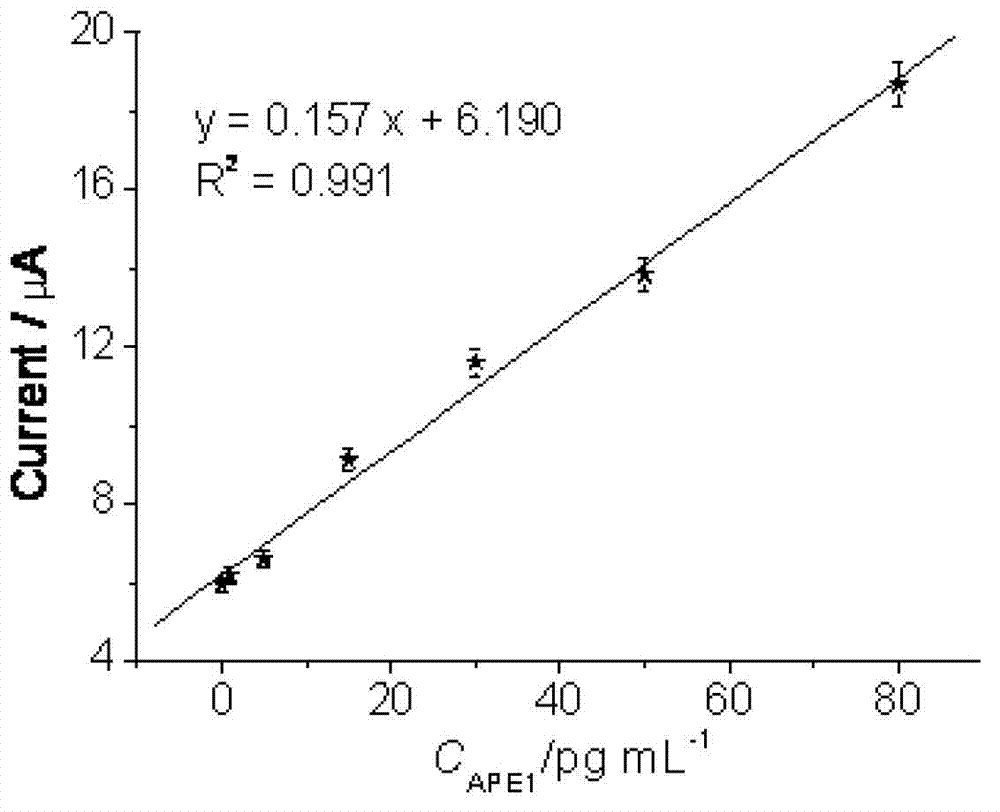
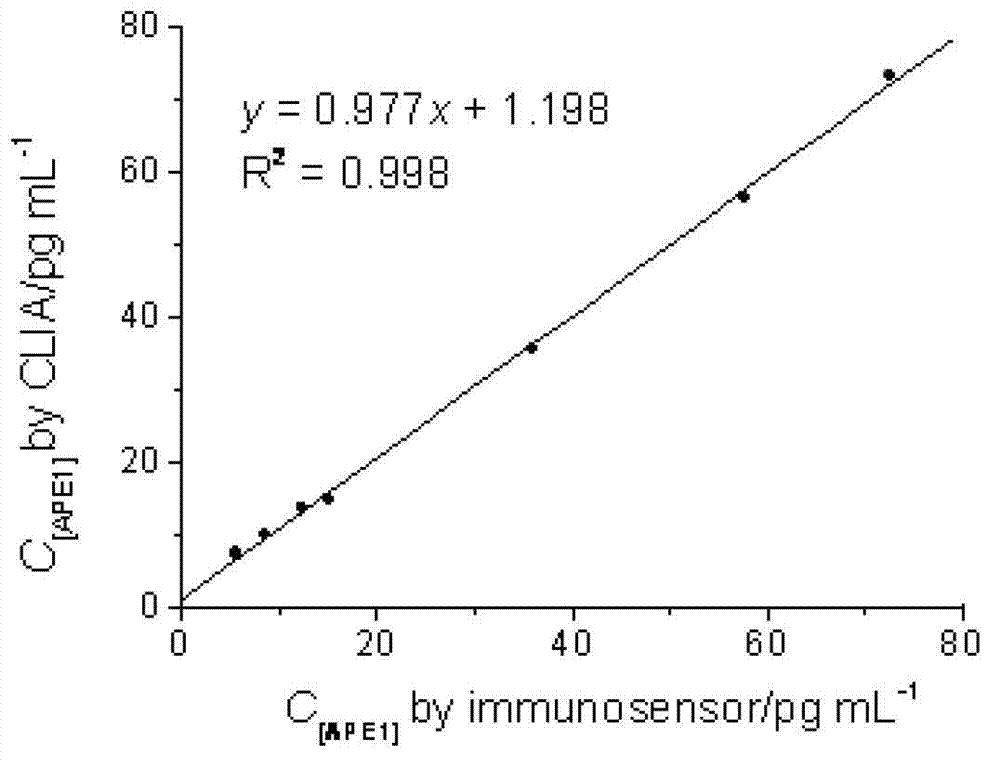
The invention relates to the field of electrochemical immunodetection, and especially relates to an ionic liquid-graphene nanocomposite, a preparation method and an electrochemical immunodetection method thereof. According to the immunodetection method, through using a double-antibody sandwich method, an apurinic / apyrimidinic endonuclease / redox factor antibody (anti-APE1) fixedly carried on the surface of an electrode carries out immunoreaction with an apurinic / apyrimidinic endonuclease / redox factor (APE1) in a sample solution, and is then combined with a room-temperature ionic liquid-graphene nanocomposite and an anti-APE1 co-coupling object marked by alkaline phosphatase (ALP) and ferrocene (Fc). Based on the electrochemical activities of the ALP-Fc-anti-APE and the room-temperature ionic liquid-graphene nanocomposite, a CV (cyclic