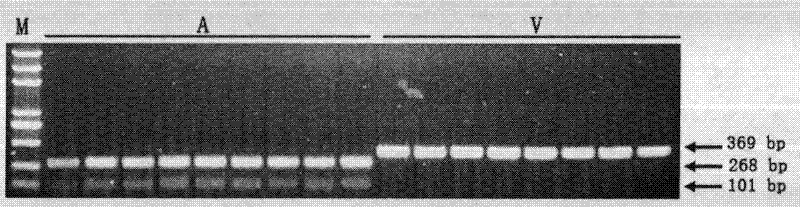
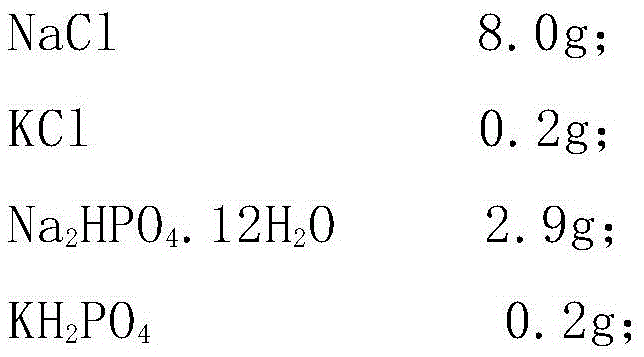
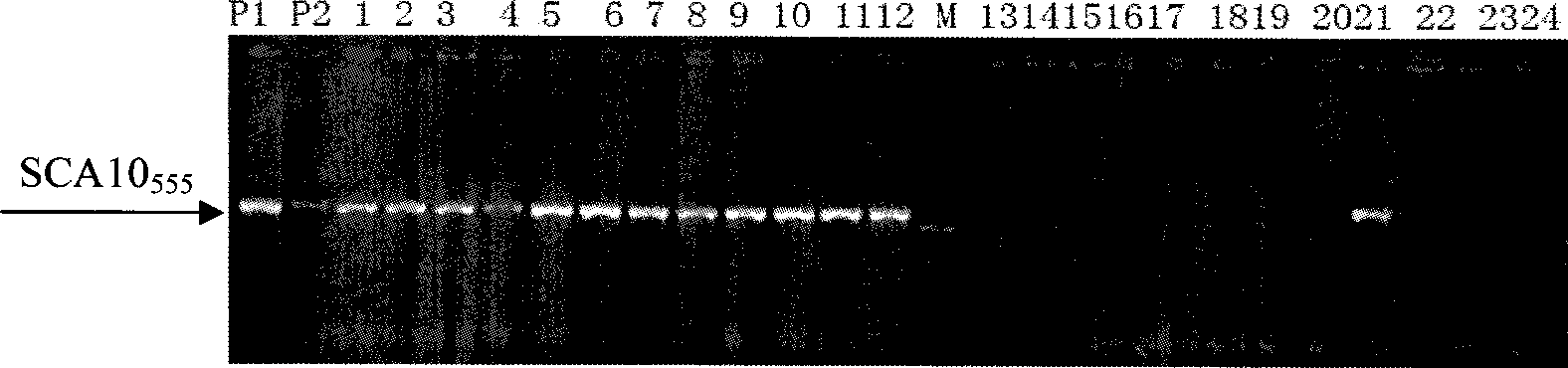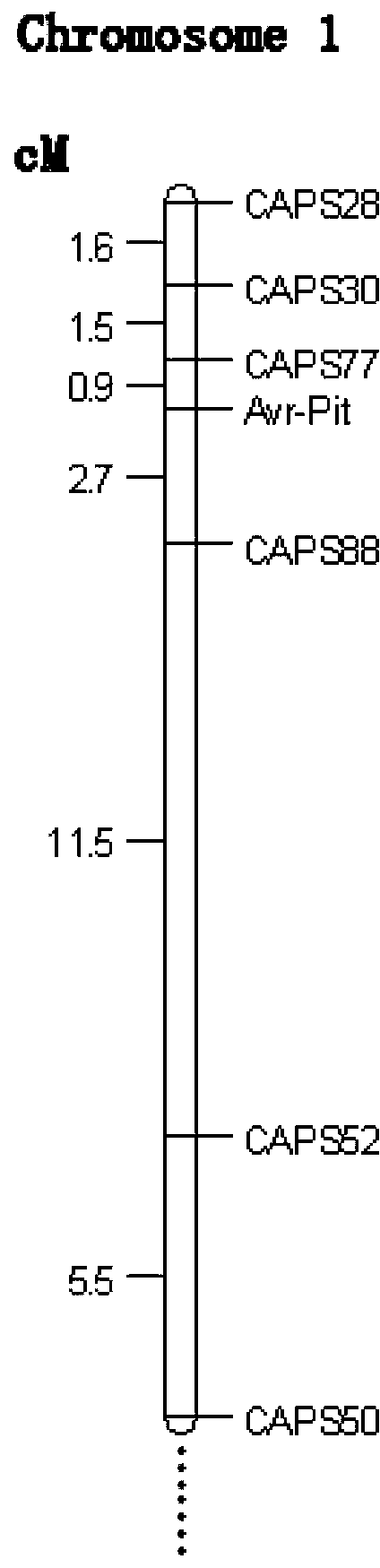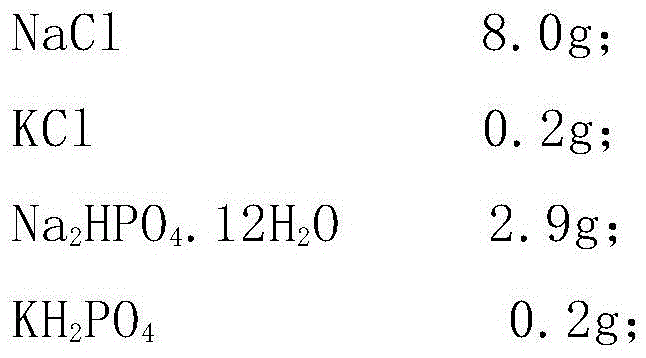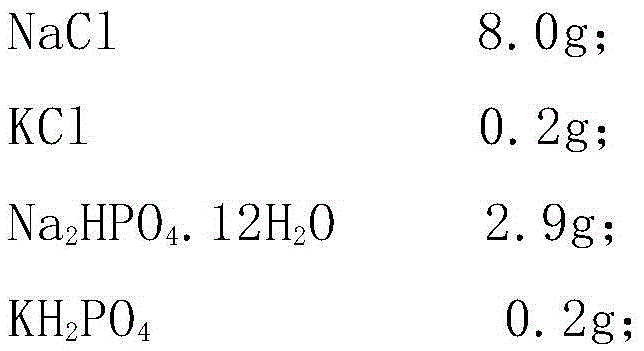Patents
Literature
91results about How to "Mark stable" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alkaline phosphatase labeled antigen-antibody diluent
The invention relates to a medical reagent, and particularly relates to an alkaline phosphatase labeled antigen-antibody diluent. The diluent is characterized by consisting of the following substances: a phosphate buffer, polyhydroxy organic compounds, amino acid and protein compounds, metal ions, a nonionic surface active agent, and a preservative. Compared with the prior art, the diluent provided in the invention can stabilize the activity of an alkaline phosphatase labeled antigen-antibody, and the alkaline phosphatase labeled antigen-antibody is prepared into a lyophilized product by using the freeze-drying method by adding the diluent into the alkaline phosphatase labeled antigen-antibody, so that the activity of the alkaline phosphatase labeled antigen-antibody can be stabilized in a long term.
Owner:AILEX TECH GRP CO LTD +1
Large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and product thereof
ActiveCN104491855AMark stableEnsure safetyMicroorganism based processesAntiviralsSucroseUltrafiltration
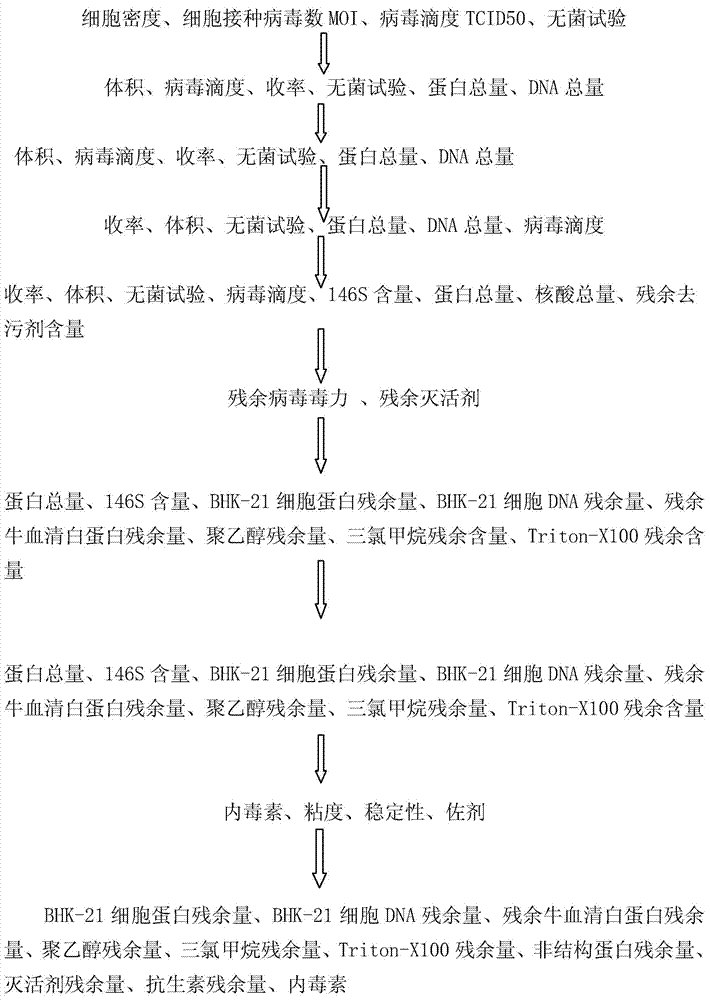
The invention discloses a large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and a product thereof. The method comprises the following steps: a)collecting a virus solution; b)performing deep filtration on a membrane, performing ultrafiltration and performing enzymolysis on nuclease; c)purifying through a strong anion exchange adsorption bed or an adsorption film; d)depositing by PEG, extracting by chloroform-isoamyl aleohl; e)inactivating; F)performing density gradient centrifugation on an inactivation liquid through cane sugar and purifying; g)performing ultrafiltration dialysis and aseptic filtration; and h)reserving a stock solution or emulsifying. The provided foot-and-mouth disease totivirus marked vaccine antigen is uniform and complete foot-and-mouth virus particle, The vaccine is injected into body, so animal infection and immunization can be completely distinguished, does not contain foot-and-mouth disease virus non-structural protein and other virus particle, and does not contain animal-based foreign protein, polypeptide and oligopeptides, animal latent anaphylactic reaction, carcinogenesis and latent risk such as mad cow disease for causing animal infectious diseases due to vaccine injection can be effectively reduced, and the vaccine has no influence on animal food safety and trade.
Owner:吕宏亮 +2
Interleukin 6 chemiluminiscence detection kit and preparation method thereof
InactiveCN107817354AHigh sensitivityImprove featuresChemiluminescene/bioluminescenceBiological testingInterleukin 6Chemiluminescent immunoassay
The invention discloses a chemiluminescent detection kit for interleukin-6 and a preparation method thereof. The kit includes: sample diluent, magnetic particles coated with interleukin-6 monoclonal antibody, interleukin-6 monoclonal antibody labeled with acridinium ester, interleukin-6 series standard solution, chemiluminescence excitation solution A, chemiluminescence excitation solution B. Cleaning solution. The kit of the invention combines the chemiluminescent technology with the immunomagnetic particle, and provides a nearly homogeneous reaction system. Compared with the prior art, the kit of the invention has the advantages of high sensitivity, strong specificity, short reaction time and the like.
