Preparation method of amphipathic asymmetric double-ion perylene bisimide dye and application thereof in marking cell membranes
A perylene imide, asymmetric technology, applied in the field of biomarkers, can solve the problems of poor stability and short action time, and achieve the effects of low toxicity, low biological toxicity and economical raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Dissolve 527mg (1mmol) of 3,4,9,10-perylenetetracarboxylic dianhydride and 130mg (1mmol) of octylamine in 15ml of methanol, add it to a 50mL three-necked bottle, and then seal the system with a rubber stopper. Under protection, stir magnetically for 30 minutes, heat up to 85°C and reflux. After 12h of reaction. After the reactant was concentrated, it was washed with ethanol to obtain a precipitate. After concentration and vacuum drying, a dark red solid was obtained.
[0037] Add the above dark red solid, 10g of imidazole, 106mg of N,N-dimethylethylenediamine (1.2mmol about 132ul) into a three-necked flask, then seal the system with a rubber stopper, and raise the temperature to 120°C for reflux reaction under the protection of nitrogen . After 12h of reaction. After the reactant was concentrated, it was filtered to obtain a precipitate, washed with a large amount of water, and separated by column chromatography on a silica gel column to obtain the second point, ...
Embodiment 2
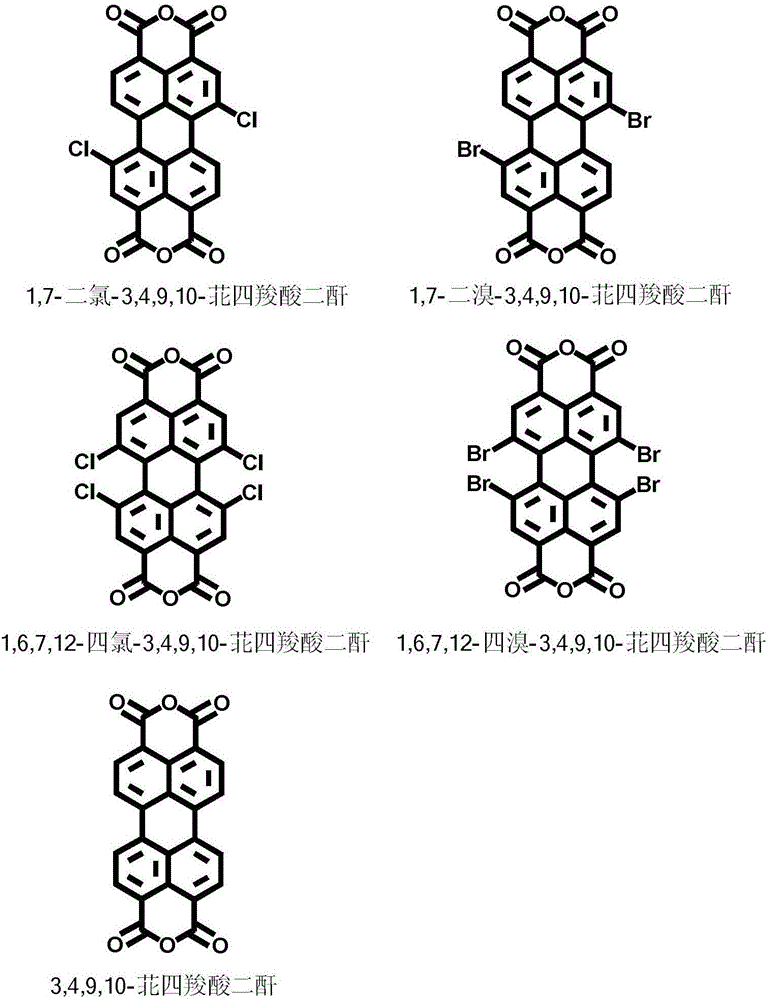
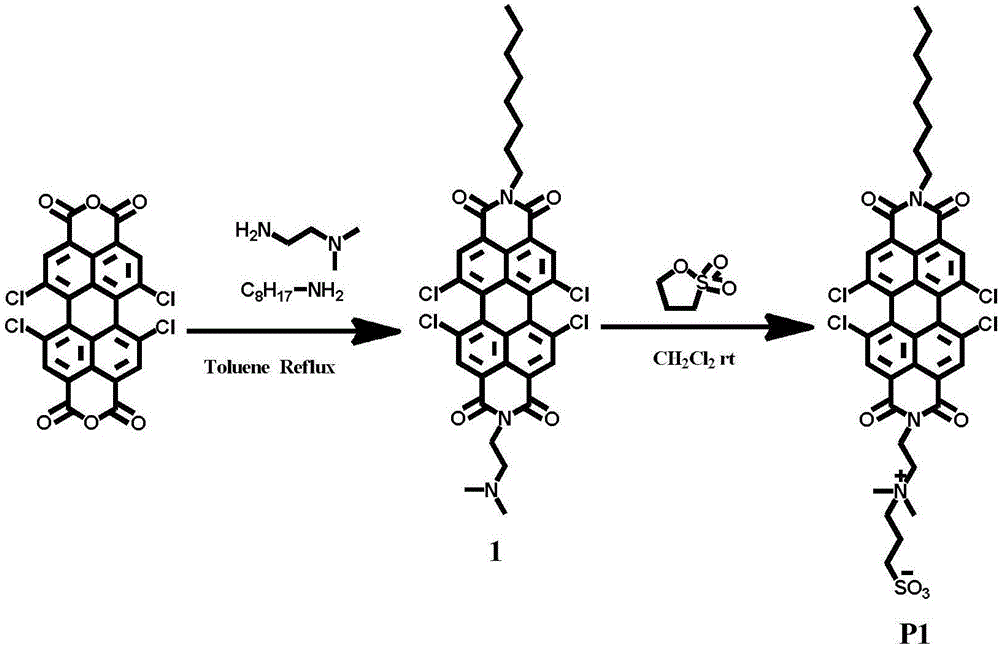
[0045] 1. Dissolve 530mg (1mmol) of 1,6,7,12-tetrachloro-3,4,9,10-perylenetetracarboxylic dianhydride and 130mg (1mmol) of octylamine in 15ml of toluene, and add it to a 50mL three-necked bottle 106mg (1.2mmol about 132ul) of N,N-dimethylethylenediamine was added to the system with a micro-injector, and then the system was sealed with a rubber stopper. Under the protection of nitrogen, magnetic stirring was carried out for 30min, and the temperature was raised to 105°C for reflux. . After 12h of reaction. After the reactant was concentrated, the second point was separated by column chromatography on a silica gel column, and the eluent was dichloromethane / n-hexane (v / v=1 / 1). After concentration and vacuum drying, 278 mg of product 2 was obtained (39% yield).
[0046]
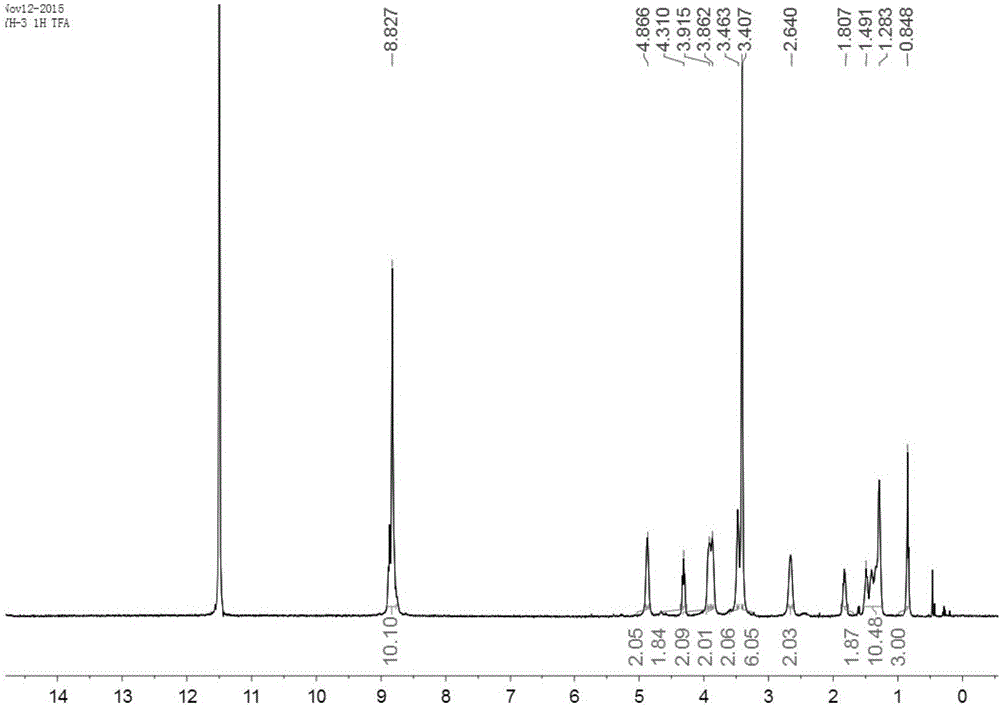
[0047] 2. Add 46mg (0.05mmol) of the above product into the polymerization tube, then add 10ml of dichloromethane, stir in a water bath at room temperature, then add 4uL 1,3-propane sultone 5.5mg (about 0.04...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com