vohammetry) catalytic current value is measured, and then the concentration of the APE1 in a detection sample is detected. The linear response range of the electrochemical immunodetection method provided by the invention is 0.1-80 pg / mL, and the lower detection limit is 0.04 pg / mL, therefore, the electrochemical immunodetection method is good in specificity and high in sensitivity.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
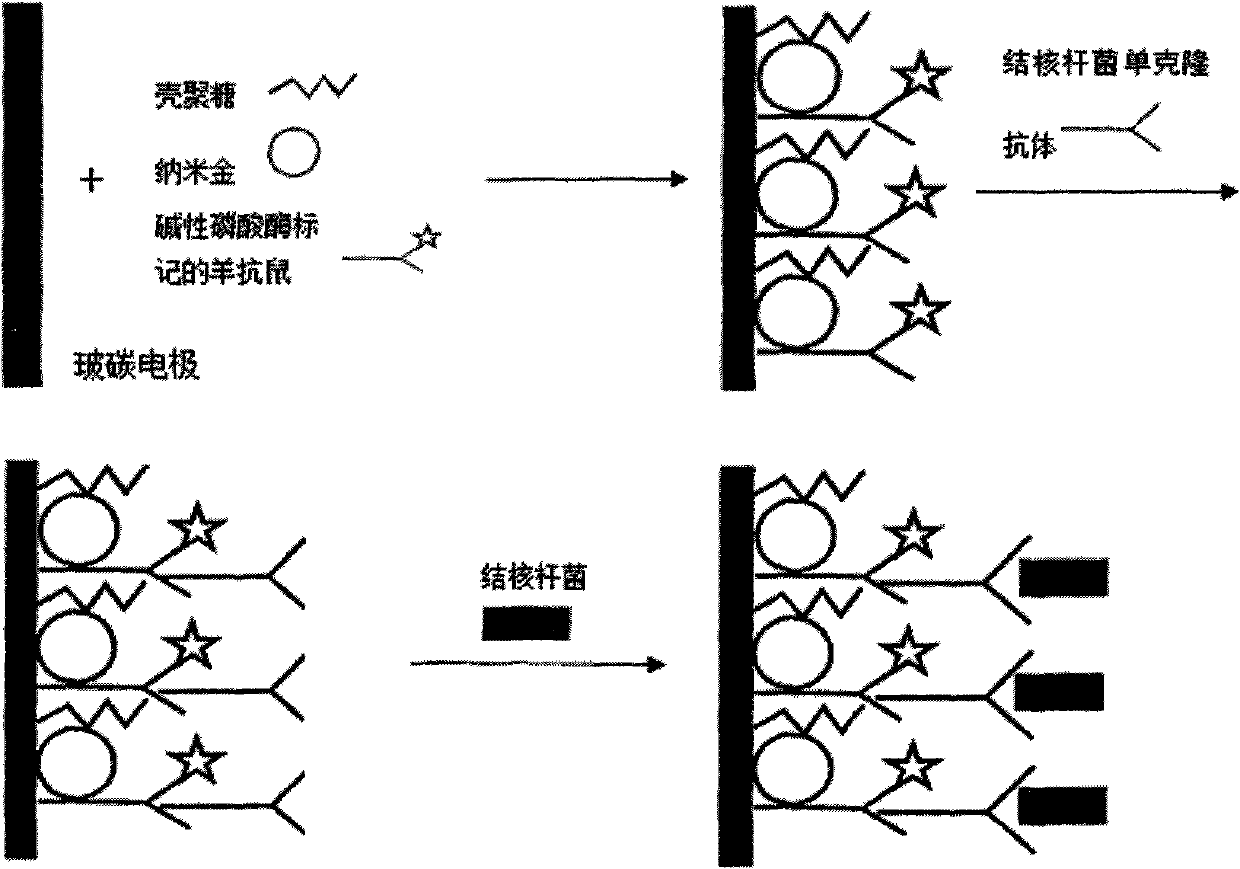
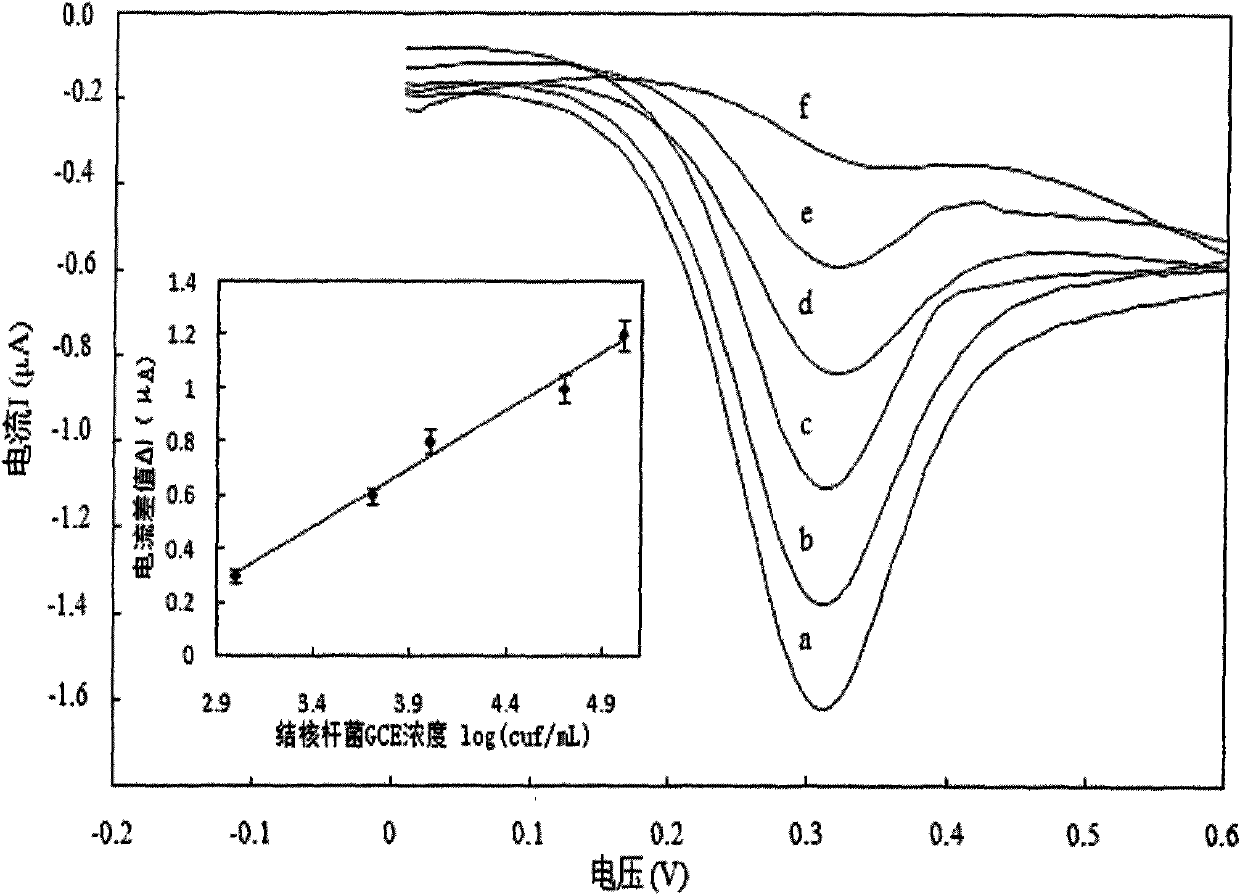
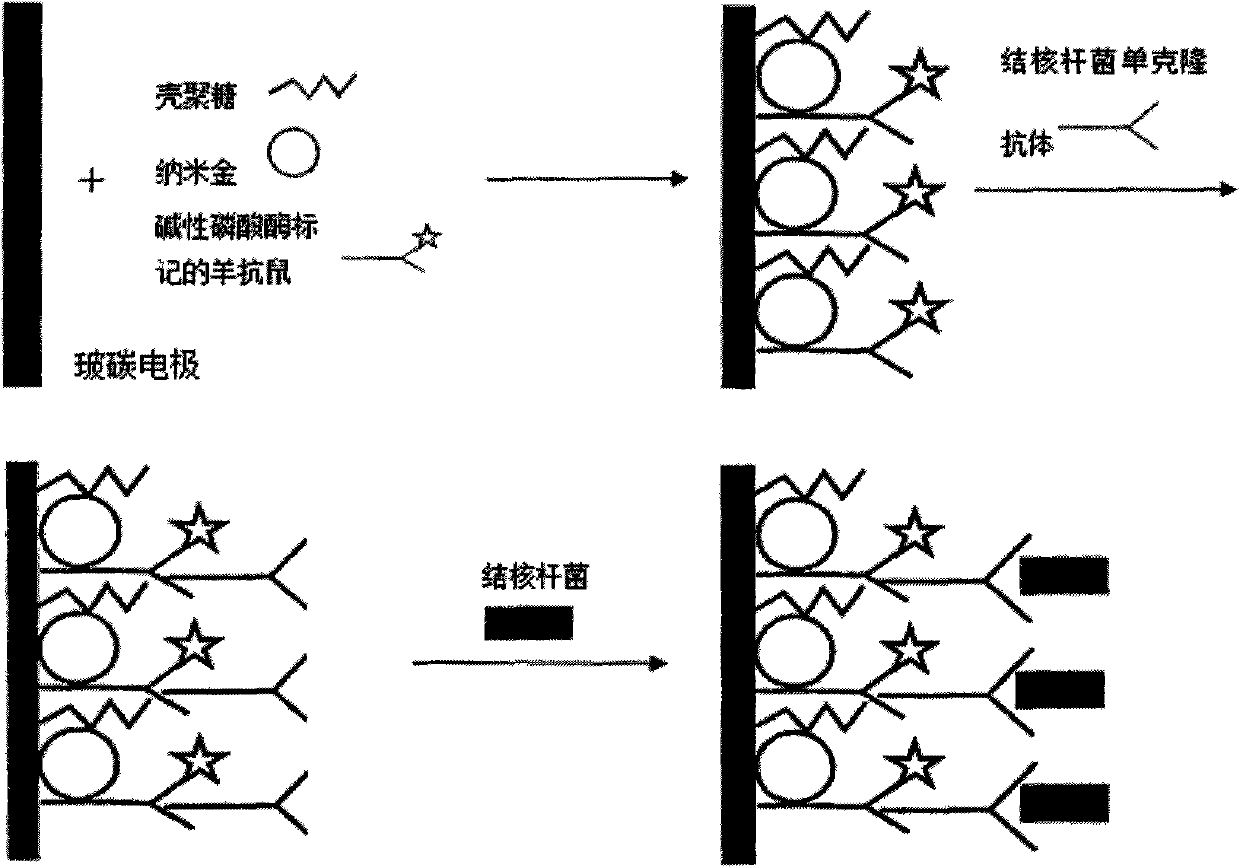
Chitosan-nano-gold enzyme immunosensor for detecting mycobacterium tuberculosis and application thereof
InactiveCN102087283AEasy to prepareMaintain biological activityBacteriaMaterial analysis by electric/magnetic meansDifferential pulse voltammetryCell wall