Owner:太原瑞盛生物科技有限公司
Chemluminescent detection kit for procalcitonin and preparation method of chemluminescent detection kit
InactiveCN107807240AHigh sensitivityImprove featuresMaterial analysisMonoclonal antibodyChemiluminescent immunoassay
The invention discloses a chemiluminescent detection kit for procalcitonin and a preparation method thereof. The kit includes: sample diluent, magnetic particles coated with procalcitonin monoclonal antibody, procalcitonin monoclonal antibody labeled with acridinium ester, procalcitonin series standard solution, chemiluminescence excitation solution A, Chemiluminescence excitation solution B, cleaning solution. The kit of the invention combines the chemiluminescent technology with the immunomagnetic particle, and provides a nearly homogeneous reaction system. Compared with the prior art, the kit of the invention has the advantages of high sensitivity, strong specificity, short reaction time and the like.
Owner:太原瑞盛生物科技有限公司
Molecular marker linked with bruchid resistance gene in mung bean
InactiveCN101358232AAssisted breeding selection with clear goalsShorten the timeMicrobiological testing/measurementPlant genotype modificationResistant genesAgricultural science
The invention relates to a molecular marker for mungbean weevil resistance gene linkage, which is used for screening mungbean weevil resistant genes. V2709, VC1973 (zhonglu No. 1) and the individual DNA of F2 separation population thereof are extracted by the CTAB method. 63 RAPD molecular markers, 100 pairs of SSR molecular markers and 28 pairs of STS molecular markers are adopted to carry out the PCR product polymorphism screening on the resistant bulk and the susceptible bulk of the two parents and the F2 progeny, and then each of F2 generation individuals is screened out the weevil resistance gene linkage marker. A RAPD primer, OPC-06 and a STS primer are found to link with a weevil resistance genes The molecular marker can target the weevil resistance gene, and the selection efficiency of weevil resistance breeding is greatly improved.
Owner:INST OF CROP SCI CHINESE ACAD OF AGRI SCI
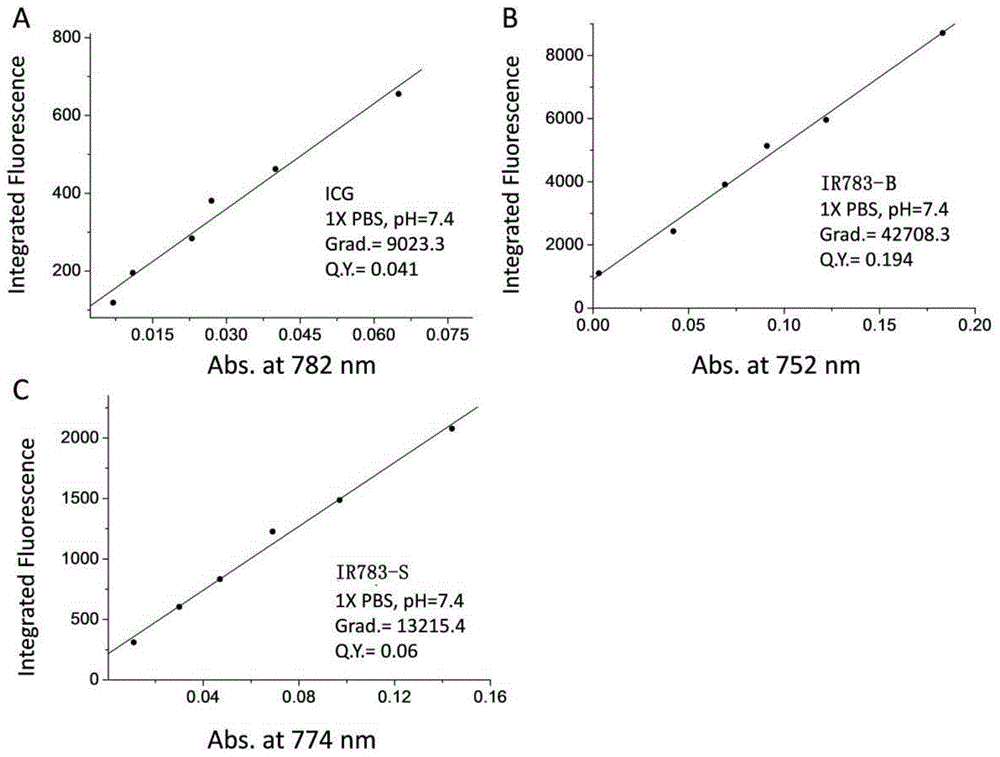
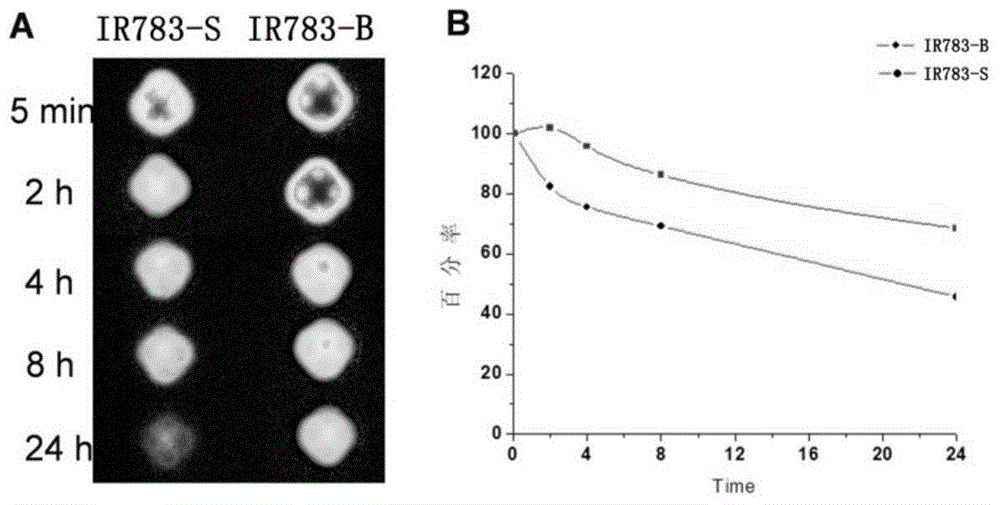
Active near infrared fluorophore as well as preparation method and application thereof
InactiveCN104673273AMild labeling conditionsHigh absorbance coefficientOrganic chemistryIn-vivo testing preparationsCarbon–carbon bondCarboxylic acid
The invention belongs to the field of molecular imaging reagents ad relates to an active near infrared fluorophore for rapidly marking bioactive molecules. A general formula of the active near infrared fluorophore is IRP-B-NHS, wherein IRP is an anthocyanin near infrared fluorophore; B is an aromatic group introduced to the secondary position of the anthocyanin fluorophore by virtue of a carbon-carbon bond; NHS is N-hydroxysuccinimide eater. A preparation method of the active near infrared fluorophore comprises the following steps: with the anthocyanin fluorophore as a parent, introducing benzene carboxylic acid into the fluorophore by virtue of the carbon-carbon bond through Suzuki-Miyaura reaction, modifying phenyl carboxylic acid to produce the N-hydroxysuccinimide eater, reacting with primary amine in a biomolecule under the physiological condition, and marking the biomolecule with the near infrared fluorophore to realize noninvasive tracing. The active near infrared fluorophore is capable of rapidly, safely, effectively and stably marking the bioactive molecules including polypeptide, proteins, antibodies or polymer molecules and has important significance of noninvasively monitoring and quantifying distribution of target active molecules in vivo.
Owner:FUDAN UNIV
Method for preparing semiconductor nano crystal of bio marker utilizing Raman signal
InactiveCN101071135AResonant Raman signal enhancementMark stableRaman scatteringSemiconductor nanocrystalsSignal enhancement
This invention belongs to nano-materials and biotechnology fields, involving the preparation of nano-materials, nano-materials in the application of biomarkers. First synthesized in the ethanol the nanocrystals of different sizes, and secondly, reduced perchlorate material and metal were dissolved in ethanol, the solution will be prepared as a mother liquor by centrifugal separation, by adding organic allocation preparation of such a Raman signals in the semiconductor nanocrystals, and through electrostatic and biological molecular docking, the implementation of the markers of biological molecules. This invention produced plasma resonance, allowing semiconductor nanocrystals Resonance Raman signal enhancement. Because of the nanocrystalline metal surface with a group of organic molecules strong coordination role, the organisms can be stable, sound marker, and the further implementation of the use of fingerprint characteristics of semiconductor nanocrystals resonance Raman signal of multi-stage unknown molecular testing.
Owner:NORTHEAST NORMAL UNIVERSITY
Method for identifying soybean male sterile cytoplasm through SNP marks of chloroplast DNA
ActiveCN104004853AMark stableImprove reliabilityMicrobiological testing/measurementBiotechnologyChloroplast
The invention provides a method for identifying soybean male sterile cytoplasm through SNP marks of chloroplast DNA. The method can also be applied to distinction of a soybean cytoplasmic male sterile line and a maintainer line containing normal cytoplasm. The method includes the following steps of extracting the seed genome DNA of the soybean cytoplasmic male sterile line and the seed genome DNA of the maintainer line to serve as amplification templates, selecting four pairs of the SNPs marks of the chloroplast DNA, and designing a pair of primers on the side wing sequences of each pair of SNPs respectively to conduct PCR amplification. The PCR products obtained through amplification conduct identification on the difference between the length of the enzyme-digested products of the sterile line and the length of the enzyme-digested products of the maintainer line through digestion of restriction enzymes. According to the method, the soybean male sterile cytoplasm and normal fertile cytoplasm can be rapidly and accurately identified. The method can also be used for detecting the maintainer line which contains fertile cytoplasm and is mixed in the sterile line containing sterile cytoplasm in the breeding process of the sterile line, and provides guarantees for the purity requirement in the production process of soybean sterile line seeds.
Owner:JILIN ACAD OF AGRI SCI
Preparation process for semiconductor nanocrystal using Raman signal to marking organism
InactiveCN1763529AResonant Raman signal enhancementMark stableRaman scatteringSemiconductor nanocrystalsOrganism
The invention relates to nano material preparation and application in the biology marker, which is characterized by the following: synthesizing different dimensions of nanocrystalline in the anhydrous alcohol; dissolving the perchlorateto and metallic reducing material in the anhydrous alcohol separately; choosing the prepared solution as mother liquid through centrifugation; preparing semiconductor nanocrystalline of Lyman signal within organic ligand; performing biology marker through butt-joint of static electricity and biological molecule. The invention generates resonance action of plasma, which executes the unknown molecule detection through resonance multi-stage Lyman signal of fingerprint feature semiconductor nanocrystalline.
Owner:NORTHEAST NORMAL UNIVERSITY
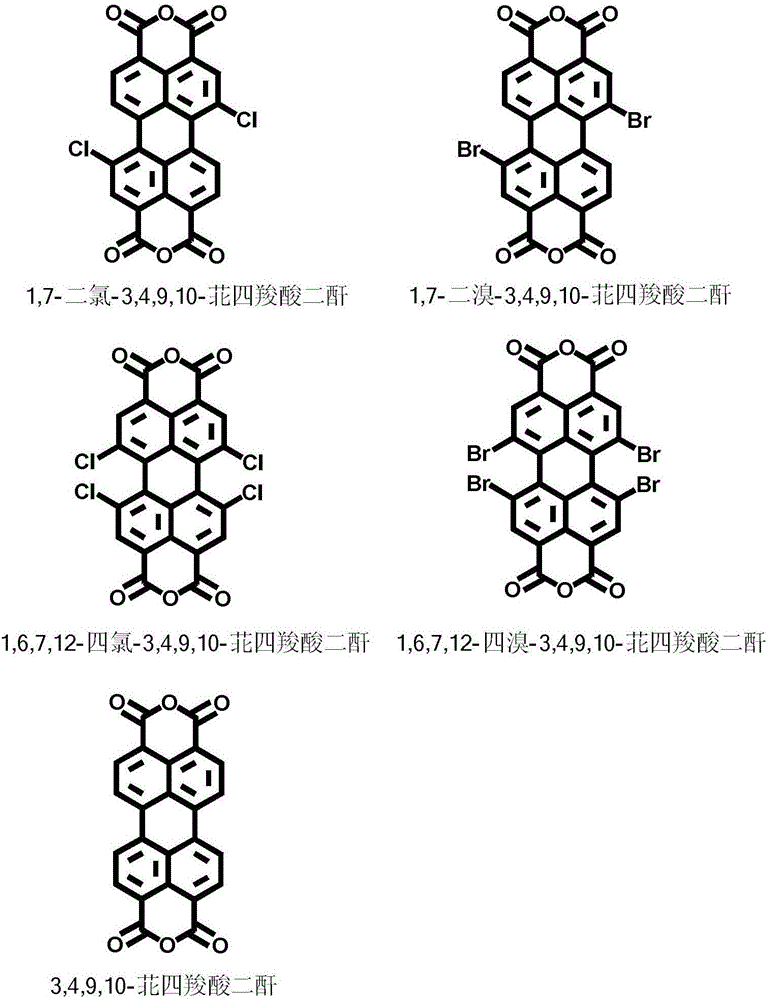
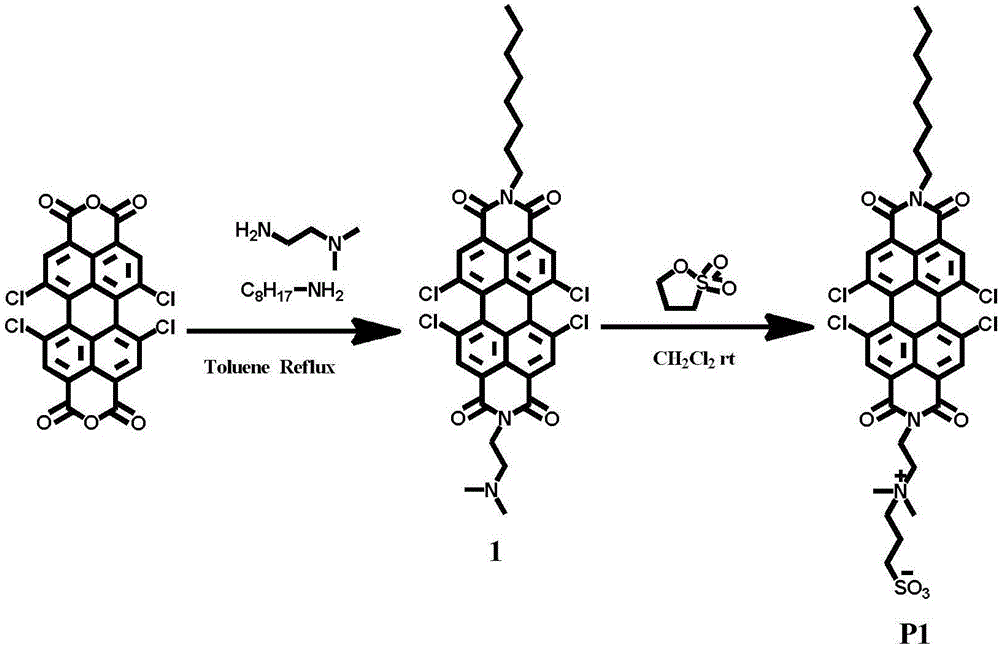
Preparation method of amphipathic asymmetric double-ion perylene bisimide dye and application thereof in marking cell membranes
ActiveCN106800796AAvoid interferenceImprove signal-to-noise ratioFluorescence/phosphorescenceAnthracene dyesSolubilityFluorescence
The invention discloses amphipathic asymmetric double-ion perylene bisimide dye, a preparation method thereof and application thereof in a living cell membrane mark fluorescence imaging aspect and belongs to the technical field of biomarker. According to the preparation method, sea island position chlorine or bromine-substitution perylene tetracarboxylic dicarboxylic anhydride or sea island position unmodified perylene tetracarboxylic dianhydride is utilized as fluorophore, and a hydrophobic alkyl chain and a hydrophilic double-ion radical are respectively led into two ends to obtain the amphipathic asymmetric double-ion perylene bisimide dye. By means of leading into the double-ion radical, water solubility and biocompatibility of a whole structure are improved, and cellular poison of the whole structure is reduced. The dye can be applied to external and internal cell membrane mark fluorescence imaging. As perylene with good photochemical stability and near-infrared emission wavelength is utilized as the fluorophore, effective marking time is greatly improved in a biological living cell membrane imaging process through an electrostatic force effect between a double-ion structure and a membrane structure, and time duration can be 72 hours or more.
Owner:BEIJING UNIV OF CHEM TECH
Brassica campestris ssp. Chinensis male sterile molecular marker-assisted selection method
ActiveCN1936021AEasy to operateLow costMicrobiological testing/measurementFermentationBrassicaBiology
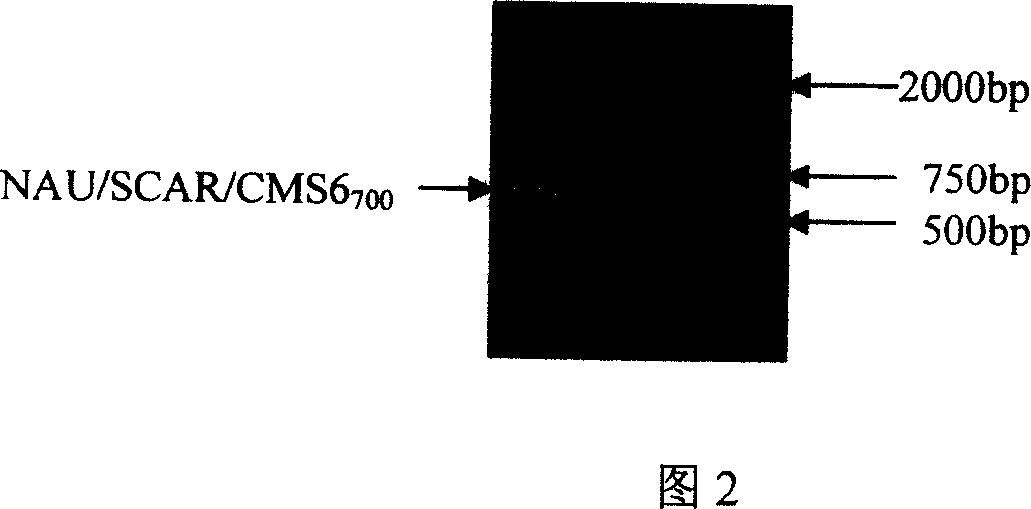
The invention relates to a non-heading cabbage male sterile molecule marking assistant selecting method. Using marking primer of OPAG20 or SCAG20 to amplify non-heading cabbage male sterile series or breeding material chondriosome DNA, if the 700bp section is amplified, the male sterile gene is existed. The invention could be used to taking selecting to PAPD and SCAR molecule marking for non-heading cabbage male sterile series 98HA and the maintainer line 98HB chondriosome DNA to improve selecting efficiency and speed up breeding process.
Owner:NANJING AGRICULTURAL UNIVERSITY
Kit and method for quantitative detection of 25-hydroxyvitamin D through TRFIA
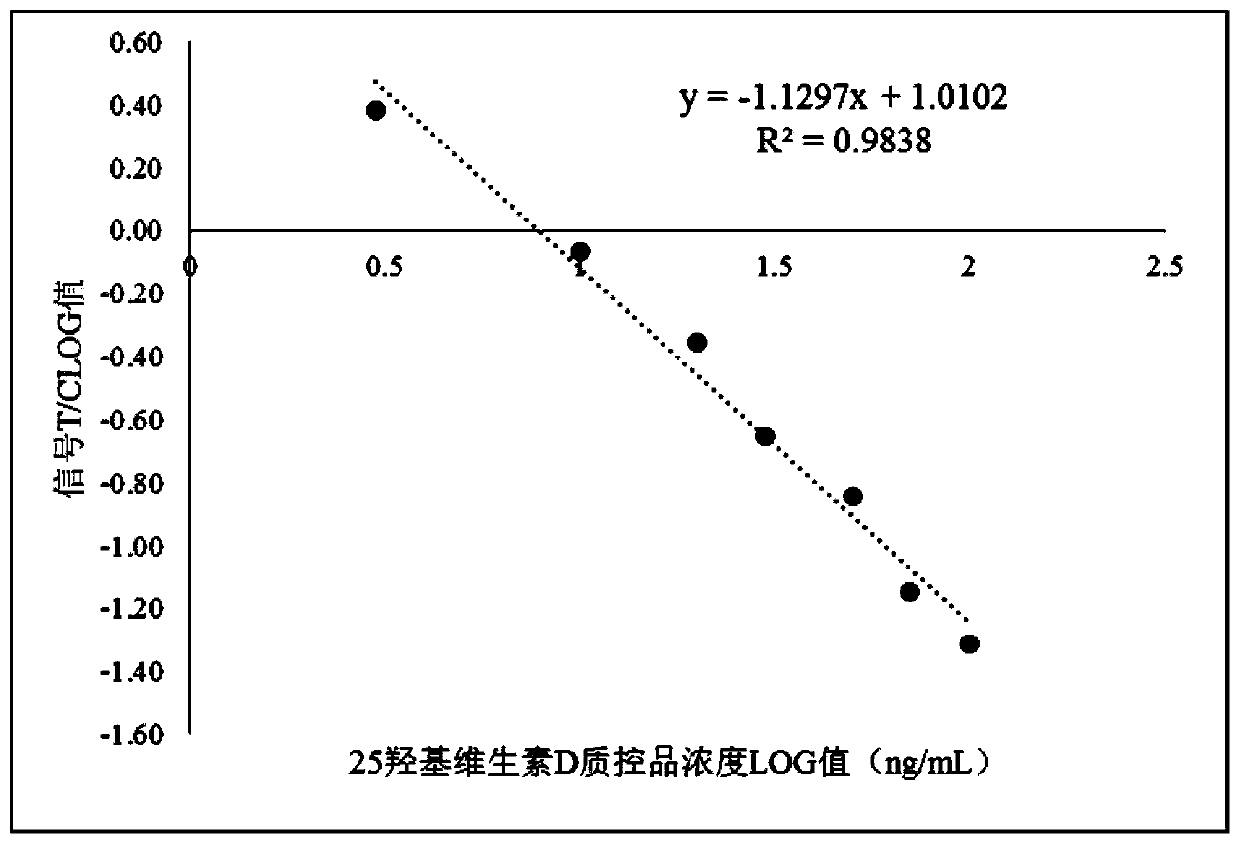
ActiveCN109975559AAvoid fluorescence interferenceLow backgroundBiological testingFluorescence/phosphorescenceRare earthBlood plasma
The invention relates to a 25-hydroxyvitamin D TRFIA (Time-Resolved Fluoroimmunoassay) kit and a detection method, which utilize the sensitivity of TRFIA technology, utilize rare earth vanadate nano fluorescent labeling materials and combine a dry-type immunofluorescence analyzer to realize high sensitivity, accurate quantification, simpleness and convenience. The kit of the invention can accurately and quantitatively detect the content of the 25-hydroxyvitamin D in serum, plasma and whole blood samples of persons, the whole blood samples can be filtered through the treatment of blood filtering membrane sample pads, the fluorescence interference of the samples can be avoided by utilization of a time resolution detection technology, the fluorescent microspheres are rare earth vanadate nanomaterials, thus the advantages of low background, strong fluorescence signals, high signal-to-noise ratio and the like are achieved, rare earth vanadate nano particles are connected with antibody through covalent bonds by labelling, a labeled product is stable, thus the kit has the characteristics of wide detection range (3-100ng / mL), high sensitivity (detection limit is 3ng / mL), high accuracy, quick and convenient detection and the like.