The invention relates to an enzyme immunosensor based on chitosan and nano-gold, and by using the specific combination of the monoclonal antibody of the cell wall of mycobacterium trberculosis with mycobacterium trberculosis, the enzyme immunosensor is applicable to the rapid detection of mycobacterium trberculosis in milk. The characteristic is that the enzyme immunosensor for detecting mycobacterium tuberculosis is prepared by the following steps: modifying the surface of a glassy carbon electrode through electrochemical deposition method by a mixed solution of chitosan gel, nano-gold solution, and goat anti-mouse antibody labeled by alkaline phosphatase; and fixing the mycobacterium trberculosis monoclonal mouse antibody on the surface of the glassy carbon electrode modified by the goat anti-mouse-chitosan-nano-gold film which is labeled by alkaline phosphatase through the specific antigen-antibody reaction. Quantitative analysis is performed by detecting the electric signal changegenerated before and after the incubation reaction of mycobacterium trberculosis and the modified electrode through differential pulse voltammetry. The preparation method of the sensor is simple, andthe sensor has the advantages of high sensitivity, short detection time, and simple operation, and is applicable to the method for the rapid detection of mycobacterium trberculosis.
Owner:HUAZHONG UNIV OF SCI & TECH
Kit for joint detection of ovarian cancer tumor markers HE4 and CA125 as well as preparation method and application thereof
ActiveCN108008132AAddresses issues that interfere with alkaline phosphatase-catalyzed luminescenceAchieving Simultaneous DetectionDisease diagnosisCoatingsPeroxidasePhosphoric acid
The invention relates to technical field of immunodetection, particularly relates to a kit for joint detection of ovarian cancer tumor markers HE4 and CA125 as well as a preparation method and application thereof. According to the kit provided by the invention, silanization treatment is performed on horse radish peroxidase, so that the possibility that the horse radish peroxidase becomes a phosphoric acid receptor in a process of catalyzing a chemiluminescent substrate to emit light by a alkaline phosphatase is blocked, the problem that the existence of horse radish peroxidase disturbs the cataluminescence of the alkaline phosphatase is solved, so that the size of the light intensity caused by the alkaline phosphatase on magnetic particles is detected by chemiluminescent substrate liquid first, the size of chromogenic absorbancy caused by the horse radish peroxidase is detected by chromogenic substrate liquid and the simultaneous detection on HE4 and CA125 of a sample is realized. Thekit provided by the invention has the advantages that the detection process is simple, the reaction time is short, the reagent dose is less, the cost is reduced, the sensitivity is high, the repeatability and stability of the reagent are good.