Owner:XIAMEN INST OF RARE EARTH MATERIALS
Shellfish pedigree breeding marking contrast method
ActiveCN105532529AReadableNo effect on growthClimate change adaptationPisciculture and aquariaZoologyPhases of clinical research
The invention relates to the technical field of aquatic organism selective breeding marking, and relates to a marking contrast method of an offshore intermediate breeding and development stage during a shellfish pedigree breeding process. The invention specifically relates to a shellfish pedigree breeding marking contrast method. According to the method, an environment-friendly plastic pad is adopted as a material for a geometric-shaped marker; different geometric holes are formed in the marker by cutting; and marking can be realized with the holes and the color of the marker. According to the method, the cost is low, and the marking is simple and clear. The marker can be accurately identified under a situation of foreign body adhesion. The recognition degree is high, and the marker meets production needs. The marker is convenient to manufacture, and is easy to carry. The marker can be widely and repeatedly applied. The marker has long preservation time and high preservation rate. Marking is carried out by tying the marker on a cultivation cage with a plastic tie, such that physiological damage to the shellfish is prevented, and the marker is stable and is prevented from falling and loss. Therefore, the stability of the marker is high. A marking rate and readable rate of the marker are both 100%, such that shellfish pedigree breeding large-scale industrialization development requirements are met. The method has an important significance in shellfish industry breeding.
Owner:孙欣
Chemiluminescence detection kit for aflatoxins M1 and preparation method of chemiluminescence detection kit
InactiveCN107688016AMark stableSmall molecular weightChemiluminescene/bioluminescenceAntigenAflatoxin M
The invention discloses a chemiluminescence detection kit for aflatoxins M1 and a preparation method of the chemiluminescence detection kit. The chemiluminescence detection kit comprises an acridiniumester marker, magnetic particles coupled with an antigen or antibody, a calibrator solution, a cleanout fluid, a chemiluminescence pre-excitation liquid A and a chemiluminescence excitation liquid B.According to the chemiluminescence detection kit for aflatoxins M1, the magnetic separation chemiluminescence technology is taken as a detection means, and the acridinium ester marking technology isused together. The chemiluminescence detection kit for aflatoxins M1 is simple and convenient to operate, mild in reaction condition and stable in lighting value and is less influenced by external conditions. Compared with the prior art, the chemiluminescence detection kit for aflatoxins M1 has the advantages of being high in sensitivity, quick and convenient in detection, high in accuracy, good in repeatability and strong in specificity.
Owner:太原瑞盛生物科技有限公司
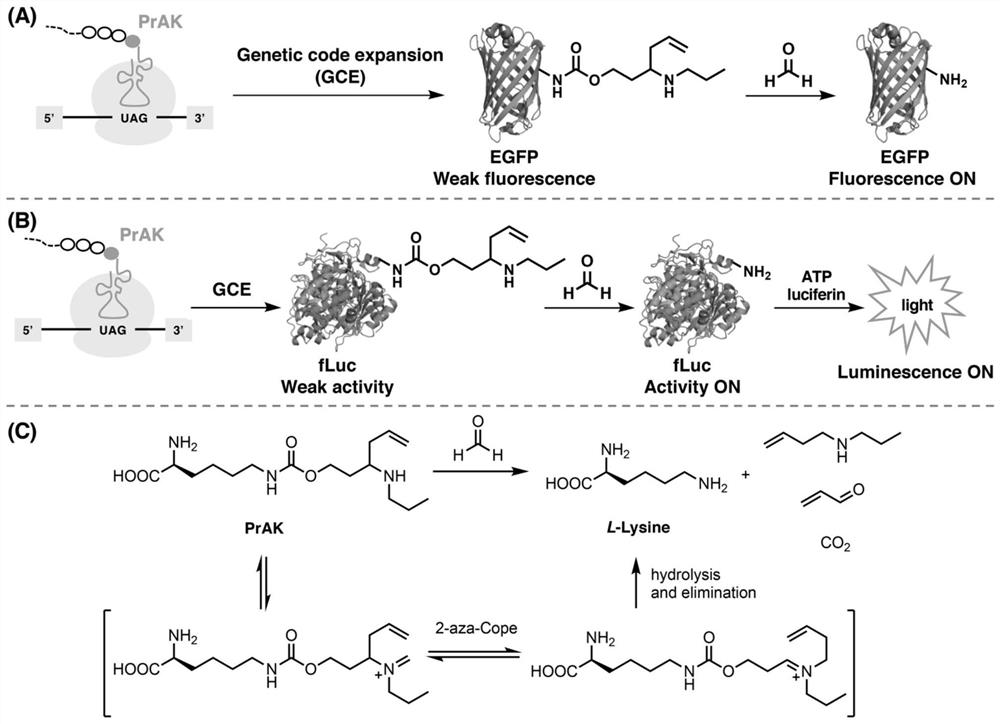
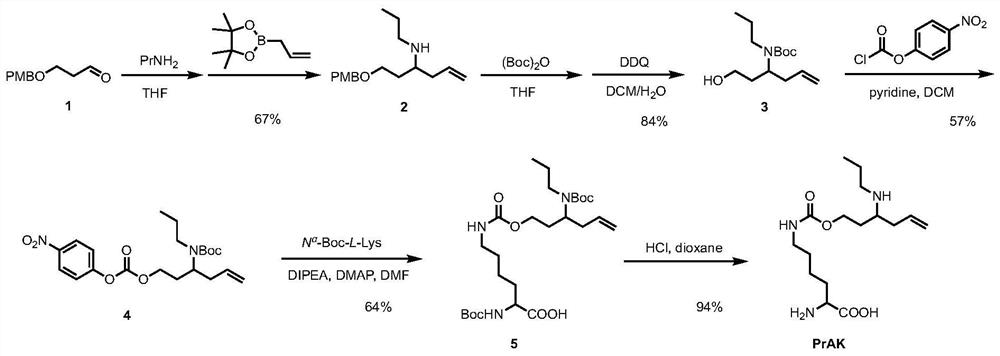
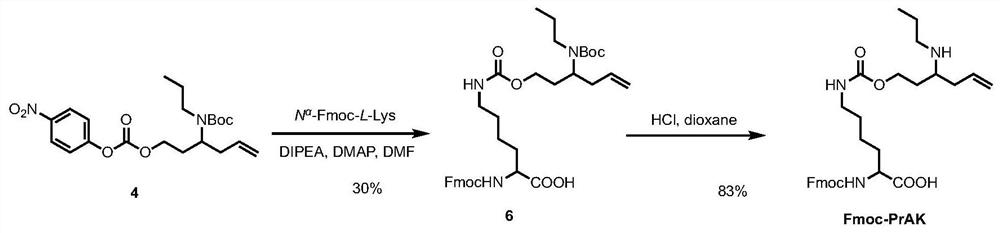
Genetically encoded formaldehyde reactive unnatural amino acid, preparation method and application thereof
ActiveCN111747869ARealize detectionQuick responseCarbamic acid derivatives preparationOrganic compound preparationLuciferasesBiological macromolecule
The invention relates to a genetically encoded formaldehyde reactive unnatural amino acid. Specifically, the invention discloses a genetically encoded formaldehyde reactive unnatural amino acid PrAK,a preparation method and application thereof in formaldehyde detection and imaging. According to the formaldehyde reactive unnatural amino acid PrAK provided by the invention, biomacromolecules such as EGFP (Enhanced Green Fluorescent Protein) and firefly luciferase (fLuc) can be specifically introduced into sites in a protein translation process; a reaction type biomacromolecule formaldehyde fluorescent probe and a bioluminescence probe are constructed, and formaldehyde in a biological sample can be effectively detected or imaged.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
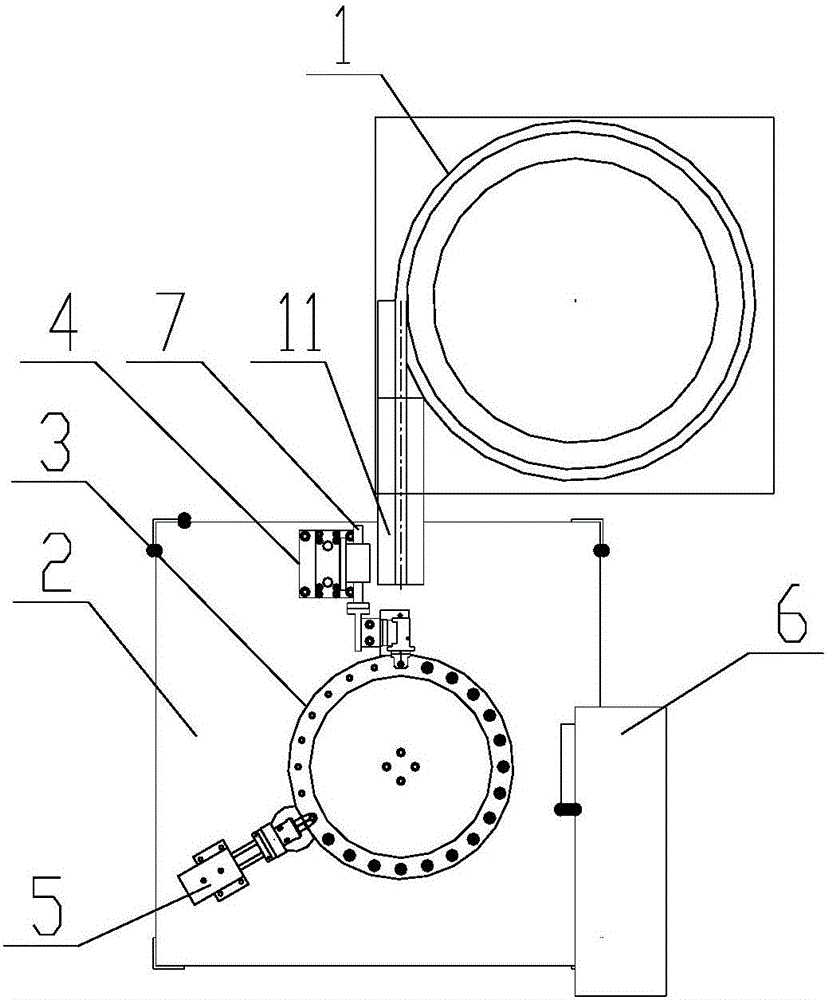
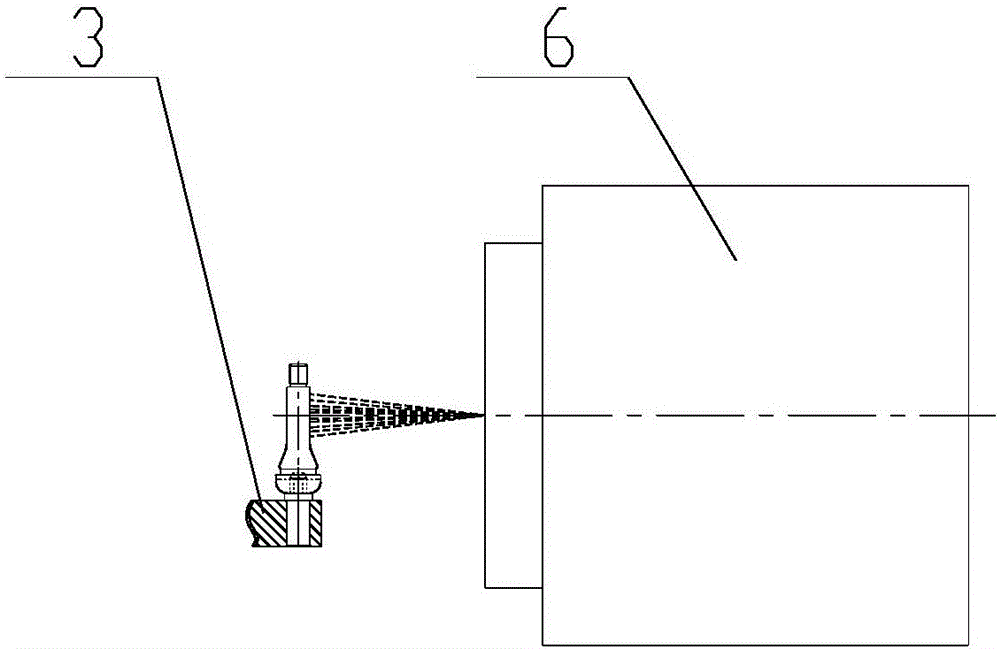
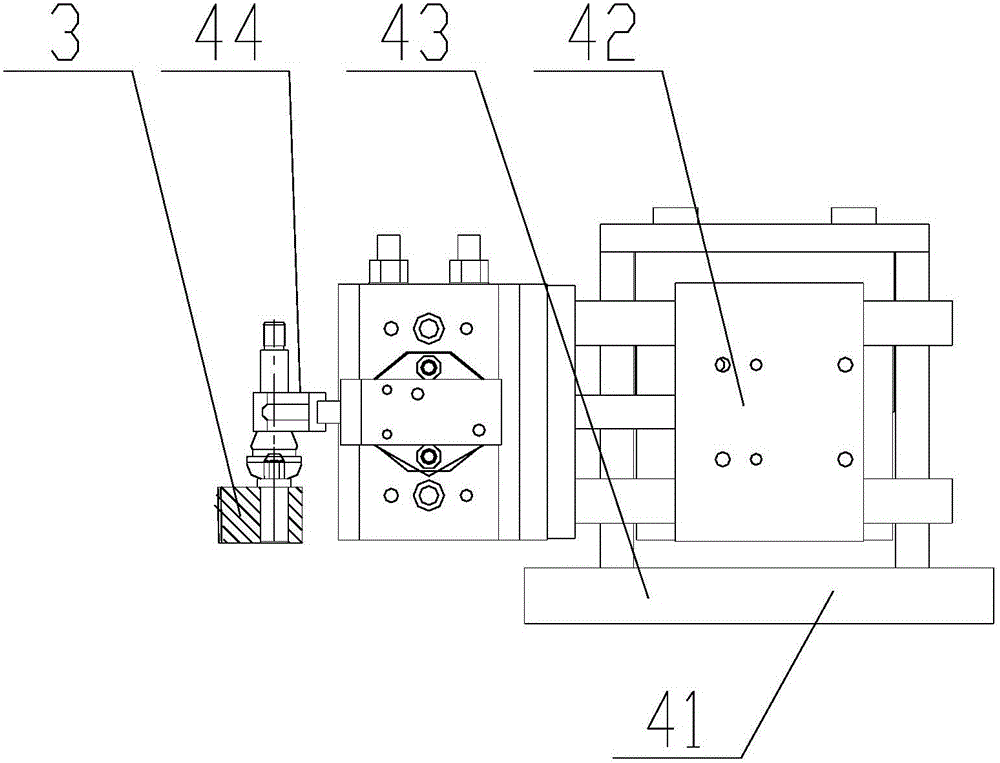
Valve stem laser marking apparatus
InactiveCN106427232AReasonable structural designImprove processing efficiencyTypewritersEngineeringManipulator
The invention discloses a valve stem laser marking apparatus. A material carrying disk is provided with a feeding station, a blanking station and a marking station in the periphery thereof. A vibration feeding mechanism is disposed on one side of the feeding station of a work bench. A feeding manipulator is arranged at the feeding station for feeding a valve stem of a blanking channel for blanking to a valve stem receiving position of the material carrying disk at the feeding station. A laser marking mechanism is disposed at the marking station for marking the valve stem which is arranged at the marking station. A blanking manipulator is disposed at the blanking station for blanking the valve stem which is at the blanking station and is marked. The valve stem laser marking apparatus has reasonable structure and design and realizes the switching of stations during feeding, marking and blanking through the rotation of the material carrying disk, such that the valve stem can conduct automatic and continuous marking, has high processing efficiency, and performs firm marking that is not easy to fall.
Owner:BAOLONG ANHUI AUTO PARTS
Method for quickly identifying genetic purity of glutinous corn hybrid
InactiveCN102505044AAccurate identificationImprove the efficiency of genetic purity identificationMicrobiological testing/measurementElectrophoresisDNA fragmentation
The invention belongs to the technical field of biology, and relates to a method for quickly identifying the genetic purity of a glutinous corn hybrid. The method comprises the following steps of: extracting genomic deoxyribonucleic acid (DNA) from glutinous corn, performing polymerase chain reaction (PCR) amplification by using the screened sequence-related amplified polymorphism (SRAP) effective primer combination NAUSRem7 / NAUSRpm1 and random amplified polymorphic DNA (RAPD) effective primers NAUSR709 and NAUSR712, respectively performing non-denaturing polyacrylamide gel electrophoresis and agarose gel electrophoresis on a PCR product obtained through amplification, and shooting a DNA electrophoresis pattern; and comparing and analyzing the size and position difference of polymorphism amplified fragments formed due to the difference of DNA fragment sequences in the electrophoresis pattern, and identifying the genetic purity of the glutinous corn F1 hybrid, namely a Suyunuo 2 seed. The detection method has the advantages of marking stability, high accuracy, no influence of the growth stage and environment of a sample to be detected, low cost, capability of being performed in thewhole growth season, and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
Molecular marker for co-separating from aroma character
InactiveCN101921761AEasy to identifyAccurate and convenient identificationMicrobiological testing/measurementDNA/RNA fragmentationWater bathsNose
The invention relates to a molecular marker for co-separating from aroma characters, belonging to the field of molecular genetics. 1-2 pieces of spires at the upper part of individual rice are taken at the tillering stage and are reserved in a refrigerator with the temperature of below 80 DEG C for later use. Rice leaves with the length of 2-4cm are taken to be placed in a centrifugal tube of 1.5ml, liquid nitrogen is added, grinding is carried out, 900 mu l of TPS solution is added, and water bath is performed for 20min at the temperature of 75 DEG C. A cover is opened to smell the odour by a nose to determine whether aroma exists. A specific PCR primer L01 or L02 is utilized to obtain the molecular marker by PCR amplification. The invention can be specially used for seed selection and genetic research of the rice aroma variety and can be used for cloning aroma genes.
Owner:SHANDONG RICE RES INST
Ground cover chrysanthemum strain type stoloniferous numerator mark auxiliary selection method
InactiveCN101368208AEasy to operateQuick extractionMicrobiological testing/measurementFermentationStrain typeScars
The invention relates to an auxiliary selecting method of a ground-cover chrysanthemum plant type creeping molecule mark, which belongs to the technical field of biology and which can be used for auxiliary selecting and breeding for the ground-cover chrysanthemum plant type creeping molecule mark. RAPD primer A-10 or an SCAR primer is used to augmentground-cover chrysanthemum or the breeding material DNA; if the fragment of 555bp can be augmented, the existence of a ground-cover chrysanthemum creeping is proofed. The method takes 152 plants of F1 segregation population obtained by taking 'early red Italy' (P1) as a female parent and takes 03(6)-12 as a male parent (P2) as the testing materials to build a plant type creeping / vertical gene pool to carry out screening on the RAPD and SCAR molecule marks, which can effectively develop the auxiliary breeding for the ground-cover chrysanthemum plant type creeping molecule mark and greatly improve the selecting efficiency, thereby quickening the breeding process.
Owner:NANJING AGRICULTURAL UNIVERSITY
Application of arabidopsis thaliana Sec14p-like gene in plant cell lipid droplet fluorescent marker
ActiveCN108530523ADoes not affect its own growth and developmentAvoid interferencePlant peptidesFluorescence/phosphorescenceFluorescencePlant cell
The invention provides application of an arabidopsis thaliana Sec14p-like gene in a plant cell lipid droplet fluorescent marker. According to the application, the arabidopsis thaliana Sec14p-like geneis used as specificity position lipid droplets of the plant cell lipid droplet marker in plant cells. The invention further provides application of the gene in fluorescent detection of the lipid droplets in the plant cells. The application comprises the following specific steps: connecting encoding protein of the gene with fluorescent protein to obtain fused protein; transforming the fused protein into a target plant to mark the plant lipid droplets. Compared with other lipid droplet fluorescent markers with similar functions, the Sec14p-like gene provided by the invention can be connected with different fluorescent protein genes according to specific requirements to be transformed into the plant, and the fused protein with different fluorescent protein is expressed, so that interferenceof plant spontaneous fluorescence is effectively avoided; the Sec14p-like gene can be stably transformed into the plant and the plant lipid droplets are stably marked for a long period; self growth and development of the plant are not influenced.
Owner:SHANGHAI JIAO TONG UNIV
Stable isotope 74Se labeled quantum dots and preparation method thereof
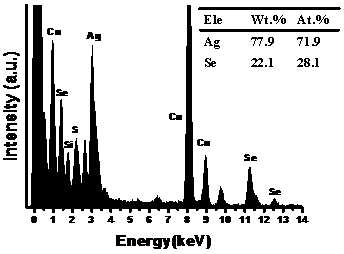
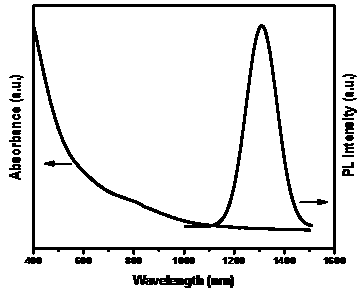
ActiveCN110105945ADoes not affect natureMark stableMaterial nanotechnologyNanoopticsStable Isotope LabelingIsotopic labeling
The invention discloses stable isotope 74Se labeled quantum dots and a preparation method thereof. The stable isotope 74Se labeled quantum dots are of a nanometer spherical structure composed of a binary compound. By means of the method, the stable isotope 74Se labeled silver selenide quantum dots and cadmium selenide quantum dots which are uniform in size and stable can be prepared, and the method can be popularized in other selenium-containing nanometer materials. The preparation method is simple in reaction condition and operation and high in repeatability, the particle size can be controlled, and the fluorescence-emission wavelength can be controlled. The stable isotope 74Se labeling method can achieve long-time labeling and tracing of the selenium in the quantum dots in the body, andthe background interference of endogenous selenium is greatly reduced.