Owner:北京惠中医疗器械有限公司 +1
Rat embryonic stem cell
ActiveUS20120142092A1Microbiological testing/measurementHybrid cell preparationEmbryoNa k atpase activity
The present invention provides a rat embryonic stem cell characterized by having the following properties of (a) expressing Oct3 / 4 gene and Nanog gene, (b) positive for alkaline phosphatase activity, (c) having an embryoid body forming ability, (d) expressing SSEA (Stage-Specific Embryonic Antigen)-1 and SSEA-4, (e) having the same number of chromosomes as does a normal rat cell, (f) capable of being subcultured and holding the undifferentiated state, (g) having in vitro pluripotency, (h) having a potential to differentiate for cells of three embryonic germ lineages, (i) having teratoma formation ability, and (j) having an ability to produce a chimeric rat, a method of establishing the aforementioned rat embryonic stem cell and the like.
Owner:SUMITOMO CHEM CO LTD
Kit for detecting osteocalcin content and testing method thereof
InactiveCN106353506AHigh detection sensitivityImprove precisionBiological testingBone gla proteinQuality control
The invention discloses a kit using a magnetic particle chemiluminiscence method to measure osteocalcin (BGP) content. The kit comprises a calibration article, a quality control article, a resisting reagent, a magnetic particle reagent and a light-emitting substrate, wherein the resisting reagent comprises an antibody marked by fluorescein isothiocyanate and coated by osteocalcin and an antibody marked by alkaline phosphatase and osteocalcin; the magnetic particle reagent is used for connecting magnetic particles with anti-goat FITC. The kit has the advantages that the chemiluminiscence technology is combined with immune magnetic particles, a reaction system close to homogeneous phase is provided, a one-step reaction mode is used, the detection sensitivity and precision of the kit are increased greatly, the detection range of the kit is increased, reaction time is shortened greatly, time from sample feeding to a detecting result is less than 35 minutes, and the detection speed of the kit is evidently higher than that of similar kits; the kit can detect multiple samples at the same time on full-automatic chemiluminescence apparatus, high-throughput and fast measuring of the osteocalcin is achieved, the kit is high in accuracy and high in specificity, and the accuracy and detection efficiency of the kit are increased greatly.
Owner:JIANGSU ZECEN BIOTECH CO LTD
Application of iridoid in preparation of anti-osteoporosis medicines
InactiveCN102389440AGood differentiation effectProliferation effect is goodOrganic active ingredientsComponent separationPharmaceutical drugAnti osteoporosis
The present invention relates to an application of iridoid in the preparation of anti-osteoporosis medicines, which belongs to the medicines technical field. The invention provides an application of iridoid which is shown in a general formula I in the preparation of medicines used for treating and / or preventing osteoporosis. Experiments found that ALP activity in UMR106 cells is taken as guidance for tracing and separating an extract of iridoid possessing general formula I, the extract and each separated component are researched from the aspects of osteoblast propagation and antioxidation activity, the iridoid possessing the general formula I has the effects of substantially promoting alkaline phosphatase activity and osteoblast differentiation, so that the iridoid has anti-osteoporosis effect and can be used for preparing the medicines used for treating or preventing osteoporosis. The component of specnuezhenide and a compound G13 possesses the effects of substantially promoting the alkaline phosphatase activity and osteoblast differentiation, and the specnuezhenide has effects for obviously promoting UMR-106 cell proliferation, a better effect is obtained when the specnuezhenide is used for anti-osteoporosis effect.
Owner:魏园
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com