Owner:SHANGHAI UNIV
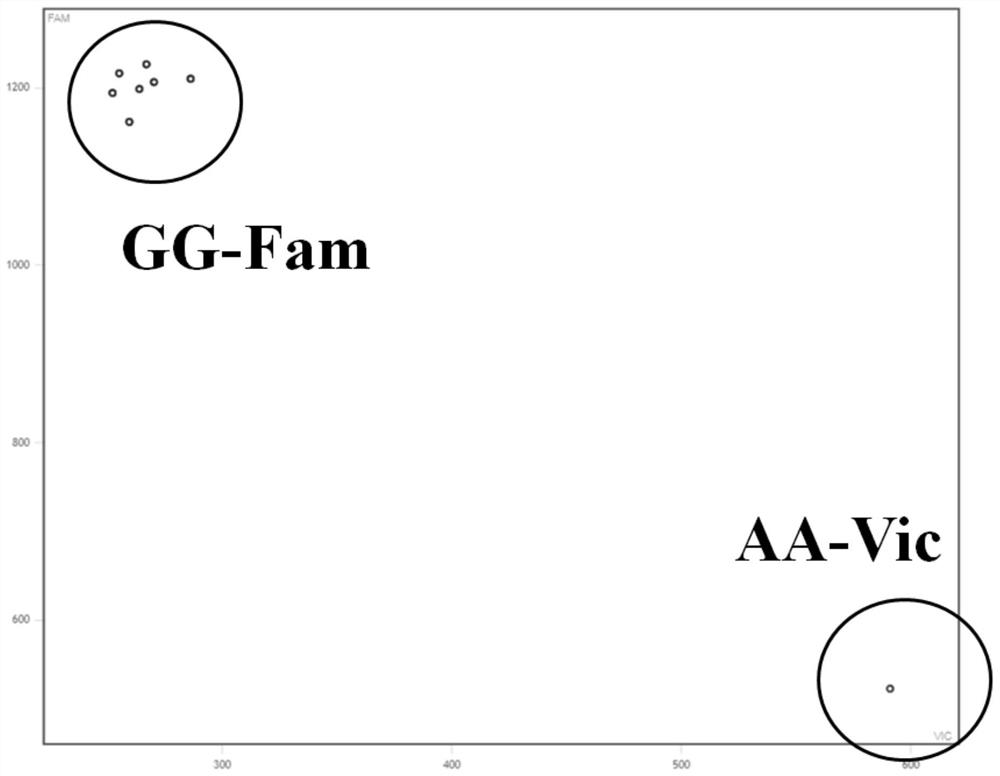
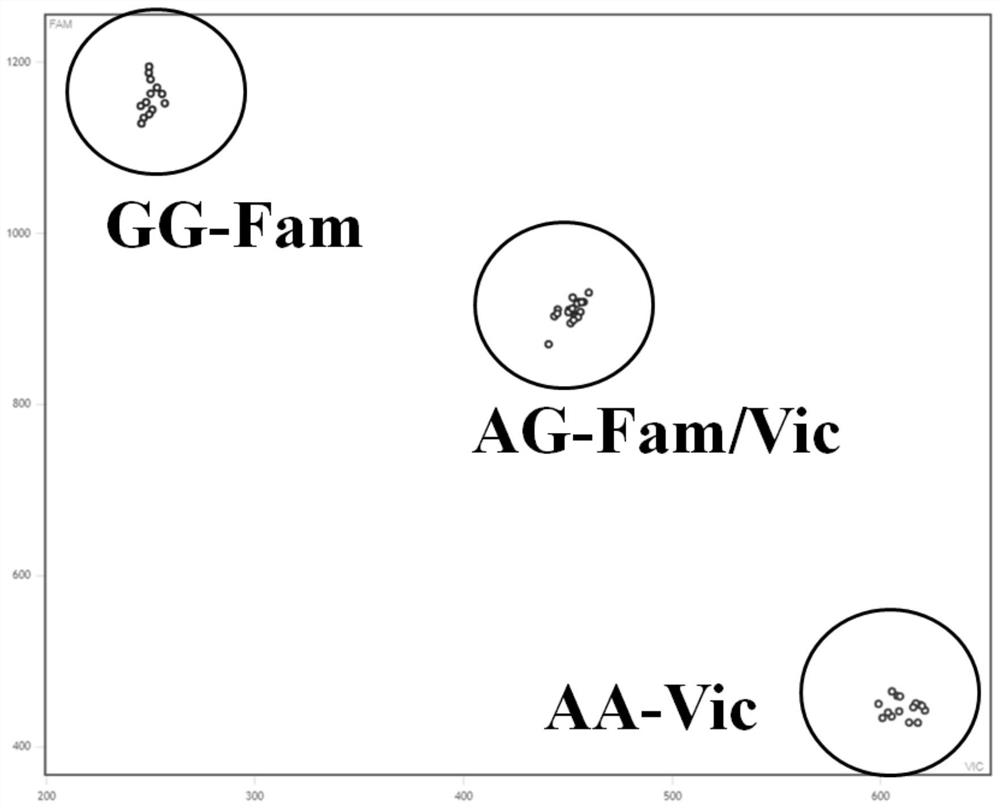
Molecular markers for avirulence gene Avr-Pit of Pyricularia grisea
InactiveCN102766691BMark stableStable molecular markerMicrobiological testing/measurementDNA/RNA fragmentationPyricularia griseaMolecular marker
Owner:NANJING AGRICULTURAL UNIVERSITY
Development and application of KASP marker of oryza sativa high-temperature-resistant gene TT1
InactiveCN113930539AAccurate detectionEasy to breedMicrobiological testing/measurementPlant genotype modificationBiotechnologyResistant genes
The invention discloses development and application of a KASP marker of an oryza sativa high-temperature-resistant gene TT1, and belongs to the technical field of gene biology. The molecular marker is a KASP marker KASP-TT1 which is closely linked with an oryza sativa high-temperature-resistant gene TT1, the KASP-TT1 is an SNP site in an oryza sativa genome, the nucleotide type of the KASP-TT1 is A or G, and the KASP-TT1 is the 101 nucleotide of SEQ ID No.4 in a sequence table. The KASP technology is applied to perform genotype identification on the oryza sativa high-temperature-resistant gene TT1, the method has the advantages of simplicity and convenience in operation, low cost, short detection period, stability in marking, environment friendliness and the like, the oryza sativa high-temperature-resistant gene TT1 can be accurately detected, and the method has important significance in promoting high-temperature-resistant oryza sativa breeding.
Owner:LIANYUNGANG ACAD OF AGRI SCI
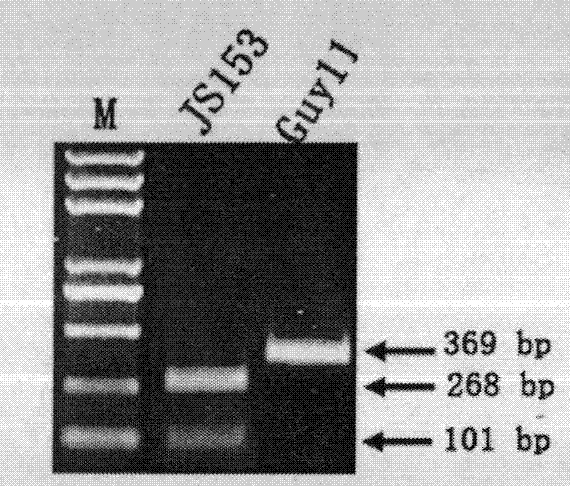
Molecular marker detection method of rice blast bacterium non-toxic genes Avr-Pit and primers thereof
InactiveCN101942521BMark stableStable molecular markerMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceCost savings
The invention discloses a method for rapidly detecting rice blast bacterium non-toxic genes Avr-Pit by utilizing molecular markers. In the invention, two pairs of specific primers are used, wherein an upstream primer in one pair of primers is ACCGCGATCAGTGGAAAACT, and a downstream primer is ATTGGCAGAGCCAGCTACTC; and an upstream primer in the other pair of primers is TTGTGTTCCTGTCAATCGCG, and a downstream primer is CGCAGCTTATATCTCGGATAG. The method in the invention has the advantages of simple operation, explicit result and time and cost saving.
Owner:NANJING AGRICULTURAL UNIVERSITY
Labeled Alkaline Phosphatase Antigen Antibody Diluent
Owner:AILEX TECH GRP CO LTD +1
Molecule labeling method for non-heading Chinese cabbage late bolting gene
ActiveCN101117645BMark stableEasy to mark tapeMicrobiological testing/measurementDNA/RNA fragmentationDNAMolecular marker
The present invention relates to a molecule marking method for a non-head Chinese cabbage late bolting gene, and the method belongs to the molecular biology field. Using the marking primer DBC16 to amplify the DNA of the non-head Chinese cabbage late bolting family or the breeding material, when the late bolting LB fragment of 252bp is amplified out, the existence of the non-head Chinese cabbage late bolting gene is proved. The present invention sieves the non-head Chinese cabbage late bolting family and the early bolting family P120 for the SSR molecule marking, and a late bolting mark LB ofthe non-head Chinese cabbage late bolting gene is obtained. The molecule marking provided by the present invention can be carried out for selecting and breeding the non-head Chinese cabbage late bolting breed, the select efficiency can be improved greatly, the breeding process can be accelerated, and in producing, the present invention can be applied to the purity identifying for the non-head Chinese cabbage late bolting breed.
Owner:南京农业大学资产经营有限公司
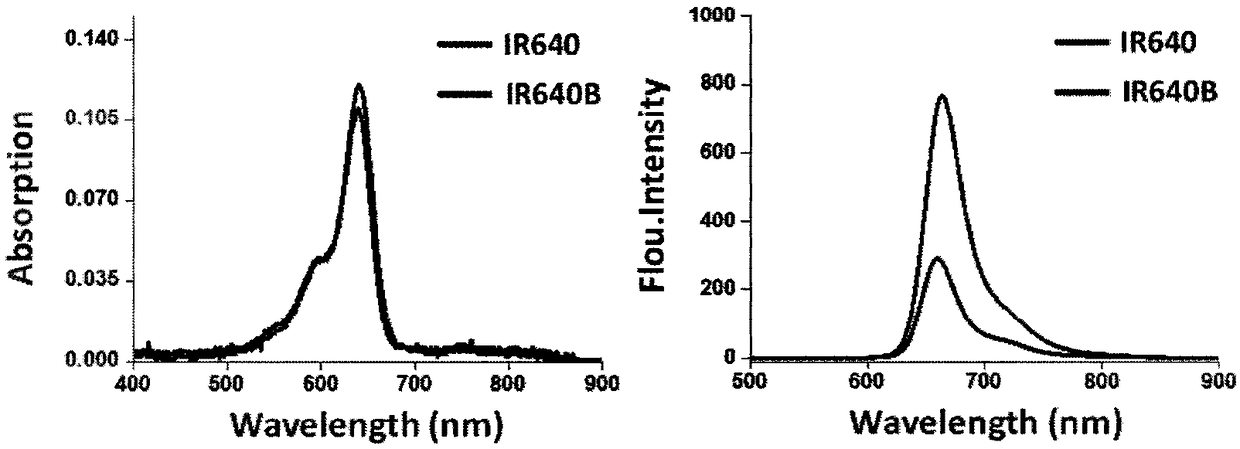
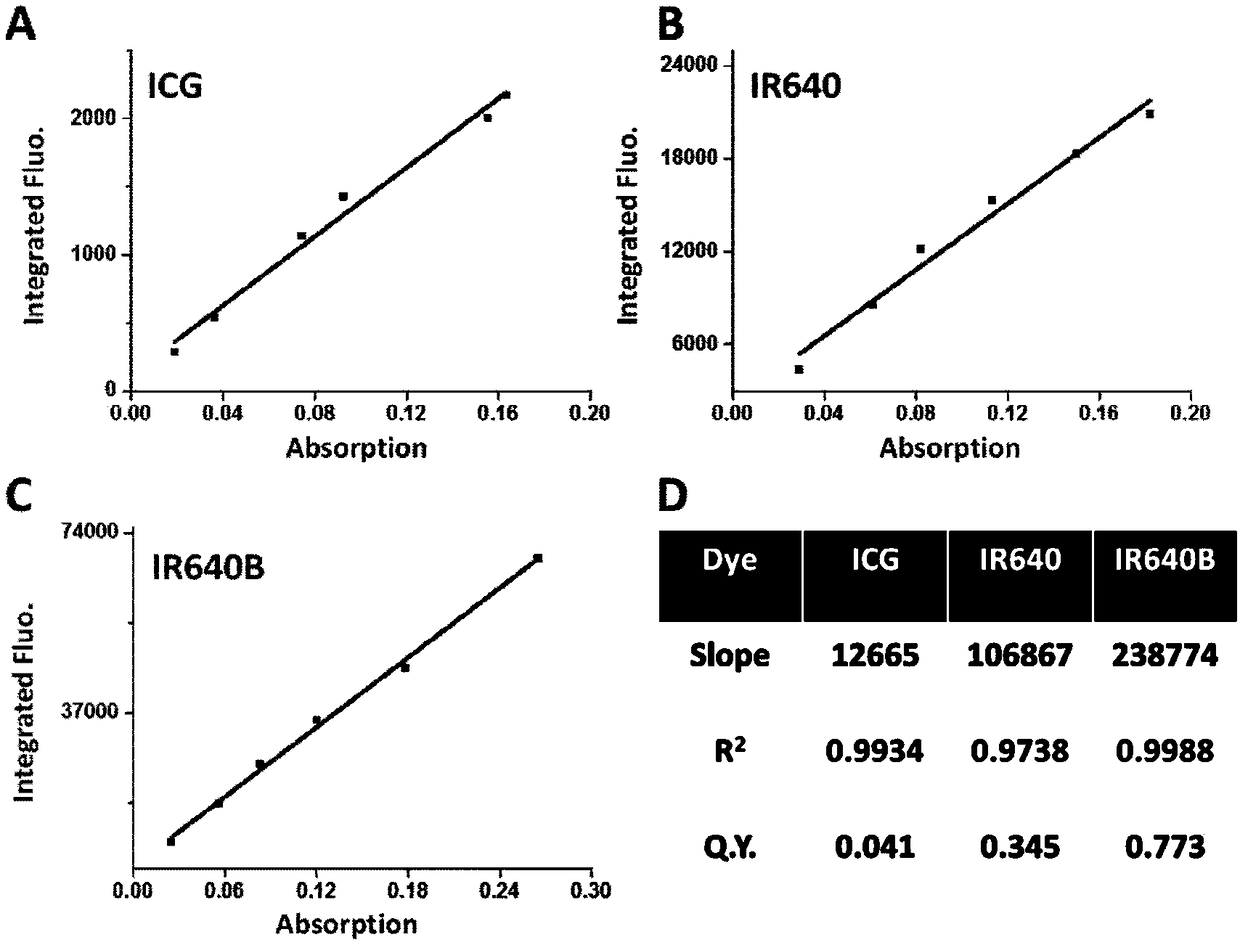
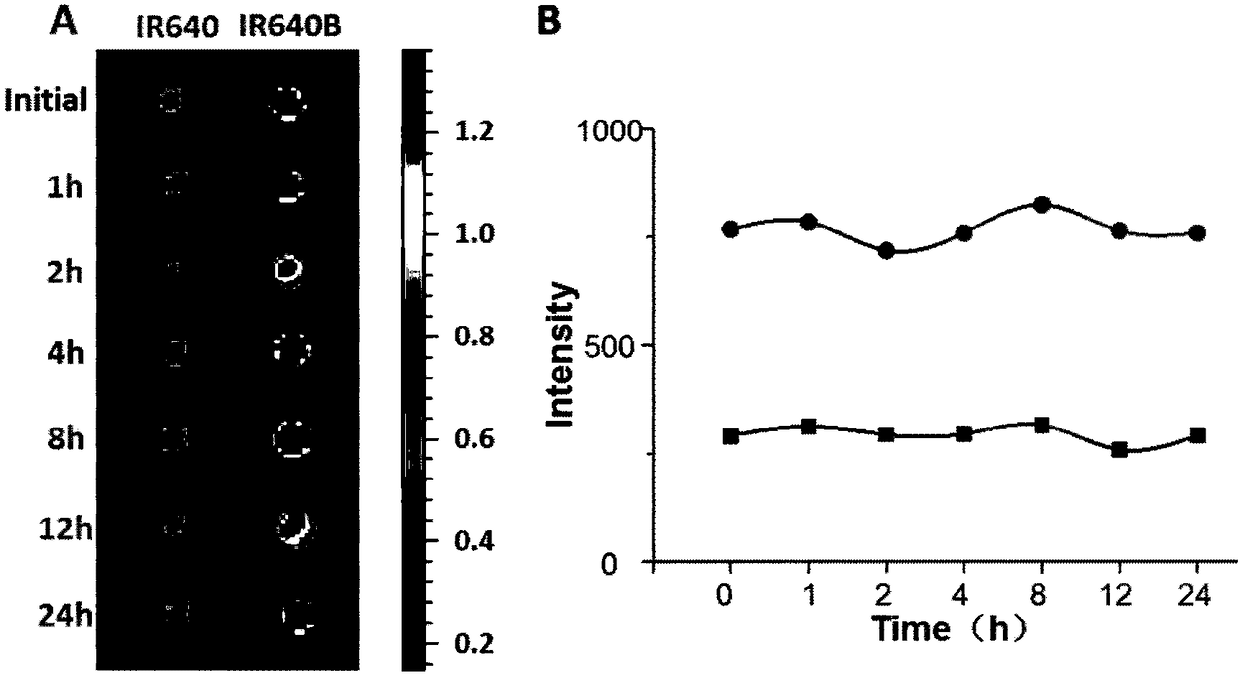
Markable dark red fluorescent active ester
InactiveCN108147992ARealize non-invasive tracingHigh labeling rateMethine/polymethine dyesPeptide preparation methodsCarbon–carbon bondFluorescence microscope
The invention belongs to the field of fluorescent imaging reagents and relates to a dark red fluorescent active ester IR640B-NHS which can be rapidly marked, and a precursor IR640B of the ester. According to the ester, 2,3,3-trimethylindole is adopted as a matrix which reacts with raw materials such as butane sultone, then a cyanine type fluorescent group with a sulfonic group is synthesized, further through a Suzuki-Miyaura reaction, phenyl carboxylic acid is introduced into a fluorescent group through a carbon-carbon bond, and the phenyl carboxylic acid is modified to generate an N-hydroxylcarboxyfluorescein diacetate succinimidyl ester. The active ester can react with a primary amino group in biological macromolecules under physiological conditions, and then dark red fluorescent groupscan be marked on target molecules through amide bonds. By adopting the ester, biological macromolecules such as polypeptide, proteins, antibodies or polymer molecules can be rapidly, safely, effectively and stably marked, in-vitro evaluation on acceptor targeting properties and intracellular distribution of target biological macromolecules can be implemented by using a fluorescence microscope, aflow cytometer and the like, and nondestructive monitoring and quantitative tracing on target molecules can be achieved through living optical imaging.
Owner:FUDAN UNIV
Method of chrysanthemum amenone form molecular marker assistant selection
ActiveCN108411026AEasy to operateImprove selection efficiencyMicrobiological testing/measurementDNA/RNA fragmentationTest materialSingle band
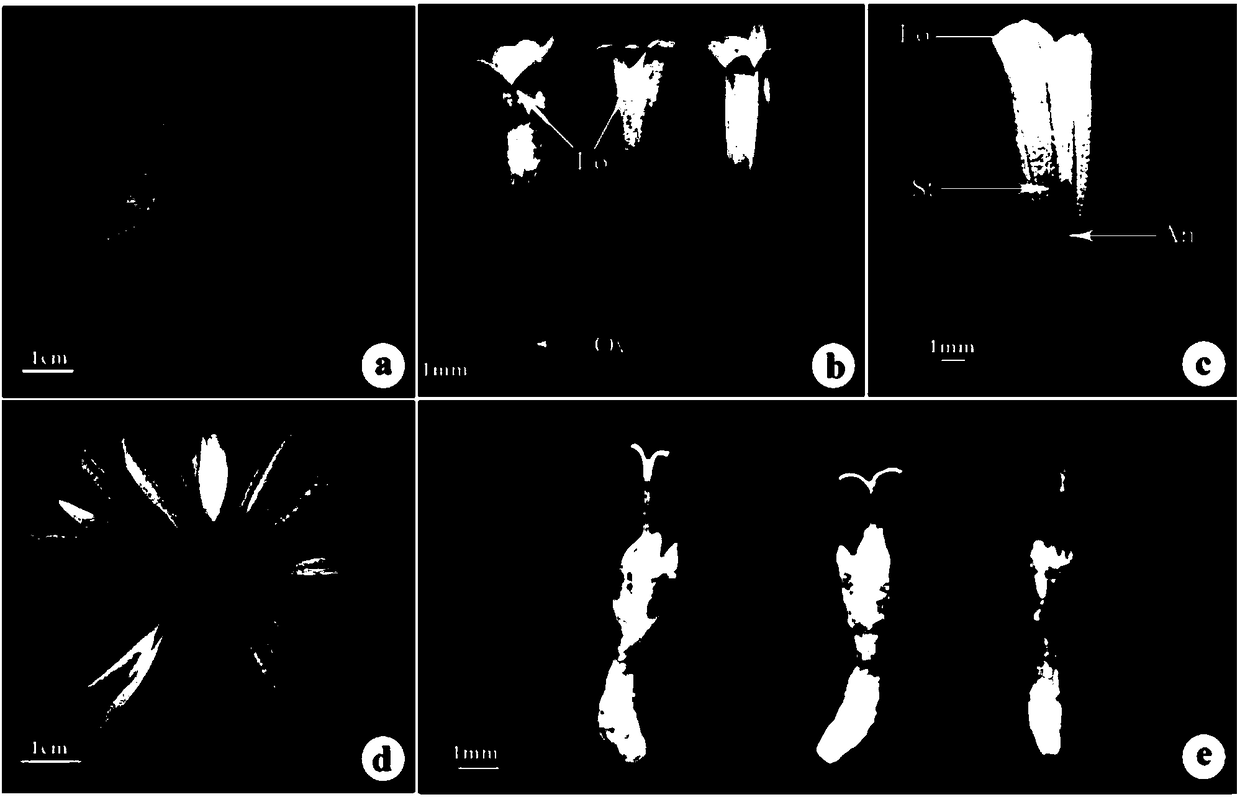
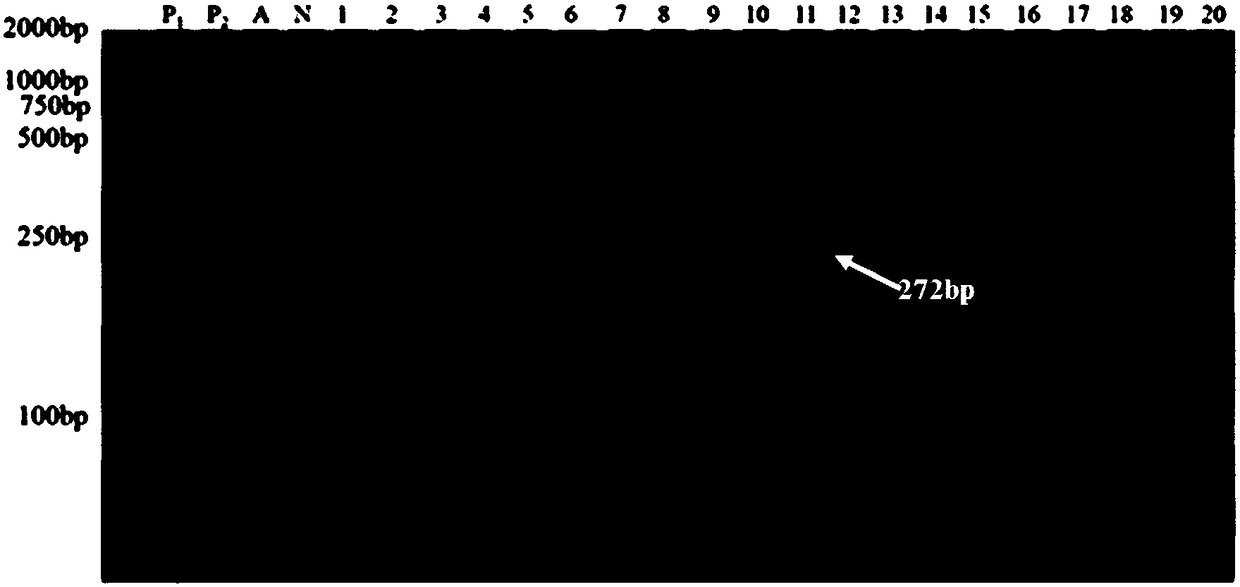
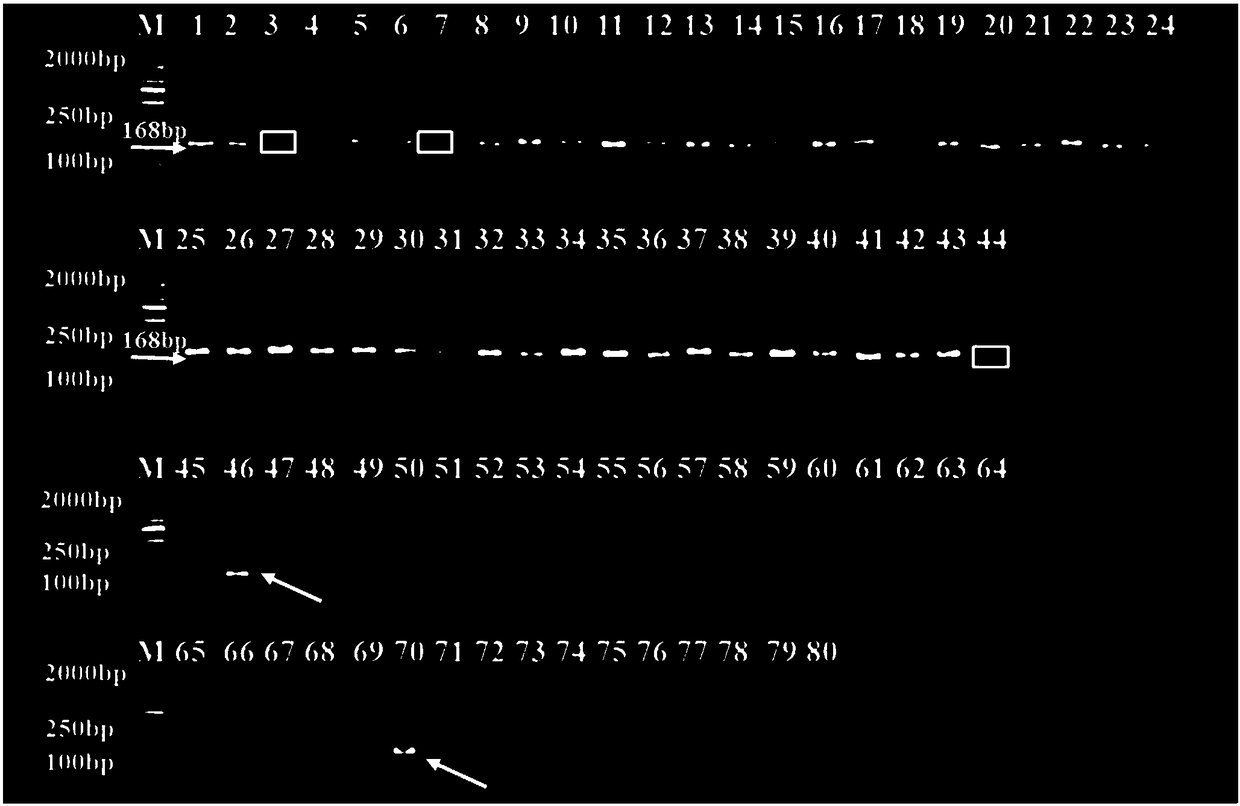
The invention belongs to the technical field of biology and discloses a chrysanthemum amenone form molecular marker assistant selection method. A molecular marker is an SRAP molecular marker or / and SCAR marker; in the method, 80 F1 segregating populations, acquired by hybridizing amenone form spray cut chrysanthemum variety Nannong Xuefeng as a female plant and non amenone form variety QX096 as amale plant, are taken as a test material; specific sites, linked to amenone form genes, are screened and controlled by using the SRAP molecular marker, wherein SRAP molecular marker primer combinationM11E1 is amplified in an amenone form chrysanthemum material to obtain a specific fragment of 272bp; by designing a specific SCAR molecular marker primer, amplification is performed in an amenone material to obtain a single band of 168bp; it is further verified in two populations of Nannong Xuefeng*QX096 and Nannong Xuefeng*Mengbai that the accuracy reaches up to 92.5 percent and 84.3 percent respectively; and the situation that the marker is successfully transformed into a SCAR molecular marker is explained. The method provided by the invention improves the selection efficiency of an amenoneform marker and accelerates the selecting process of a new variety of the amenone form chrysanthemum.
Owner:NANJING AGRICULTURAL UNIVERSITY
Special chip for detecting breast cancer related gene expression
ActiveCN113846159AEasy expression screeningLow costMicrobiological testing/measurementCancer researchRelated gene
The invention relates to a special chip for detecting breast cancer related gene expression. A pair of primers for RT-PCR is used as probes to be fixed on a substrate, a gene chip is used for completing simultaneous detection of multi-gene expression profiles at one time, and the detection of breast cancer specific gene expression conditions can be rapidly completed by using an RNA sample.
Owner:BEIJING BIONAXIN BIOTECH CO LTD
Brassica campestris ssp. Chinensis cytoplasmic male sterile gene molecular marking method
ActiveCN1936022AImprove selection efficiencyAuxiliary selection is convenientMicrobiological testing/measurementFermentationBrassicaMolecular genetics
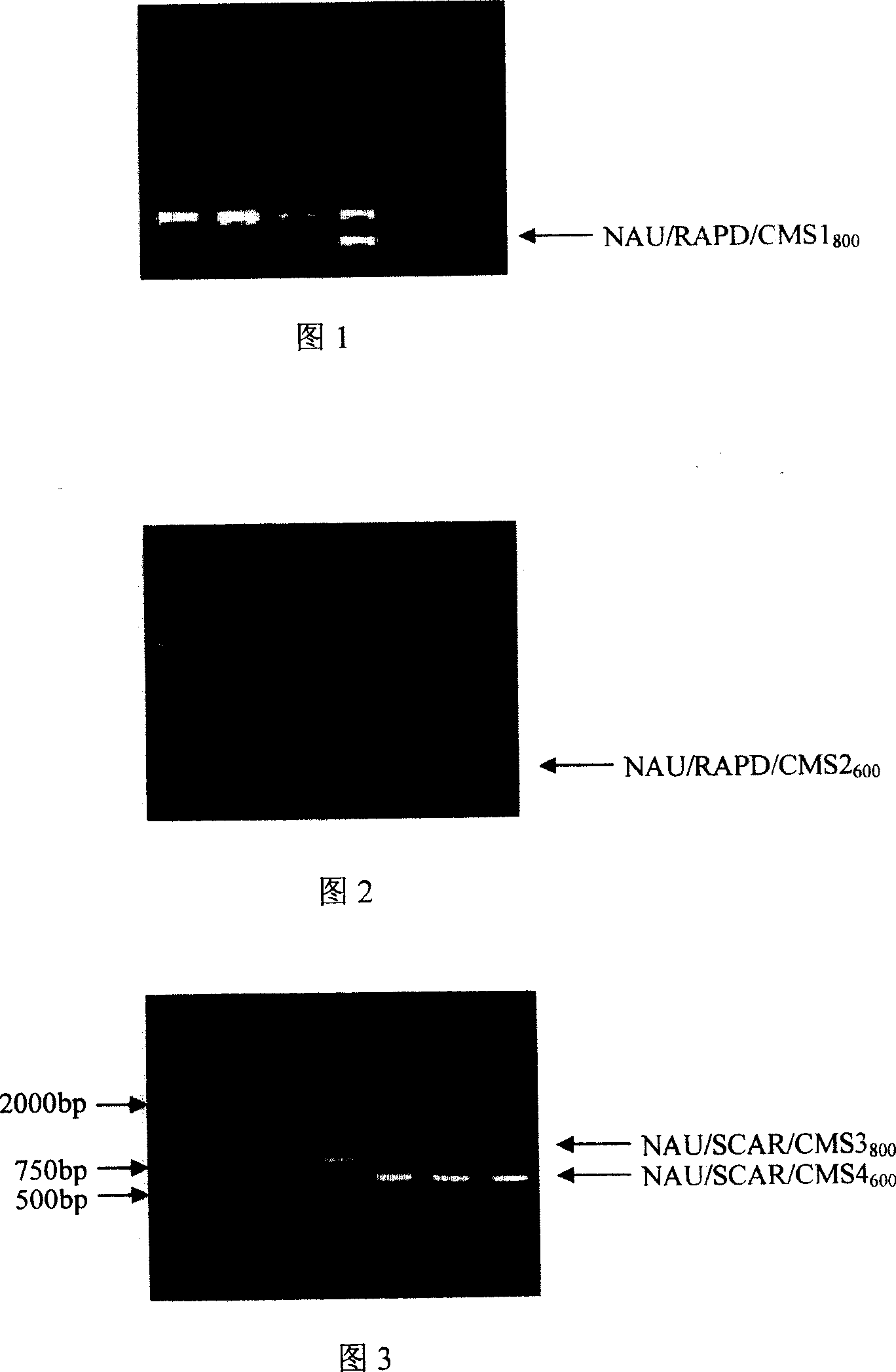
The invention relates to the molecule marking method for non-heading cabbage male sterile gene that takes selecting of PAPD and SCAR molecule mark for non-heading cabbage male sterile series 98HA and maintainer line 98HB to gain two marks of non-heading cabbage male sterile genes--NAU / RAPD / CMS1800 and NAU / RAPD / CMS2600 and transforming it to two SCAR marks NAU / SCAR / CMS3800 and NAU / SCAR / CMS4600. The invention could improve selecting efficiency and speed up breeding process.
Owner:NANJING AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com